Introduction
Meibomian gland dysfunction (MGD) is believed to be the leading cause of dry eye disease (DED), which afflicts tens of millions Americans (1). Of particular interest, the most common pharmaceutical treatment for the management of MGD in the United Statesis the off-label use of topical azithromycin (2). This macrolide antibiotic is presumed to be effective because of its anti-inflammatory and anti-bacterial actions, which may suppress the MGD-associated posterior blepharitis and growth of lid bacteria (3). However, there are no published, peer-reviewed data demonstrating that azithromycin has the ability to act directly on the human meibomian gland to enhance this tissue’s function, and to ameliorate the pathophysiology of MGD.
We hypothesize that azithromycin can act directly on human meibomian gland epithelial cells to stimulate their differentiation, enhance the quality and quantity of their lipid production, and promote their holocrine secretion. Our purpose was to begin to test our hypothesis.
Material and Methods
Immortalized human meibomian gland epithelial cells (IHMGEC; passages 20–22) were cultured in the presence or absence of 10% fetal bovine serum, as previously reported (4).Cells were treated with the ethanol vehicle or azithromycin (10µg/ml; Santa Cruz Biotechnology) for varying time periods. Cellular morphological appearance was recorded, cells were counted with a hemocytometer, and lipid accumulation was assessed by staining cells with LipidTOX green neutral lipid stain (Invitrogen, Grand Island, NY), according to reported methods (4). Staining fluorescent intensities were quantified by using ImageJ (http://rsbweb.nih.gov/ij/index.html).Statistical analyses were performed with Student’s t-test (two-tailed, unpaired).
Results
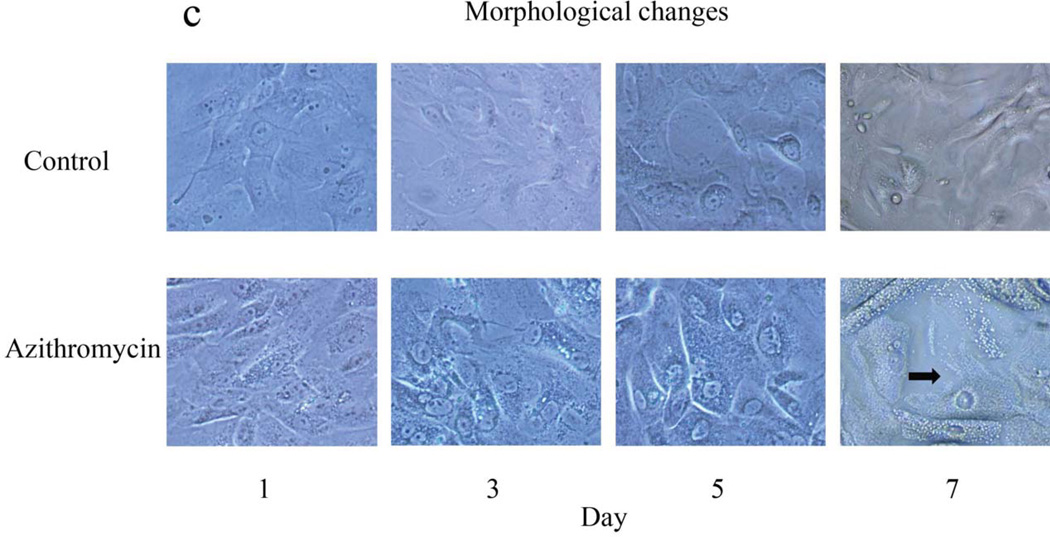
Our results show that azithromycin induces a striking, time-dependent accumulation of lipid in IHMGEC (Figure 1a). Within 3 days of azithromycin exposure, the number, size and staining intensity of intracellular lipid-containing vesicles had markedly increased, as compared to those of vehicle-treated control cells. This azithromycin effect on lipids appeared to become maximal at days 3 to 7 of the study (Figure 1b).
Figure 1.
Effect of azithromycin on the lipid accumulation and morphology of IHMGEC. Cells were treated with ethanol vehicle or azithromycin in serum-containing media for 7 days. Results are representative of three separate experiments. (a) Appearance of cellular lipids. Cells were fixed and stained with LipidTOX Green Neutral Lipid Stain and DAPI (red nuclear stain; Invitrogen). Magnification, × 400. (b) LipidTOX staining intensity. Data (mean ± SE) are reported as fold-change compared to control values on the same day. *Significantly (p < 0.005) greater than control. (c) Cellular morphology. Pictures were taken (magnification × 200) prior to LipidTOX staining. Azithromycin-induced cellular maturation and vesicle accumulation was often followed by cell disruption and vesicle release (arrow, day 7).
Evaluation of cellular morphology indicated that azithromycin may promote terminal maturation of IHMGEC, given that vesicle accumulation was often followed by a cell break-up and vesicle release (Figure 1c).
In contrast to these effects, azithromycin reduced the proliferation of IHMGEC. As shown in Figure 2, this result was found irrespective of whether IHMGEC were cultured under proliferation or differentiation conditions.
Figure 2.
Influence of azithromycin on IHMGEC proliferation. Cells were cultured in the absence (a) or presence (b) of serum for up to 7 days. Cell numbers at day 0 represent the baseline and data are reported as mean ± SE. Similar results were found in two additional studies. *Significantly (p < 0.005) less than control.
Discussion
This study supports our hypothesis that azithromycin can act on human meibomian gland epithelial cells and stimulate their lipid accumulation. This azithromycin effect appears to be paralleled by a cellular maturation, a decreased proliferation, and a holocrine-like secretion.
This azithromycin action is quite notable, because MGD is thought to be the most common cause of DED (1). Typically, the meibomian glands produce and release a lipid mixture that promotes the stability and prevents the evaporation of the tear film, thereby playing an essential role in ocular surface health. Conversely, MGD destabilizes the tear film and increases its evaporation. MGD is caused primarily by hyperkeratinization of the terminal duct epithelium and reduced secretion quality, and leads to cystic dilatation of glandular ducts, acinar cell death and lipid deficiency (1). The end result is DED, characterized by a vicious cycle of tear film hyperosmolarity and ocular surface stress, and leading to increased friction, inflammation and damage to the eye (5). The impact of moderate to severe DED is analogous to conditions such as dialysis and severe angina, and is associated with significant pain, role limitations, low vitality and poor general health (5).
Given our finding that azithromycin stimulates the function and differentiation of human meibomian gland epithelial cells in vitro, it is possible that this antibiotic may prove beneficial as a treatment for MGD and its associated DED in vivo.
Acknowledgments
This research was supported by NIH grant EY05612 and the Margaret S. Sinon Scholar in Ocular Surface Research Fund, and the Guoxing Yao & Yang Liu Research Fund. Yang Liu had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Authors’ contributions: DAS conceived the study. DAS and YL designed and supervised the experiments. YL performed the experiments. DAS, YL, WRK and JD analyzed and interpreted data. DAS and YL wrote and revised the paper. WRK and JD critiqued the paper.
Conflict of interest disclosures: None
References
- 1.Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalenceand treatment. Ocul Surf. 2009;7(2 Suppl):S1–S14. doi: 10.1016/s1542-0124(12)70620-1. [DOI] [PubMed] [Google Scholar]
- 3.Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O'Brien T, Rolando M, Tsubota K, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 201;52(4):2050–2064. doi: 10.1167/iovs.10-6997g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Kam WR, Ding J, Hatton MP, Sullivan DA. Effect of growth factors on the proliferation and gene expression of human meibomian gland epithelial cells. Invest Ophthalmol Vis Sci. 2013;54(4):2541–2550. doi: 10.1167/iovs.12-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan DA, Hammitt KM, Schaumberg DA, Sullivan BD, Begley CG, Gjorstrup P, Garrigue J-S, Nakamura M, Quentric Y, Barabino S, Dalton M, Novack GD. Report of the TFOS.ARVO Symposium on global treatments for dry eye disease: An unmet need. Ocul Surf. 2012;10(2):108–116. doi: 10.1016/j.jtos.2012.02.001. [DOI] [PubMed] [Google Scholar]