Abstract
Tetrahydrobiopterin (BH4) is important for normal brain development as congenital BH4 deficiencies manifest movement disorders at different childhood ages. BH4 transitions from very low levels in fetal brains to higher ‘adult’ levels postnatally, with the highest levels in the thalamus. Maternal supplementation with BH4-precursor, sepiapterin, reduces postnatal motor deficits and perinatal deaths following 40-min fetal hypoxia-ischemia (HI) at 70% gestation, suggesting brain BH4 is important in improving function after HI. We tested the hypothesis that the intrinsically low concentrations of BH4 made fetal neurons vulnerable to added insults. Brains were obtained from either naïve fetal rabbits or after 40-min HI, at 70% (E22) and 92% gestation (E29). Neuronal cultures were prepared from basal ganglia, cortex and thalamus, regions with different intrinsic levels of BH4. Cultures were grown with or without added BH4 to 48 hours. Cell survival and mitochondrial function were determined by flow cytometry. At E22, thalamic cells had the lowest survival rate in a BH4-free milieu, in both control and HI groups, while BH4 supplementation ex vivo increased neuronal survival in only HI cells. Neuronal survival was similar in all regions without BH4 at E29. BH4 supplementation increased cell survival and cells with intact mitochondrial membrane potential, from basal ganglia and cortex, but not thalamus. After E29 HI, however, the benefit of BH4 was limited to cortical neurons. We conclude that BH4 is important for fetal neuronal survival following HI especially in the premature thalamus. Supplementation of BH4 has a greater benefit at an earlier gestational age.
Keywords: Tetrahydrobiopterin, anoxia, fetus, thalamus, cortex, basal ganglia, premature, neurons, brain, cell survival
Introduction
Tetrahydrobiopterin (BH4) serves as cofactor for many important enzymes in the brain involved in biogenic amines and nitric oxide production. A deficient level of BH4 is a known etiological factor in several neurological syndromes characterized by developmental delay, movement disorders and seizures. Deficiency of BH4 caused by autosomal dominant guanosine triphosphate cyclohydrolase deficiency (GTPCH), the first enzyme in the biosynthetic pathways, manifests as the syndrome of DOPA-responsive dystonia with dysfunction of the nigrostriatal dopaminergic neurons [1]. Gene mutations of next two enzymes in the de novo biosynthetic pathway, 6-pyruvoylpteridine and sepiapterin reductase, are also associated with movement disorders that manifest to different degrees with onset at different ages. Most importantly, BH4 treatment after the onset of symptoms can ameliorate some of the movement disorders [2]. Increasing brain BH4 may improve dopamine production. Recently, it has been proposed that BH4 may also show some benefit in autistic children [3, 4].
We have shown that there is a developmentally low level of BH4 in fetal rabbit brains at preterm gestation when compared to near-term or postnatal rabbit brain [5]. BH4 levels increased developmentally in all brain regions during pregnancy but concentrations varied significantly in different brain regions. BH4 levels were highest in the thalamus and lowest in the cortex at term gestation [5]. The significance of these developmental variations remains somewhat elusive. The manifestation and onset of congenital deficiency disorders suggest that an intrinsic threshold of BH4 may be necessary for maintenance of normal healthy cells, and that different regions required different levels of BH4 for normal development.
We have shown that sepiapterin administration to pregnant rabbit dams at preterm gestation increased fetal brain BH4 levels [5]. This maternal intervention prevented severe motor deficits and perinatal deaths following global hypoxia-ischemia (HI) at 70% gestation (22 days or E22) [5]. This response suggests that increasing BH4 may be a key factor to neuron survival. Our hypothesis was that the intrinsically low concentrations of BH4 made fetal neurons vulnerable to additional insults. We compared neuronal survival in cell culture conditions with neurons from three brain regions with different intrinsic levels of BH4, and also compared naïve animals with those after an in vivo HI insult. We also tested whether ex vivo supplementation of BH4 could equalize the differences in cell survival and mitochondrial function. Herein, we show that in primary culture, thalamic neurons from preterm fetal brains are the most sensitive to BH4 deprivation (in both control and HI groups) in contrast to those from near-term brains. Supplementation of BH4 in culture media significantly increased the number of surviving neurons from preterm thalamus, after fetal HI, implicating a developmental vulnerability of preterm neurons.
Materials and Methods
Animals
This study was approved by the Animal Review Committee of the NorthShore University HealthSystem Research Institute. Animals received humane care and were treated in compliance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
Surgery
The surgical procedure has been described previously [6]. Briefly, In vivo global HI of fetuses was induced by uterine ischemia in timed pregnant New Zealand white rabbits. The dams were anesthetized with intravenous fentanyl (75 μg/kg/hr) and droperidol (3.75 mg/kg/hr), and bag and mask ventilation was provided to maintain normal arterial pH (7.35–7.45), Pco2 (32–45 torr), and Po2 (70–100 torr). Uterine ischemia, which resulted in fetal HI, was induced with a 4 F Fogarty balloon catheter (Baxter Health Care Corporation, Santa Ana, CA). The catheter was introduced into the left femoral artery; advanced 10 cm into the descending aorta to above the uterine and below the renal arteries, and the balloon was inflated with 300 μl of saline. At the end of the procedure (40 minutes later), the balloon was deflated and the catheter was removed.
Primary neuron culture
Fetal brains were extracted from either naïve animals or after HI insult in utero. The cortex, basal ganglia and thalamus were dissected. Cell suspensions were prepared according to the procedure described previously [7]. Briefly, after the meninges were removed, the brain sample was placed in 0.025% trypsin and incubated on a rotating shaker at 37°C for 45 min. The brain suspension was spun at 300 X g for 10 min, the trypsin aspirated, and the cells were washed with HBSS before limited trituration (limited to 30 times) in Neurobasal® Media (Life Technologies, Carlsbad, CA). The brain suspension was then passed through a sterile 70 μ filter to produce a single-cell suspension. This procedure with gentle enzyme digest and limited trituration was chosen from many combinations because it caused minimal cell death as determined by propidium iodide staining.
After the cells were obtained from each brain region they were pooled with the cells from their litter mates. Each litter thus gave a number=2 or 3 regions. Cells were counted and plated in six well plates as described previously [7]. Cell cultures were grown in Neurobasal® Medium supplied with 10% B27 supplement (Invitrogen, Carlsbad, CA) at 37°C with 21% O2 and 5% CO2. For BH4 supplementation in wells, desferroxamine was added to the medium to a final concentration of 0.1 mM and incubated for one hour, followed by addition of BH4 to a final concentration of 0.1 μM. Desferroxamine was necessary for ensuring stability of BH4 in solution. Another dose of BH4 was added four hours later to bring the final concentration to 0.2 μM. After 24 hours of incubation, the supernatant was collected for flow cytometry analysis and fresh medium was added with the same final concentration of BH4 (0.2 μM). After 48 hours of incubation from the initial culture, the supernatant and attached cells were collected [7] and again subjected to flow cytometric analysis.
Groups
We investigated the influence of four independent factors:
As BH4 level increases with gestational age, preterm (E22) and near-term (29 days gestation, E29) brains (term 31.5 days) were studied to compare fetal brains with different endogenous BH4 levels.
As HI is known to cause a decrease in BH4 levels [5], we compared naive rabbits (controls) with HI rabbits.
As the thalamus is known to have the highest BH4 level among all regions at birth [5], cortex and basal ganglia were compared to thalamus to find out the regional fate of neuronal cultures in medium without BH4.
The primary cell cultures were grown without BH4 (Neurobasal media per se does not have BH4) and then compared to similar cultures with BH4 supplementation.
The saline group only received an equal volume of saline.
Flow cytometry analysis
Immediately after preparation, an initial aliquot of cell suspension was analyzed by flow cytometry. This initial cell count was used to ensure that equal numbers of cells went into each well. Subsequently, the supernatant in the wells was collected at 24 and 48 hour in addition to the attached cells in the wells at 48 hour, to obtain a total cell count of surviving cells (Figure 1).
Figure 1.
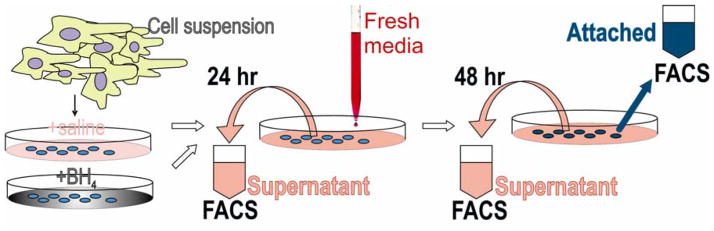
Experimental Plan and Flow cytometric Analysis
Cell suspensions were analyzed and subpopulations of 1) living cells defined by negative propidium iodide staining (PI −), 2) neurons, cells positive for cholera toxin, 3) neurons with functioning mitochondria, cells positive for both cholera toxin and Rhodamine 123 (Cholera + Rhodamine +) as described previously [7], and 4) cells with high mitochondrial function that had higher red than green fluorescence with JC-1 staining. JC-1 was used in a concentration of 5 μg/ml for assessing mitochondrial function [8]. The healthy cells with high mitochondrial function have higher Δψm and JC-1 spontaneously forms complexes known as J-aggregates with intense red fluorescence.
Neuronal survival ex vivo was estimated by two indices that differ in the denominator used in calculating the index: First, the number of attached cells at 48 hours was divided by initial number of cells loaded on each well (seeded cells); thus, the numbers of Cholera + Rhodamine + cells were divided by the initial seeded live cells that were negative for PI. This index was labeled as ‘Healthy neurons % seeded” for brevity and clarity.
The second parameter used to estimate neuronal survival was the efficiency of attachment by estimating the ratio of attached cells over total cells in the dish. Total cells included estimations of floating cells in supernatant at 24 and 48 h plus attached at 48 h. This index was labeled as ‘Attachment efficiency of cells’ for all cells counted by flow cytometry. Similarly, ‘Attachment efficiency of functioning mitochondria neurons’ was labeled for Cholera + Rhodamine + cells and ‘Attachment efficiency of high mitochondria cells’ for JC-1 red>green stained cells. These indices provide a little more information on the functional properties of living cells by estimating the attachment efficiency.
Statistical analysis
All values were expressed as Mean ± SEM. For power estimations of number of kits, we used a futility design for the most efficient use of animals [9] and taking α = 0.1, power = 0.85, effect size equal to standard deviation of cell death from previous studies. Results were analyzed using ANOVA and multiple comparisons were done with Tukey’s Studentized Range Test, using SAS v9.2 (SAS Institute Inc, North Carolina, USA). Paired t-test results were used for comparison between brain regions and for changes with BH4 supplementation. Instead of correcting for multiple comparisons, actual p values are shown for comparison.
Results
The responses of three brain regions with different intrinsic BH4 levels to cell culture conditions in BH4-free media were compared.
Preterm Thalamic Neurons Show Less Survival in BH4-free Culture Conditions
We first investigated survival rates of cells cultured from naïve rabbit fetal brain at an age (E22), when BH4 levels in all regions were low [5]. At this age, brain BH4 levels were similar to those in the prototypical model used to study BH4 deficiency, the hph-1 mouse with low GTPCH activity [10].
Healthy neurons % seeded
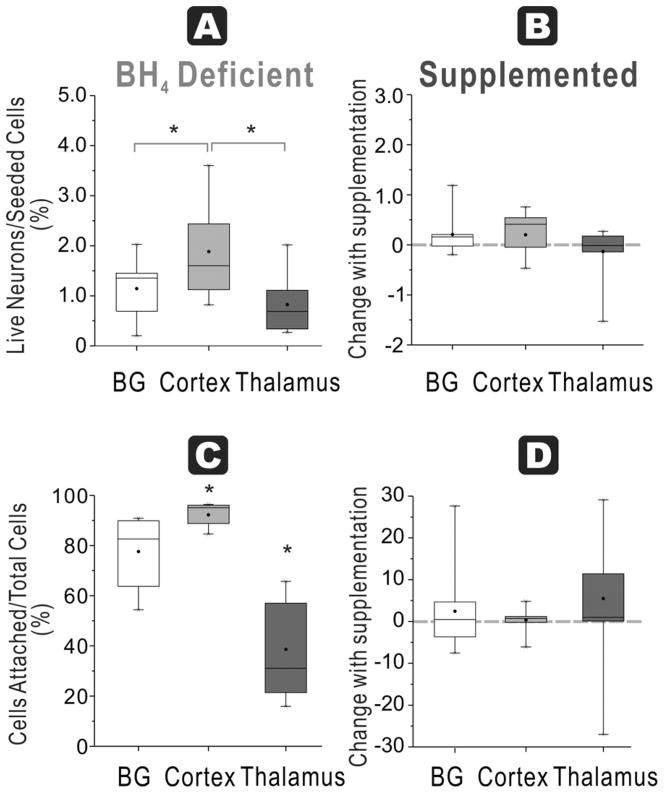
At 48 h, cortex cultures from naïve fetuses in BH4-free media had the highest number of ratio of attached live neurons/seeded cells (2 ± 0.3 %), which was significantly higher than basal ganglia (1 ± 0.2 %) and thalamus (1 ± 0.2 %) groups (Figure 2A). Thalamus had the lowest number of cells.
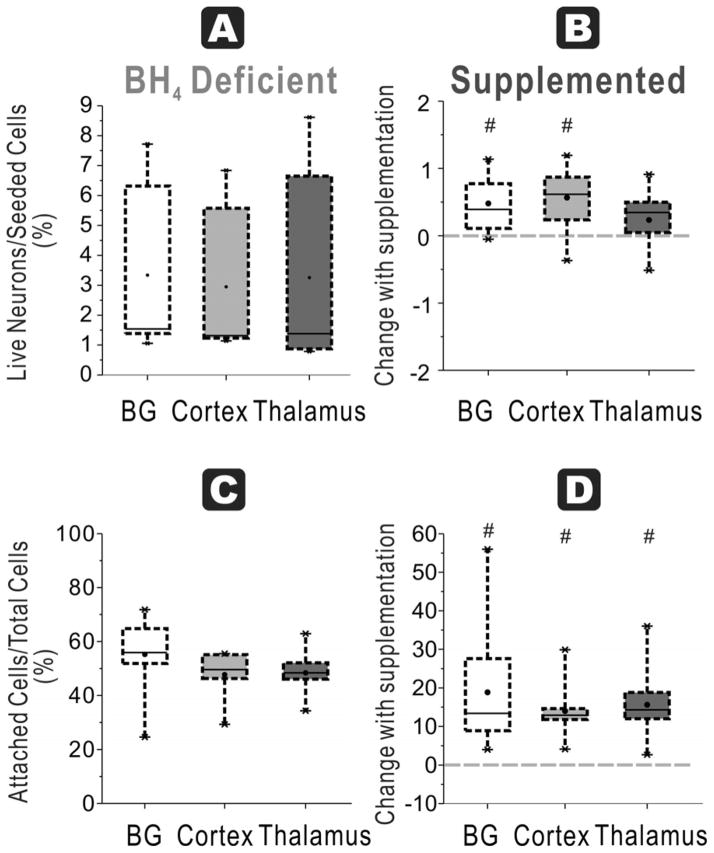
Figure 2. Regional susceptibility to BH4 deficiency for neuronal survival and function at E22.
A. Healthy neurons % seeded: In E22 brains, the ratio of live neurons (Cholera toxin and Rhodamine positive cells after 48h culture) divided by total cells (from flow cytometer count of initial cell suspension) show the highest number in cortex compared to basal ganglia and thalamus in a BH4 deficient environment (ANOVA p=0.0153; * p=0.0354 cortex vs basal ganglia, p=0.0028 vs. thalamus, paired t-test). B. With BH4 supplementation, there are no increases in recovery of the ratio (shown as a % change) in any of the groups. C. Attachment efficiency of cells: The efficiency of attachment given by the percentage of attached cells (at 48 h) over the total cells obtained in supernatants at 24 h and 48 hr and attached cells show that the lowest attachment in the thalamus group (ANOVA p<0.0001, *p<0.0001 vs cortex and p=0.007 vs basal ganglia, paired t-test); basal ganglia is lower than cortex (p=0.0116, paired t-test). D. Supplementation with BH4 did not increase the efficiency of attachment in any of the groups (dashed line shows 0 change).
In HI animals, thalamus cultures again had the lowest ratio (1 ± 0.2 %) compared to basal ganglia (2 ± 0.4 %) and cortex (3 ± 0.5 %, n=11/group, Figure 3A). HI did not show any appreciable change in this ratio compared to naïve in any of the regions except for basal ganglia (p=0.042). Thus, there was no loss of cells that attached with HI in any of the regions.
Figure 3. HI at E22 and BH4 dependency on neuronal survival and function.
A. Healthy neurons % seeded: In the HI group for E22, ratio of live neurons (Cholera toxin and Rhodamine positive cells after 48h culture) divided by total cells (from flow cytometer count of initial cell suspension) show the lowest number in thalamus in a BH4 deficient environment (ANOVA p=0.0172; *p =0.0009 vs basal ganglia and p=0.0012 vs cortex, paired t-test). B. Healthy neurons % seeded With BH4 supplementation, this ratio was significantly increased in thalamus, but not in basal ganglia and cortex groups (# p=0.0004, paired t-test). C. Attachment efficiency of cells: In the HI group, the efficiency of attachment given by the percentage of attached cells (at 48 h) over the total cells obtained in supernatants at 24 h and 48 hr and attached cells show that the lowest attachment in the thalamus group (ANOVA p=0.0003; * p=0.0004 vs. basal ganglia and p=0.0021 vs cortex, n=11, paired t-test). D. Attachment efficiency of cells: Supplementation with BH4 increase the efficiency of attachment in both basal ganglia (# p=0.0398) and thalamus (# p=0.0056, n=11, paired t-tests).
Attachment efficiency of cells
This ratio had a similar pattern to ‘Healthy neurons % seeded’. This was most likely due to the fact that the majority of surviving cells were neurons at 48 hours. In the naïve fetus, thalamus had the lowest survival ratio of attached cells/total cells (39 ± 7 %) compared to basal ganglia (78 ± 5 %) and cortex (92 ± 2 %, n= 11, Figure 2C). In the HI group, thalamus once again had the lowest ratio (24 ± 6 %) compared to basal ganglia (65 ± 6 %) and cortex (59 ± 8%, n =11/group, Figure 3C).
Comparing naïve and HI groups, only cortex showed a significant decrease with HI (p<0.002). Thus, there was less attachment in the cortex following in vivo HI.
Attachment efficiency of functioning mitochondria neurons
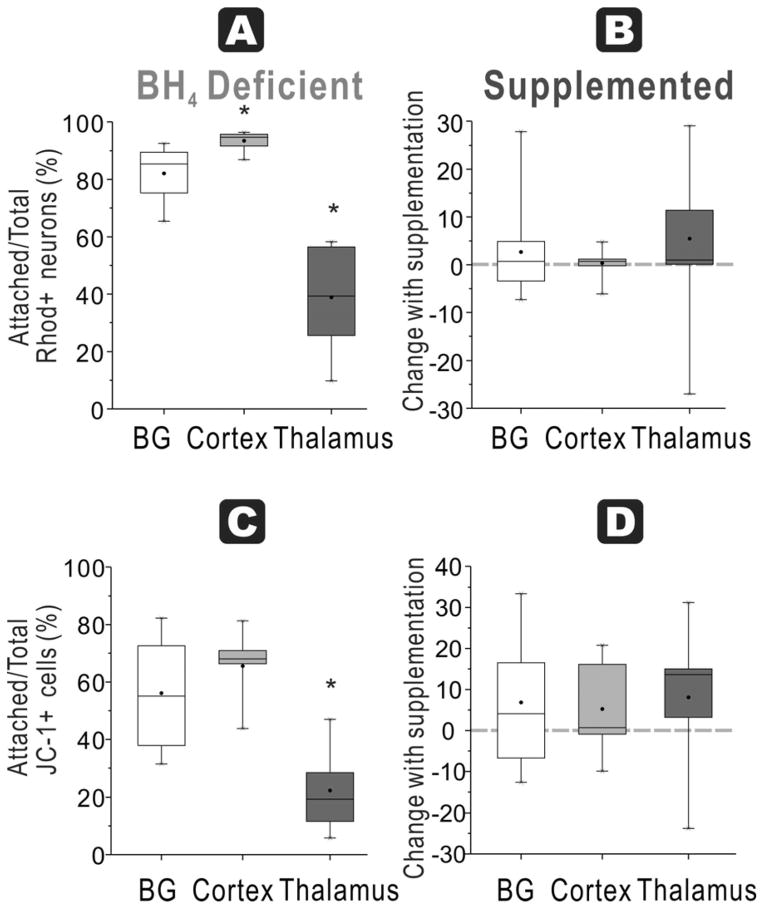
In naïve fetuses, thalamus had significantly lower ratio of attached functioning mitochondria neurons/total neurons (39±6%) compared to basal ganglia (82±3%) and cortex (94±1%, n=9/group, Figure 4A).
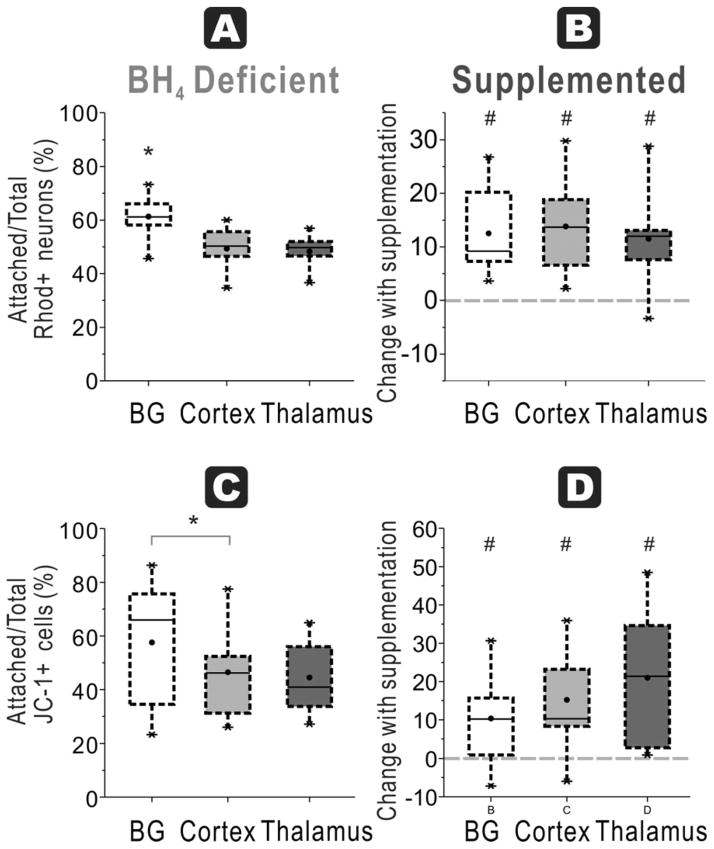
Figure 4. BH4-dependent mitochondrial function and regional susceptibility at E22.
A. Attachment efficiency of functioning mitochondria neurons: In E22 brains, the ratio of attached neurons with functioning mitochondria (Rhodamine + Cholera + cells at 48 h) over total neurons with functioning mitochondria (in supernatant at 24 and 48 h plus attached at 48 h) was the highest in the cortex in a BH4 deficient environment (ANOVA p<0.0001; *p=0.0057 cortex vs basal ganglia, p<0.0001 cortex vs. thalamus, p=0.0003 basal ganglia vs. thalamus, paired t-test). B. With BH4 supplementation, there are no increases in recovery of the ratio (shown as a % change) in any of the groups. C. Attachment efficiency of high mitochondria cells: Thalamus neurons from E22 rabbit fetal brain had the lowest ratio of attached cells high mitochondrial function (JC healthy -1 red>green fluorescent cells at 48 h) to total cells with high mitochondrial function (in supernatant at 24 and 48 h plus attached at 48 h) without BH4 supplementation (ANOVA p<0.0001; * p=0.0068 vs. basal ganglia and p=0.0001 vs. cortex, paired t-test). D. There were no differences in this ratio in any of the groups with BH4 supplementation.
In the HI group, thalamus again had the lowest ratio (24 ± 6 %) compared to basal ganglia (69 ± 6 %) and cortex (61 ± 7%, n =11/group, Figure 5A). Comparing naïve and HI groups, cortex again showed a significant decrease of cell attachment with HI (p<0.001).
Figure 5. HI at E22 and BH4-dependent regional mitochondrial function.
A. In the HI group for E22, the ratio of attached neurons with functioning mitochondria (Rhodamine + Cholera + cells at 48 h) over total neurons with functioning mitochondria (in supernatant at 24 and 48 h plus attached at 48 h) was the lowest in thalamus in a BH4 deficient environment (ANOVA p<0.0001; * p=0.0002 vs basal ganglia and p=0.0007 vs cortex, paired t-test). B. Attachment efficiency of functioning mitochondria neurons: With BH4 supplementation, this ratio significantly increased in thalamus (# p=0.0099, paired t-test) but not in basal ganglia and cortex. C. Attachment efficiency of high mitochondria cells: In the E22 HI group, thalamus had the lowest ratio of attached cells with healthy mitochondria (JC-1 red>green fluorescent cells at 48 h) to total cells with healthy mitochondria (in supernatant at 24 and 48 h plus attached at 48 h) without BH4 supplementation (ANOVA p=0.0313; *p=0.0098 thalamus vs basal ganglia, paired t-test). D. With BH4 supplementation, there are no increases in recovery of this ratio in any of the groups.
Attachment efficiency of high mitochondria cells
Assessing the efficiency of attachment of cells with JC-1 red>green labeled cells, naïve fetal thalamus again had the lowest ratio (22 ± 5%) compared to basal ganglia (56 ± 7%) and cortex (66 ± 4 %, n=9, Figure 4C). In HI brains, there was a similar but non-significant trend for this ratio with 48 ± 8% for basal ganglia, 34 ± 8% for cortex and 19 ± 3 % for thalamus (n=7–8/group, Figure 5C).
Near-Term Thalamic Neurons are More Resistant to Hypoxic Injury
Healthy neurons % seeded
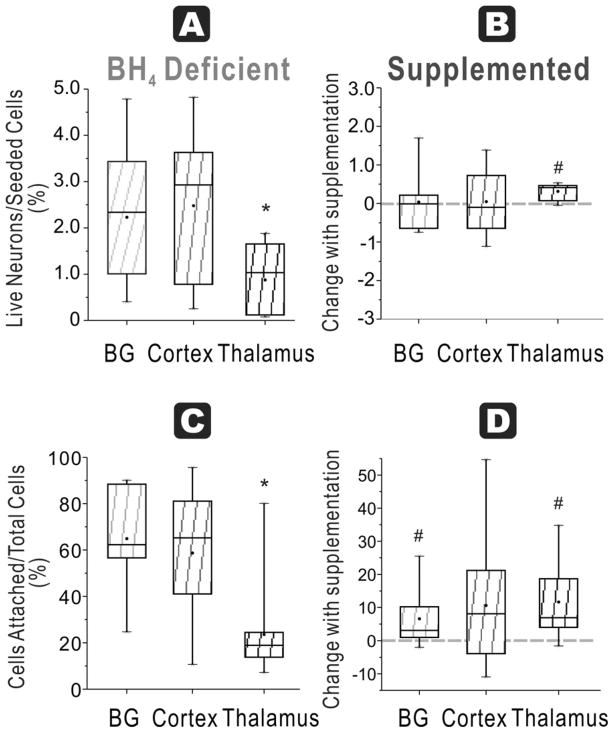
In naïve fetuses at E29, the ratio of attached live neurons/seeded cells was not significantly different among basal ganglia 3 ± 1%, cortex 3 ± 1 % or thalamus 3 ± 1 % (n=9/group, Figure 6A).
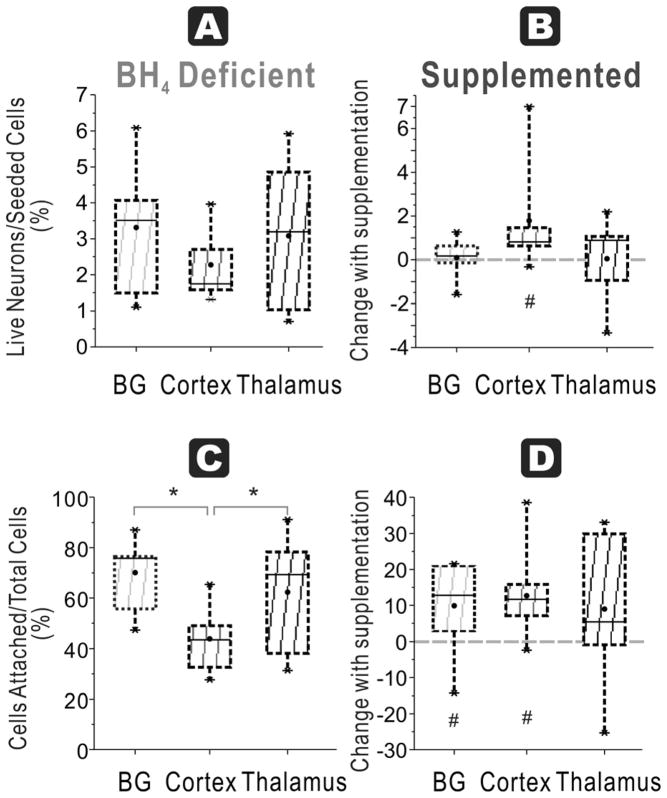
Figure 6. Most regions show improvement with BH4 in neuronal survival and function at E29.
A. Healthy neurons % seeded: In E29 brains (depicted by dashed lines), the ratio of live neurons (Cholera toxin and Rhodamine positive cells after 48h culture) divided by total cells (from flow cytometer count of initial cell suspension) was not different among cortex, basal ganglia and thalamus in a BH4 deficient environment. B. Healthy neurons % seeded: With BH4 supplementation, this ratio significantly increased in both basal ganglia and cortex (# p=0.0109 and p=0.0103 respectively, paired t-test) but not in thalamus group. C. Attachment efficiency of cells: The efficiency of attachment given by the percentage of attached cells (at 48 hr) over the total cells obtained in supernatants at 24 h and 48 hr and attached cells at 48 hr showed no difference attachment among the thalamus, cortex and basal ganglia. D. Attachment efficiency of cells: Supplementation with BH4 increased the efficiency of attachment in all of the groups (#BG p=0.0084, Cortex p=0.0006, thalamus p=0.0012, paired t-test).
In HI group, there was again no difference among the three regions, basal ganglia 3 ± 1 %, cortex 2 ± 0% and thalamus 3 ± 1 % (n=9/group, Figure 7A). Again, HI did not show any appreciable decrease in the yield of attached cells after cell culture except in the cortex (p<0.049).
Figure 7. HI at E29 and BH4 dependency on neuronal survival and function specific to cortex.
A. Healthy neurons % seeded: In the HI group for E29, ratio of live neurons (Cholera toxin and Rhodamine positive cells after 48h culture) divided by total cells (from flow cytometer count of initial cell suspension showed no difference among the three regions. B. Healthy neurons % seeded: With BH4 supplementation, this ratio was significantly increased in cortex (#, p=0.04911, n=9/group, paired t-test), but not in basal ganglia and thalamus. C. Attachment efficiency of cells: In the HI group, the efficiency of attachment given by the percentage of attached cells (at 48 h) over the total cells obtained in supernatants at 24 h and 48 hr and attached cells showed that cortex had the least cells (ANOVA p=0.0132; * p=0.0015 cortex vs. basal ganglia, p=0.0399 cortex vs. thalamus, n=9/group, paired t-test). D. Attachment efficiency of cells: Supplementation with BH4 increased the efficiency of attachment in both basal ganglia and cortex (# p =0.0421 and p=0.0126 respectively, n=9, paired t-test), but not in thalamus.
Attachment efficiency of cells
In naïve fetuses, there was no significant difference among basal ganglia (55 ± 5 %), cortex (48 ± 3 %) and thalamus (48 ± 3 %, Figure 6C). In the HI group, basal ganglia (70 ± 5 %) and thalamus (62 ± 8 %) were significantly higher than cortex (44 ± 5 %, Figure 7C).
Attachment efficiency of functioning mitochondria neurons
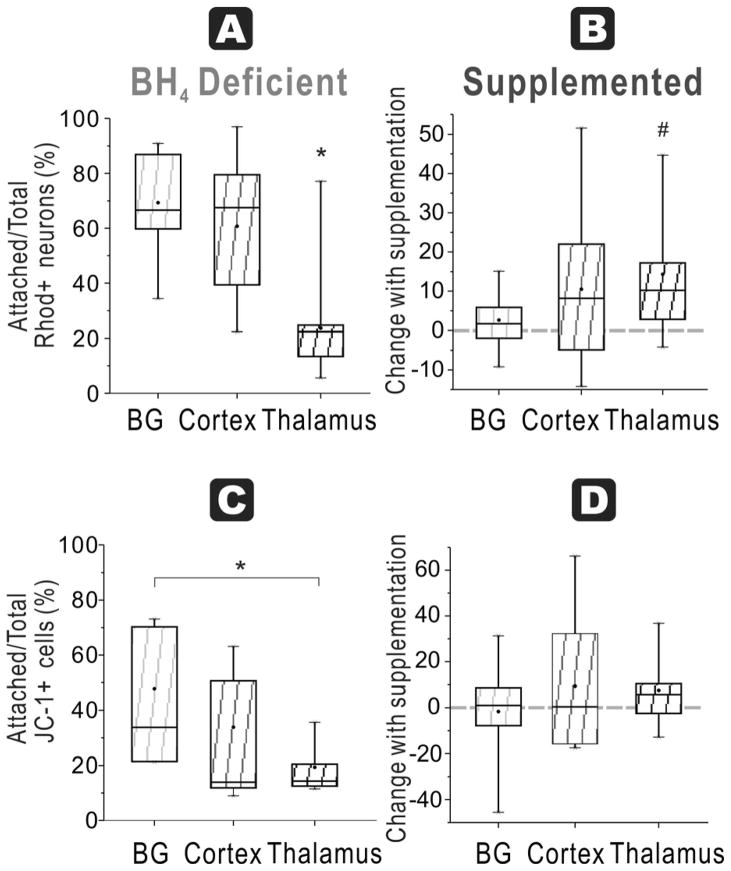
In naïve fetuses, the ratio in basal ganglia (61 ± 3 %) was significantly higher compared to cortex (49 ± 3%) and thalamus (48 ± 2%, n=9/group), with no difference between thalamus and cortex (Figure 8A).
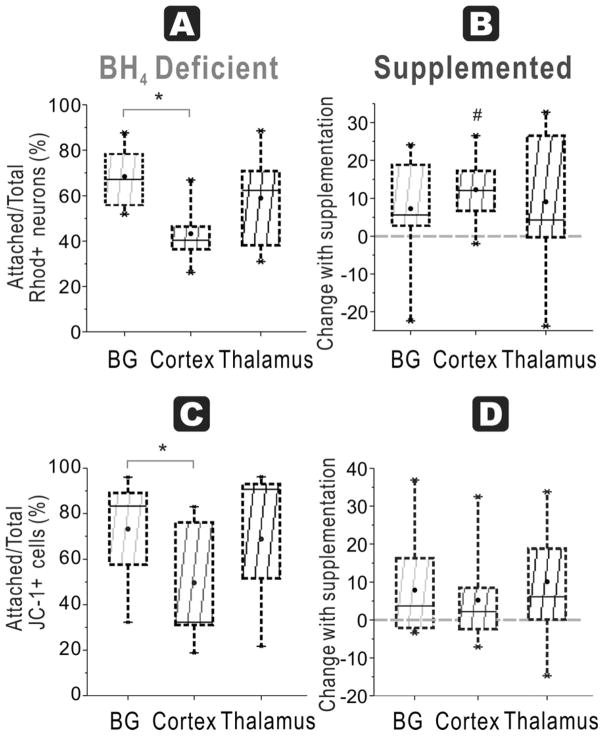
Figure 8. BH4-dependent mitochondrial function in all regions at E29.
A. In E29 brains, the ratio of attached neurons with functioning mitochondria (Rhodamine + Cholera + cells at 48 h) over total neurons with functioning mitochondria (in supernatant at 24 and 48 h plus attached at 48 h) show the highest number in basal ganglia compared to cortex and thalamus in a BH4 deficient environment (ANOVA p=0.0030; *p=0.0017 basal ganglia vs. cortex, p=0.0072 vs. thalamus, n=9/group, paired t-test). B. Attachment efficiency of functioning mitochondria neurons: With BH4 supplementation, there are significant increases in recovery of the ratio (shown as a % change) in all groups (# basal ganglia p=0.0017, Cortex p=0.0013, thalamus p=0.0045, n=9/group, paired t-test). C. Attachment efficiency of high mitochondria cells: Basal ganglia neurons from E29 rabbit fetal brain had higher ratio of attached cells with high mitochondrial function (JC-1 red>green fluorescent cells at 48 h) to total cells with high mitochondrial function (in supernatant at 24 and 48 h plus attached at 48 h) compared to cortex, without BH4 supplementation (* p=0.0234, n=9/group, paired t-test but ANOVA not significant). D. Attachment efficiency of high mitochondria cells: With BH4 supplementation, there were significant increases in recovery of this ratio in all the groups (# basal ganglia p=0.0314, Cortex p=0.0102, thalamus p=0.0079, n=9/group, paired t-test).
In HI group, basal ganglia (68 ± 4 %) was significantly higher than cortex (43 ± 5 %) with no difference between thalamus (59 ± 7 %) and other regions (n=9/group, Figure 9A).
Figure 9. HI at E29 and BH4-dependent mitochondrial function specific to cortex.
A. Attachment efficiency of functioning mitochondria neurons: In the HI group for E29, the ratio of attached neurons with functioning mitochondria (Rhodamine + Cholera + cells at 48 h) over total neurons with functioning mitochondria (in supernatant at 24 and 48 h plus attached at 48 h) in basal ganglia was higher than cortex in a BH4 deficient environment (ANOVA p=0.0137; * p=0.0077 basal ganglia vs. cortex, paired t-test). B. Attachment efficiency of functioning mitochondria neurons: With BH4 supplementation, this ratio significantly increased in cortex (# p=0.0046, paired t-test) but not in basal ganglia and thalamus. C. Attachment efficiency of high mitochondria cells: Cortex had a lower ratio of attached cells with healthy mitochondria (JC-1 red>green fluorescent cells at 48 h) to total cells with healthy mitochondria (in supernatant at 24 and 48 h plus attached at 48 h) compared to basal ganglia without BH4 supplementation (* p=0.0179, paired t-test although ANOVA not significant). D. With BH4 supplementation, there were no increases in recovery of this ratio in any of the groups.
Attachment efficiency of high mitochondria cells
In naïve fetuses basal ganglia (58 ± 8 %) again had significantly higher ratio than cortex (47 ± 6 %) and thalamus (45 ± 5 %, Figure 8C). In HI group, basal ganglia had the highest ratio (73 ± 8%), which was significantly higher than that of cortex, but not thalamus (Figure 9C).
BH4 Supplementation Improves Cell Survival
Supplementation of neuronal cultures with BH4 caused differential effects depending on age. In naïve fetuses at E22, BH4 supplementation did not increase any of the indices from any of the regions (Figure 2B, D & Figure 4B, D). This is in contrast to the naïve fetuses at E29 (see later). In the HI group, at E22, BH4 supplementation improved neuronal survival in the thalamus in 3 of the 4 indices: ‘Healthy neurons % seeded’ (paired t test, p=0.0004, n=11/group, Figure 3B), ‘Attachment efficiency of cells’ (p=0.0056, n=11/group, Figure 3D) and ‘Attachment efficiency of functioning mitochondria neurons’ (p=0.0099, n=11/group, Figure 5B). BH4 supplementation only improved ‘Attachment efficiency of cells’ in basal ganglia (p=0.0398, n=11/group) while there was no difference in other indices. There was no difference in cortex with BH4 supplementation.
At E29, the response to BH4 supplementation in all groups was different from the response in E22. In naïve E29 fetuses, BH4 supplementation significantly increased neuronal survival in all indices in all regions at 48h, except for thalamus for ‘Healthy neurons % seeded’. Basal ganglia and cortex had an increased ratio (p=0.0109 and 0.0103, respectively, n=11/group, Figure 6B). For ‘Attachment efficiency of cells’, BH4 supplementation significantly increased ratio in basal ganglia (p=0.0084), cortex (p=0.0006) and thalamus (p=0.0012, n=11/group, Figure 6D). ‘Attachment efficiency of functioning mitochondria neurons’ increased in all three regions with BH4 supplementation (p=0.0017, 0.0013 and p=0.0045 for basal ganglia, cortex and thalamus respectively, n=9/group, Figure 8B). ‘Attachment efficiency of high mitochondria cells’ also increased in basal ganglia (p=0.0314), cortex (p=0.0102) and thalamus (p=0.0079, n=11/group, Figure 8D). The implications of these results is that in normal development, older gestation neurons have increased vulnerability if they are exposed to a state of BH4 deficiency and this vulnerability can be reversed with BH4 supplementation.
In contrast to the naïve fetus at E29 and the HI fetus at E22, brain regions responded differently to exogenous BH4 supplementation following HI at E29. BH4 supplementation improved neuronal survival in the cortex in 3 of the 4 indices: ‘Healthy neurons % seeded’ (p=0.0491, n=9/group, Figure 7B), ‘Attachment efficiency of cells’ (p=0.0126, n=9/group), and ‘Attachment efficiency of functioning mitochondria neurons’ (p=0.0046, n=9/group). BH4 supplementation only improved ‘Attachment efficiency of cells’ in basal ganglia (p=0.0420, n=9/group) while there was no difference in other indices. There was no difference in thalamus with BH4 supplementation, in contrast to the thalamus after E22 HI.
Discussion
The main findings of this study are that the different levels of BH4 found in different fetal brain regions reflect a differential vulnerability of developing neurons to injury and survival. This is the first study to show that at a premature gestation (70%. E22), neurons obtained from the thalamus region are most sensitive to ex vivo BH4-free conditions. The translational implication is that an added insult, such as HI that reduces BH4 levels in vivo, would make the neurons increasingly vulnerable to cell death. This contention is supported by the rescue of neurons in the thalamus by ex vivo BH4. The other distinctive finding of this study was that with increasing gestation, the thalamus at near-term gestation (E29) was no longer different from other regions to the response to BH4 rescue. This would suggest that during development the attainment of a threshold amount of BH4 may be enough for survival. BH4 supplementation ex vivo improved neuronal survival and cell functions in all regions in non-HI E29 animals. Following HI at E29, only cortex showed definite benefit from BH4 treatment, suggesting that the fall in BH4 with HI, renders the region with the lowest vulnerability to injury.
We have previously shown that BH4 levels in fetal rabbit brains showed a differential development in different regions [5]. In E22 fetuses, both BH4 and GTPCH levels were lower than those in E29 [5]. In general, BH4 level increases with gestation from very low levels in prematurity but the levels at near-term are still lower than newborn levels. The region with the highest absolute BH4 level at all gestational ages is the thalamus. It is possible that neurons in the thalamus need more BH4 to thrive. The greater susceptibility of thalamic neurons to death following HI likely reflects the specific need for BH4 in this region.
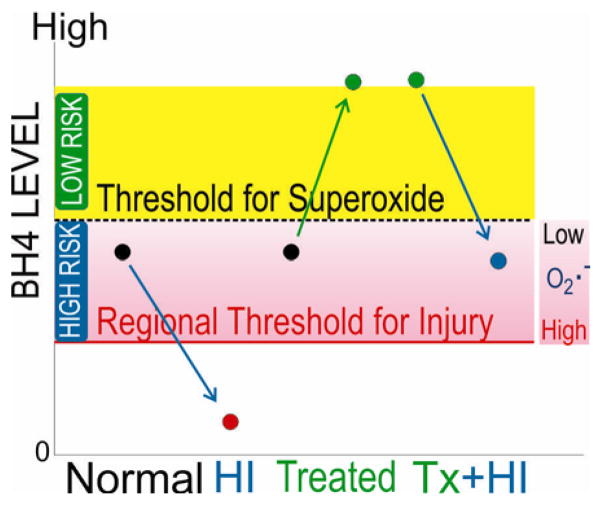
Generally brain cells develop normally from a stage of low to higher BH4 without incident. However, an added insult during the stage of low levels could trigger cell death pathways. In our study, we used two insults: ex vivo cell culture conditions and in vivo HI. Note that cell culture for 48 hours probably selects the cells that could survive in a milieu of very low BH4. In this situation, supplementation would not improve survival, as was the case in our study for E22. The exact biochemical mechanisms of cell death and survival that involve BH4 in neurons have not been elucidated. One of the potential pathways for improving survival may be related to nitric oxide (NO)-dependent mechanisms. BH4 is a cofactor for nitric oxide synthase (NOS). BH4 deficiency decreases NO-formation but increases superoxide, a process known as NOS uncoupling [11–13]. Thus, uncoupling of NOS at very low BH4 levels [11] may lead to cellular dysfunction [14]. We have found that mRNA levels of neuronal NOS (nNOS) are developmentally regulated and regional expression mirrored the findings of BH4 levels, with the thalamus having the highest expression of enzyme. Hypoxia induced increased expression of nNOS in brain [15]. With increased nNOS in HI, NOS uncoupling might increase with lower BH4 levels. Increased oxidative stress may exceed the cell’s ability to cope beyond a threshold level, leading to the proposal of a double-hit hypothesis depicted in Figure 10, which refines the hypothesis originally proposed by Dr. Vasquez-Vivar [5].
Figure 10. Double-hit hypothesis as proposed by Vasquez-Vivar and Tan.
BH4=Tetrahydrobiopterin, O2·− =superoxide, Tx=treated, HI=hypoxia-ischemia. Note that even in high risk phase another insult other than HI can result in injury.
Adding BH4 restores normal activity of nNOS and inhibits production of superoxide by the enzyme [11]. We and others have shown that oxidative stress increases during HI. Recently we showed that MRI could identify the fetuses at risk for postnatal motor deficits with a combination of HI changes and a distinct reperfusion-reoxygenation injury [16]. MRI identified reperfusion-reoxygenation injury was associated with an increase in superoxide production and critical oxidative stress during HI and reperfusion-reoxygenation determined future motor deficits [16]. In the present study, BH4 supplementation had more benefits in HI group than in the control group. The next study would be to investigate superoxide production in cells from different regions, with and without supplemented BH4 in vivo. Since cell specific responses to variations in BH4 steady state levels may be dependent on the expression levels of NOS, and the possibility of increasing co-factor requirements, a detailed study of nitrosative and oxidative stress warrants further investigation during H-I and in the reperfusion-reoxygenation period [16], especially in those fetuses that are destined to have motor deficits.
It is well known that the brain cells in the fetus have different pathways of cell death than in the adult [17, 18]. Previous studies using non-physiological cell concentrations probably erroneously concluded that BH4 is cytotoxic. Dopaminergic cells undergo apoptosis and oxidative stress from added BH4 in culture media [19–21] but the cells were initially grown in BH4-free conditions and added levels were thousand-fold higher BH4 than normal. Most of these studies also employed tumor cell lines [19, 20, 22, 23], which in contrast to primary fetal cultures grow well in BH4-free conditions, and may lack critical enzyme systems which are important for normal development. Increasing BH4 levels by supplying its precursor sepiapterin in similar cells that overexpress nNOS resulted in attenuation of apoptosis by 1-methyl-4-phenylpyridinium [24]. In mouse primary brain cells that were initially grown in BH4-free conditions, supplemented BH4 increased apoptosis, but the concentrations used were much higher than in our studies, 1–50 μM [21, 25]. In contrast, sepiapterin 20 μM, increased intracellular BH4 and protected against neuronal death following glutathione depletion in nigrostriatal cultures [26]. Sepiapterin, 40 μM, has been shown to be protective against toxicity in nigral cultures by raising the tissue BH4 content to 33 pmol/mg protein [27]. In our study, we investigated cell survival and estimation of cell function using attachment efficiency, both of which have not been studied before. Our concentrations of BH4 used for supplementation (0.2 μM) were derived from the physiological postnatal brain concentrations in rabbits, determined in our previous study [5]. It is possible that BH4 is needed for better mitochondrial function in stressful situations but the exact mechanisms need to be elucidated.
In conclusion, early BH4 deprivation in prematurity may be a risk factor for neuronal survival and function, especially in the thalamus region. Supplementation of BH4 has greater benefit at earlier gestational age E22 than later E29. Developing neurons need a dual hit to be injured.
Table 1.
| E22 Naïve | Basal Ganglia | Cortex | Thalamus | E22 H-I | Basal Ganglia | Cortex | Thalamus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
|
| |||||||||||||
| Healthy neurons % seeded | |||||||||||||
|
| |||||||||||||
| Without BH4 | 1.1 | 0.2 | 1.9 | 0.3 | 0.8 | 0.2 | 2.2 | 0.4 | 2.5 | 0.5 | 0.9 | 0.2 | |
| With BH4 | 1.4 | 0.2 | 2.1 | 0.3 | 0.7 | 0.1 | 2.3 | 0.5 | 2.5 | 0.4 | 1.2 | 0.2 | |
|
| |||||||||||||
| Attachment efficiency of cells | |||||||||||||
|
| |||||||||||||
| Without BH4 | 78 | 5 | 92 | 2 | 39 | 7 | 65 | 6 | 59 | 8 | 24 | 6 | |
| With BH4 | 84 | 3 | 88 | 3 | 46 | 6 | 69 | 6 | 69 | 7 | 35 | 6 | |
|
| |||||||||||||
| Attachment efficiency of functioning mitochondria neurons | |||||||||||||
|
| |||||||||||||
| Without BH4 | 82 | 3 | 94 | 1 | 39 | 6 | 69 | 6 | 61 | 7 | 24 | 6 | |
| With BH4 | 85 | 3 | 94 | 1 | 44 | 6 | 70 | 6 | 71 | 6 | 38 | 7 | |
|
| |||||||||||||
| Attachment efficiency of high mitochondria cells | |||||||||||||
|
| |||||||||||||
| Without BH4 | 56 | 6 | 65 | 4 | 22 | 5 | 48 | 8 | 34 | 8 | 19 | 3 | |
| With BH4 | 63 | 3 | 71 | 4 | 30 | 5 | 50 | 10 | 39 | 11 | 27 | 5 | |
| E29 Naïve | E29 H-I | ||||||||||||
|
| |||||||||||||
| Healthy neurons % seeded | |||||||||||||
|
| |||||||||||||
| Without BH4 | 3.3 | 1.0 | 2.9 | 0.8 | 3.3 | 1.1 | 3.3 | 0.6 | 2.3 | 0.3 | 3.1 | 0.6 | |
| With BH4 | 3.8 | 1.0 | 3.5 | 0.9 | 3.5 | 1.0 | 3.4 | 0.5 | 4.1 | 0.7 | 3.1 | 0.4 | |
|
| |||||||||||||
| Attachment efficiency of cells | |||||||||||||
|
| |||||||||||||
| Without BH4 | 55 | 5 | 48 | 3 | 48 | 3 | 70 | 5 | 44 | 5 | 62 | 8 | |
| With BH4 | 74 | 2 | 62 | 3 | 64 | 3 | 80 | 4 | 57 | 5 | 71 | 3 | |
|
| |||||||||||||
| Attachment efficiency of functioning mitochondria neurons | |||||||||||||
|
| |||||||||||||
| Without BH4 | 61 | 3 | 49 | 3 | 48 | 2 | 68 | 4 | 43 | 4 | 59 | 7 | |
| With BH4 | 74 | 2 | 63 | 3 | 60 | 3 | 76 | 5 | 56 | 4 | 68 | 5 | |
|
| |||||||||||||
| Attachment efficiency of high mitochondria cells | |||||||||||||
|
| |||||||||||||
| Without BH4 | 58 | 7 | 46 | 6 | 45 | 5 | 73 | 8 | 50 | 9 | 69 | 10 | |
| With BH4 | 68 | 6 | 62 | 5 | 65 | 4 | 81 | 4 | 55 | 8 | 79 | 5 | |
Mean and SEM of different brain regions at different ages with and without H-I and with and without BH4 supplementation. Bold numbers indicate significant differences with other region(s) by paired t-test.
Highlights.
Study if high BH4 levels, e.g. thalamus, influence neuronal survival ex vivo
Preterm thalamic neurons have the lowest survival in BH4-free milieu
Ex vivo added BH4 improves survival in preterm thalamus after hypoxia-ischemia
At near-term, ex vivo added BH4 improved survival in all regions in controls.
After hypoxia-ischemia, added BH4 improved in only cortical near-term neurons
Acknowledgments
Financial support for the conduct of the research from NIH NS043285, NS051402 (Tan), NS081936 (Tan, Vasquez-Vivar), HD057307 (Derrick). Funding agency had no involvement in preparation, in study design; collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations
- BH4
tetrahydrobiopterin
- DOPA
levodopa
- HI
hypoxia-ischemia
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- NOS
nitric oxide synthase
- E22,E29
22, 29 days gestation
- PI
propidium iodide
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lei Yu, Email: yulei_gt@hotmail.com.
Jeannette Vásquez-Vivar, Email: jvvivar@mcw.edu.
Rugang Jiang, Email: rjiang@northshore.org.
Kehuan Luo, Email: kluo@northshore.org.
Matthew Derrick, Email: mderrick1096@gmail.com.
Reference List
- 1.Ichinose H, Nomura T, Sumi-Ichinose C. Metabolism of tetrahydrobiopterin: its relevance in monoaminergic neurons and neurological disorders. Chem Rec. 2008;8:378–85. doi: 10.1002/tcr.20166. [DOI] [PubMed] [Google Scholar]
- 2.Fink JK, Ravin P, Argoff CE, Levine RA, Brady RO, Hallett M, et al. Tetrahydrobiopterin administration in biopterin-deficient progressive dystonia with diurnal variation. Neurology. 1989;39:1393–5. doi: 10.1212/wnl.39.10.1393. [DOI] [PubMed] [Google Scholar]
- 3.Frye RE, DeLatorre R, Taylor HB, Slattery J, Melnyk S, Chowdhury N, et al. Metabolic effects of sapropterin treatment in autism spectrum disorder: a preliminary study. Transl Psychiatry. 2013;3:e237. doi: 10.1038/tp.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danfors T, von Knorring AL, Hartvig P, Langstrom B, Moulder R, Stromberg B, et al. Tetrahydrobiopterin in the treatment of children with autistic disorder: a double-blind placebo-controlled crossover study. J Clin Psychopharmacol. 2005;25:485–9. doi: 10.1097/01.jcp.0000177667.35016.e9. [DOI] [PubMed] [Google Scholar]
- 5.Vasquez-Vivar J, Whitsett J, Derrick M, Ji X, Yu L, Tan S. Tetrahydrobiopterin in the prevention of hypertonia in hypoxic fetal brain. Ann Neurol. 2009;66:323–31. doi: 10.1002/ana.21738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, et al. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derrick M, He J, Brady E, Tan S. The in vitro fate of rabbit fetal brain cells after acute in vivo hypoxia. J Neurosci. 2001;21:RC138. doi: 10.1523/JNEUROSCI.21-07-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green DR, Reed JC. Mitochondria and apoptosis. [Review] [59 refs] Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 9.Tilley BC, Palesch YY, Kieburtz K, Ravina B, Huang P, Elm JJ, et al. Optimizing the ongoing search for new treatments for Parkinson disease: using futility designs. Neurology. 2006;66:628–33. doi: 10.1212/01.wnl.0000201251.33253.fb. [DOI] [PubMed] [Google Scholar]
- 10.Hyland K, Gunasekara RS, Munk-Martin TL, Arnold LA, Engle T. The hph-1 mouse: a model for dominantly inherited GTP-cyclohydrolase deficiency. Ann Neurol. 2003;54(Suppl 6):S46–S48. doi: 10.1002/ana.10695. [DOI] [PubMed] [Google Scholar]
- 11.Vasquez-Vivar J, Hogg N, Martasek P, Karoui H, Pritchard KAJ, Kalyanaraman B. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem. 1999;274:26736–42. doi: 10.1074/jbc.274.38.26736. [DOI] [PubMed] [Google Scholar]
- 12.Vasquez-Vivar J, Kalyanaraman B. Generation of superoxide from nitric oxide synthase. FEBS Lett. 2000;481:305–6. doi: 10.1016/s0014-5793(00)02001-9. [DOI] [PubMed] [Google Scholar]
- 13.Vasquez-Vivar J, Martasek P, Hogg N, Karoui H, Masters BS, Pritchard KA, Jr, et al. Electron spin resonance spin-trapping detection of superoxide generated by neuronal nitric oxide synthase. Methods Enzymol. 1999;301:169–77. doi: 10.1016/s0076-6879(99)01080-0. [DOI] [PubMed] [Google Scholar]
- 14.Vasquez-Vivar J. Tetrahydrobiopterin, superoxide, and vascular dysfunction. Free Radic Biol Med. 2009;47:1108–19. doi: 10.1016/j.freeradbiomed.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward ME, Toporsian M, Scott JA, Teoh H, Govindaraju V, Quan A, et al. Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. J Clin Invest. 2005;115:3128–39. doi: 10.1172/JCI20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drobyshevsky A, Luo K, Derrick M, Yu L, Du H, Prasad PV, et al. Motor deficits are triggered by reperfusion-reoxygenation injury as diagnosed by MRI and by a mechanism involving oxidants. J Neurosci. 2012;32:5500–9. doi: 10.1523/JNEUROSCI.5986-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005;12:162–76. doi: 10.1038/sj.cdd.4401545. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Carlsson Y, Basso E, Zhu C, Rousset CI, Rasola A, et al. Developmental shift of cyclophilin D contribution to hypoxic-ischemic brain injury. J Neurosci. 2009;29:2588–96. doi: 10.1523/JNEUROSCI.5832-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi HJ, Jang YJ, Kim HJ, Hwang O. Tetrahydrobiopterin is released from and causes preferential death of catecholaminergic cells by oxidative stress. Mol Pharmacol. 2000;58:633–40. [PubMed] [Google Scholar]
- 20.Choi HJ, Lee SY, Cho Y, No H, Kim SW, Hwang O. Tetrahydrobiopterin causes mitochondrial dysfunction in dopaminergic cells: implications for Parkinson’s disease. Neurochem Int. 2006;48:255–62. doi: 10.1016/j.neuint.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Lee SY, Moon Y, Hee CD, Jin CH, Hwang O. Particular vulnerability of rat mesencephalic dopaminergic neurons to tetrahydrobiopterin: Relevance to Parkinson’s disease. Neurobiol Dis. 2007;25:112–20. doi: 10.1016/j.nbd.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Choi HJ, Kim SW, Lee SY, Hwang O. Dopamine-dependent cytotoxicity of tetrahydrobiopterin: a possible mechanism for selective neurodegeneration in Parkinson’s disease. J Neurochem. 2003;86:143–52. doi: 10.1046/j.1471-4159.2003.01808.x. [DOI] [PubMed] [Google Scholar]
- 23.Choi HJ, Lee SY, Cho Y, Hwang O. JNK activation by tetrahydrobiopterin: implication for Parkinson’s disease. J Neurosci Res. 2004;75:715–21. doi: 10.1002/jnr.20012. [DOI] [PubMed] [Google Scholar]
- 24.Shang T, Kotamraju S, Zhao H, Kalivendi SV, Hillard CJ, Kalyanaraman B. Sepiapterin attenuates 1-methyl-4-phenylpyridinium-induced apoptosis in neuroblastoma cells transfected with neuronal NOS: role of tetrahydrobiopterin, nitric oxide, and proteasome activation. Free Radic Biol Med. 2005;39:1059–74. doi: 10.1016/j.freeradbiomed.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Cardaci S, Filomeni G, Rotilio G, Ciriolo MR. p38(MAPK)/p53 signalling axis mediates neuronal apoptosis in response to tetrahydrobiopterin-induced oxidative stress and glucose uptake inhibition: implication for neurodegeneration. Biochem J. 2010;430:439–51. doi: 10.1042/BJ20100503. [DOI] [PubMed] [Google Scholar]
- 26.Gramsbergen JB, Sandberg M, Moller DA, Kornblit B, Zimmer J. Glutathione depletion in nigrostriatal slice cultures: GABA loss, dopamine resistance and protection by the tetrahydrobiopterin precursor sepiapterin. Brain Res. 2002;935:47–58. doi: 10.1016/s0006-8993(02)02451-4. [DOI] [PubMed] [Google Scholar]
- 27.Madsen JT, Jansen P, Hesslinger C, Meyer M, Zimmer J, Gramsbergen JB. Tetrahydrobiopterin precursor sepiapterin provides protection against neurotoxicity of 1-methyl-4-phenylpyridinium in nigral slice cultures. J Neurochem. 2003;85:214–23. doi: 10.1046/j.1471-4159.2003.01666.x. [DOI] [PubMed] [Google Scholar]