Abstract
The objective of this study was to develop a Korean version of the Assessment of Spondyloarthritis International Society-Health Index/Environmental Factor (ASAS HI/EF) and to evaluate its reliability and validity in Korean patients with axial spondyloarthritis (SpA). A total of 43 patients participated. Translation and cross-cultural adaptation of the ASAS HI/EF was performed according to international standardized guidelines. We also evaluated validity by calculating correlation coefficients between the ASAS-HI/EF score and the clinical parameters. Test-retest reliability was excellent. The correlations among the mean ASAS-HI score and all tools of assessment for SpA were significant. When it came to construct validity, the ASAS HI score was correlated with nocturnal back pain, spinal pain, patients's global assessment score, the Bath ankylosing spondylitis disease activity index (BASDAI), Bath ankylosing spondylitis functional index (BASFI), Bath ankylosing spondylitis metrology index (BASMI) and EuroQoL visual analogue scale (EQ VAS) (r = 0.353, 0.585, 0.598, 0.637, 0.690, 0.430, and -0.534). The ASAS EF score was also correlated with the patient's global assessment's score, BASDAI, BASFI, BASMI, and EQ VAS score (r = 0.375, 0.490, 0.684, 0.485, and -0.554). The Korean version of the ASAS HI/EF can be used in the clinical field to assess and evaluate the state of health of Korean axial SpA patients.
Graphical Abstract
Keywords: Spondylitis, Ankylosing; Spondylarthropathies; Validation Studies
INTRODUCTION
Ankylosing spondylitis (AS) is characterized by inflammation of the axial skeleton, sacroiliac joints, and, to a lesser degree, peripheral joints and certain extra-articular organs, including the eyes, skin, and cardiovascular system (1). The most unique feature in AS is subchondral eburnation and the presence of syndesmophytes, which possibly lead to ankylosis and spinal fusion. Pain, stiffness, and bony ankylosis cause variable degrees of restricted mobility of the spine with consequent loss of functional capacity (2), and impairment and disability are important components of the patient's perception of the disease (3).
The Assessment of Spondyloarthritis International Society (ASAS) Health Index (HI) has been developed to measure health based on the International Classification of Functioning, Disability and Health (ICF) (4, 5). The ICF core set for AS served as the underlying concept to capture the whole range of functioning and disability in patients with axial spondyloarthritis (SpA) (4, 6). The methodology of the ASAS HI has been described (5).
In short, the ASAS HI is based on an item pool which has been developed by linking items to the ICF core set for AS. The origin of the items is either existing questionnaires already in use for patients with axial SpA (axSpA) or items from questionnaires which are linked to the ICF. Some of the items have been rephrased to obtain a consistent item structure (expressed in the first person and in the present tense). In parallel, patients with AS proposed during a patient meeting important items to be included in the final measure or proposed new items which are not adequately represented in existing questionnaires. The final item pool with 251 items in 44 categories has been tested in two international cross-sectional studies with the aim of reducing the item pool. The final ASAS HI measure contains 17 dichotomous items addressing the ICF categories of pain, emotional functions, sleep, sexual functions, mobility, self care, community life and employment. In addition, a set of 9 environmental factors (EF) has been proposed which addresses the categories of support/relationships, attitudes and health services. These EF items can act as a barrier or a facilitator and may influence the health of patients with axial SpA. The items of the ASAS HI can also be the starting point for developing a disease-specific utility instrument that will enable the calculation of disease specific Quality Adjusted Life Years (QALYs), that can further be used in economic evaluations.
This study is one of the global projects. The objective was the translation of the ASAS HI and the EF item set into the Korean language.
MATERIALS AND METHODS
Subjects and clinical assessment
A total of 43 patients (13 patients with non-radiographic axial SpA and 30 patients with AS) were enrolled. We recruited outpatients diagnosed as having non-radiographic axial SpA by the ASAS classification criteria (6) and diagnosed as having AS by the modified New York criteria (7). Patients were excluded from this study if they had an injury of the lumbar vertebrae or femur, had a previous history of fracture of the bones, or a condition which involved a bone disease. Demographics and disease-related characteristics including age, gender, and disease duration since the occurrence of disease specific symptoms were assessed. Clinical assessments were also performed. These included the Bath ankylosing spondylitis disease activity index (BASDAI) (8), Bath ankylosing spondylitis functional index (BASFI) (9), Bath ankylosing spondylitis metrology index (BASMI) (10), and the patient's global assessment (PGA), spinal pain, and night back pain score (11). We used the Korean version of the EuroQoL (EQ) visual analogue scale (VAS) to measure the patients' quality of life (12).
Translation steps of the Korean version of ASAS HI/EF
Translation and cross-cultural adaptation of the ASAS HI and EF were performed according to the published recommendations (13). The proposed steps contained 5 stages: translation, synthesis of translation, back translation, expert committee review and pre-testing in a field test. First, three persons who had a different background (e.g. medical and non-medical) independently performed a forward translation of the instrument from the source language to the Korean language. The translators synthesized their results. Back translation was then performed by three independent bilingual native English speakers, blinded to the English original version. The expert committee agreed upon the final wording of the Korean version. The initial (forward and back) translators and two persons very familiar with cross-cultural adaptation participated in this expert meeting. The final version (APPENDIX) was tested with axial SpA patients with respect to their sex, age, disease duration, and educational level. After completion of the questionnaire, each question was discussed with the patient to check whether all items had been fully understood and to assess whether the patients had problems with the formulation.
Reliability
The reliability of the ASAS HI and EF were assessed by the test-retest method at a one week interval. This was an estimate of the instrument's reproducibility over time, assuming that no change in conditions had taken place. It was measured using the intra-class correlation (ICC).
Statistical analyses
Descriptive statistics were performed. Using Spearman's correlation coefficient, the validity was assessed by comparing the correlation of the ASAS HI/EF and clinical parameters in all patients. All statistical tests were two-sided and P values less than 0.05 were considered to indicate statistical significance. Statistical analysis was performed using SPSS for Windows (version 18.0; SPSS, Chicago, IL, USA).
Ethics statement
Information about the study and confidentiality was given to each patient, and informed consent was obtained. The study received approval by the institutional review board of Chonnam National University Hospital (IRB No. CNUH-2013-068).
RESULTS
The clinical characteristics of the subjects are presented in Table 1. All the patients fulfilled the questionnaires by themselves. Because the final wording needs to be understood by people with a lower level of education, education levels were stratified as elementary school (two patients, 4.6%), middle school (six patients, 13.9%), high school (thirteen patients, 30.2%), college (nine patients, 20.9%), and university (thirteen patients, 30.2%). The mean age (SD) of the patients was 36.7 (11.3) yr. Most were males (83.7%). Duration of disease was 51.4 (50.7) months. Mean scores (SD) of BASDAI, BASFI, BASMI, and EQ VAS were 3.1 (1.9), 1.2 (1.9), 2.0 (1.7), and 66.1 (19.0), respectively. Mean time consumption (SD) of ASAS HI and EF were only 75.4 (31.7) and 64.5 (19.2) sec.
Table 1.
Baseline demographics and clinical characteristics of subjects
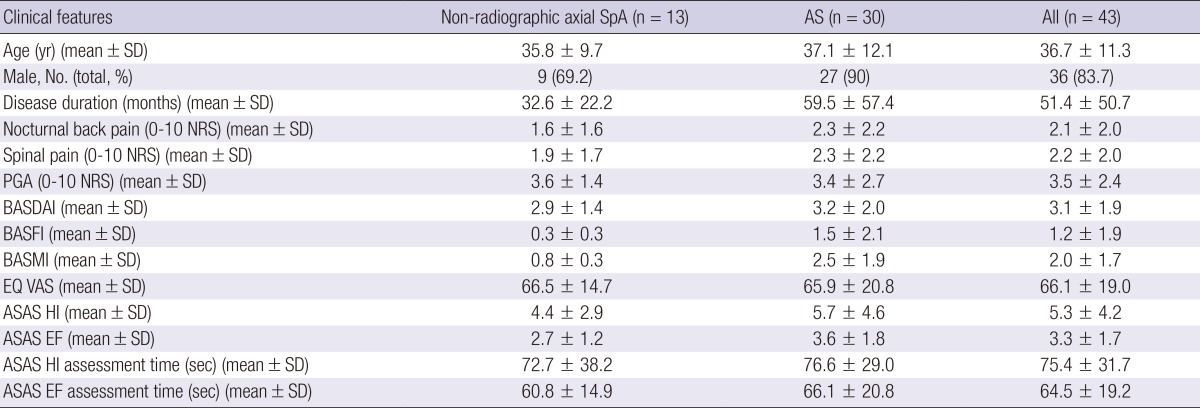
SpA, spondyloarthritis; AS, ankylosing spondylitis; NRS, numerical rating scale; PGA, patient's global assessment; BASDAI, Bath ankylosing spondylitis disease activity index; BASFI, Bath ankylosing spondylitis functional index; BASMI, Bath ankylosing spondylitis metrology index; EQ VAS, EuroQoL visual analogue scale; ASAS HI, The Assessment of Spondyloarthritis International Society Health Index; EF, Environmental Factors.
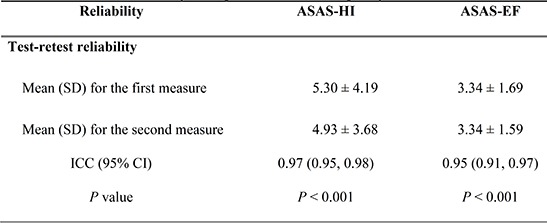
Test-retest reliability was assessed using the ICCs recorded from the first and second interview of the ASAS HI and EF. The ICCs were 0.97 (95% confidence interval [CI], 0.95-0.98; P<0.001) for the ASAS HI and 0.95 (95% CI, 0.91-0.97; P<0.001) for the ASAS EF (Table 2).
Table 2.
Test-retest reliability in 43 patients with axial spondyloarthritis
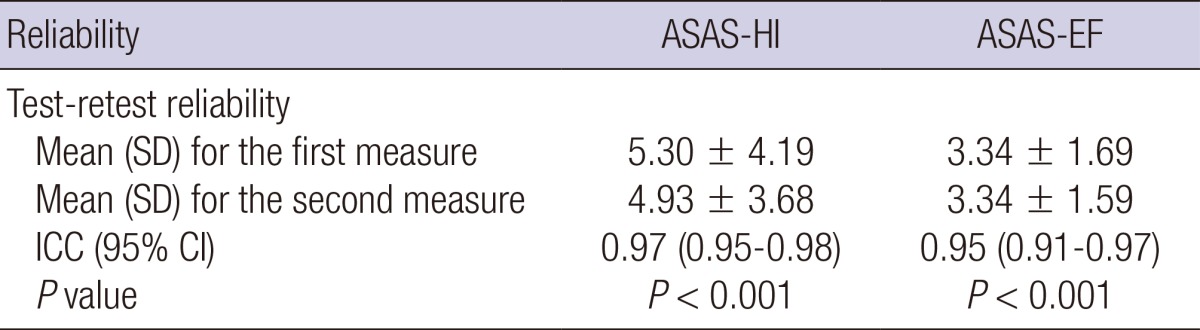
ASAS HI, The Assessment of Spondyloarthritis International Society Health Index; ASAS EF, The Assessment of Spondyloarthritis international Society Environmental factors; ICC, intra-class correlation; SD, standard deviation.
To assess construct validity, the ASAS HI, and EF were compared with the disease specific clinical parameters and quality of life. The ASAS HI score was correlated with nocturnal back pain, spinal pain, PGA, BASDAI, BASFI, BASMI, and EQ VAS (r=0.353, 0.585, 0.598, 0.637, 0.690, 0.430 and -0.534). The ASAS EF score was also correlated with PGA, BASDAI, BASFI, BASMI, and EQ VAS score (r=0.375, 0.490, 0.684, 0.485, and -0.554). Nevertheless, there were no significant correlations between ASAS HI/EF and disease duration (Table 3).
Table 3.
Correlation coefficient between ASAS-HI/EF score and other clinical factors
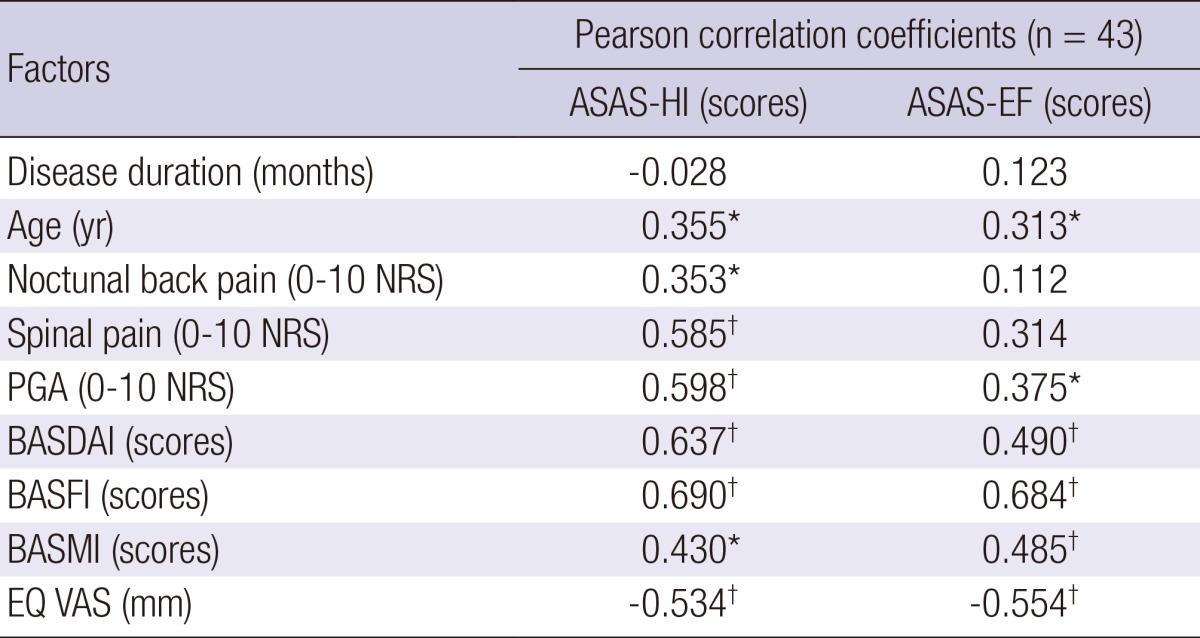
*P<0.05, †P<0.01. ASAS HI, The Assessment of Spondyloarthritis International Society Health Index; ASAS EF, The Assessment of Spondyloarthritis International Society Environmental Factors; PGA, patient's global assessment; BASDAI, Bath ankylosing spondylitis disease activity index; BASFI, Bath ankylosing spondylitis functional index; BASMI, Bath ankylosing spondylitis metrology index; EQ VAS, EuroQol visual analogue scale; NRS, numerical rating scale.
DISCUSSION
The Comprehensive ICF Core Set for comprehensive classification and the Brief ICF Core Set for clinical studies in AS are now available. The core sets aim to present new references for defining the functioning in AS and facilitate clinicians' and researchers' efforts to incorporate a patient-oriented, multilevel and comprehensive view of functioning in AS (4). The purpose of this project was to assess the relevance, acceptability, comprehensiveness and understandability of questionnaire items for SpA patients in Korean. The ASAS HI and EF were translated into Korean, according to international standardized guidelines. After the expert committee's agreement upon the final wording of the Korean version, face validity was also performed by feedback from the patients in case the initial translators needed to go back to the translation and modify it. All final translations were done in such a way so that the wording would be understood by lay people including people with a lower level of education. There was one problematic item in the translation step. Regarding EF Item 1, it was decided to allow the inclusion of the term "children or other relatives" in the translation by the ASAS committee. The test-retest reliability of the ASAS HI and EF was high, with an excellent ICC. These results were interpreted as showing appropriate reliability.
To reflect the multidimensional domains of a disease's impact, there is a need to include both patient-assessed specific and generic health-related quality of the outcome measures and range of motion measures in the evaluation of patients with AS (11, 14). We found significant correlations between ASAS HI and spinal pain, global health score, BASDAI, BASFI, and BAS MI. ASAS EF also has good correlation with the global health score, BASDAI, BASFI, and BASMI. The EQ VAS is a generic measure of health status that does not focus on regional disabilities (15). Both ASAS HI and EF have significant correlations with EQ VAS. The results of the study support the applications of the Korean version of ASAS HI and EF in studies of assessing and evaluating Korean SpA patients.
Patient-reported outcome has become popular as an evaluation tool and is increasingly being used. The most important advantages are that questionnaires do not require the time of a doctor and they can be completed by the patient and returned by mail without the patient attending the hospital. Surprisingly, we registered less than 2 min in filling out the each instrument. These instruments will be cost effective and suitable in Korean studies with large populations, such as in registry studies. The translated version was made available freely without copyright or payment for use. The translation of the ASAS HI and the EF items set can be used in clinical trials and clinical practice as a new measure in patients with axial SpA to assess and evaluate the state of health in Korean AS (or axial SpA) patients.
In summary, despite the linguistic differences, the Korean ASAS HI and EF represents a standardized patient-based instrument for assessing the status of health in patients with axial SpA. Future clinical practice should include the ASAS HI and EF to improve the quality of medical care associated with axial SpA.
Appendix 1
I. Korean version _ASAS Health Index

Appendix 2
I. Korean version_Environmental Factors related to ASAS Health Index

Appendix 3

Footnotes
The authors have no conflicts of interest to disclose.
This study was supported by grants from the National Research Foundation of Korea (NRF) Grant funded by the Ministry of Education, Science, and Technology (grant No. 2011-0008867 and 2011-0011332), and by Chonnam National University, 2011.
References
- 1.Khan MA, van der Linden SM. A wider spectrum of spondyloarthropathies. Semin Arthritis Rheum. 1990;20:107–113. doi: 10.1016/0049-0172(90)90023-9. [DOI] [PubMed] [Google Scholar]
- 2.Dougados M, Gueguen A, Nakache JP, Nguyen M, Amor B. Evaluation of a functional index for patients with ankylosing spondylitis. J Rheumatol. 1990;17:1254–1255. [PubMed] [Google Scholar]
- 3.Ruof J, Stucki G. Comparison of the dougados functional index and the Bath Ankylosing Spondylitis Functional Index: a literature review. J Rheumatol. 1999;26:955–960. [PubMed] [Google Scholar]
- 4.Boonen A, Braun J, van der Horst Bruinsma IE, Huang F, Maksymowych W, Kostanjsek N, Cieza A, Stucki G, van der Heijde D. ASAS/WHO ICF Core Sets for ankylosing spondylitis (AS): how to classify the impact of AS on functioning and health. Ann Rheum Dis. 2010;69:102–107. doi: 10.1136/ard.2008.104117. [DOI] [PubMed] [Google Scholar]
- 5.Kiltz U, van der Heijde D, Cieza A, Boonen A, Stucki G, Ustün B, Braun J. Developing and validating an index for measuring health in patients with ankylosing spondylitis. Rheumatology (Oxford) 2011;50:894–898. doi: 10.1093/rheumatology/keq315. [DOI] [PubMed] [Google Scholar]
- 6.Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, Braun J, Chou CT, Collantes-Estevez E, Dougados M, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 7.Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 8.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–2291. [PubMed] [Google Scholar]
- 9.Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, Jenkinson T. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21:2281–2285. [PubMed] [Google Scholar]
- 10.Jones SD, Porter J, Garrett SL, Kennedy LG, Whitelock H, Calin A. A new scoring system for the Bath Ankylosing Spondylitis Metrology Index (BASMI) J Rheumatol. 1995;22:1609. [PubMed] [Google Scholar]
- 11.Zochling J, Braun J. Assessments in ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2007;21:699–712. doi: 10.1016/j.berh.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Kim MH, Cho YS, Uhm WS, Kim S, Bae SC. Cross-cultural adaptation and validation of the Korean version of the EQ-5D in patients with rheumatic diseases. Qual Life Res. 2005;14:1401–1406. doi: 10.1007/s11136-004-5681-z. [DOI] [PubMed] [Google Scholar]
- 13.Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000;25:3186–3191. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 14.Van der Heijde D, Bellamy N, Calin A, Dougados M, Khan MA, van der Linden S. Preliminary core sets for endpoints in ankylosing spondylitis: assessments in Ankylosing Spondylitis Working Group. J Rheumatol. 1997;24:2225–2229. [PubMed] [Google Scholar]
- 15.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]


