Abstract
Although prostate-specific antigen (PSA) is a very useful screening tool, prostate biopsy is still necessary to confirm prostate cancer (PCA). However, it is reported that PSA is associated with a high false-positive rate and prostate biopsy also has various procedure-related complications. Therefore, the authors have devised a nomogram, which can be used to estimate the risk of PCA, using available clinical data for men with a serum PSA less than 10 ng/mL. Prostate biopsies were obtained from 2,139 patients from January 1998 to March 2011. Of them, 1,171 patients with a serum PSA less than 10 ng/mL were only included in this study. Patient age, PSA, free PSA, prostate volume, PSA density and percent free PSA ratio were analyzed. Among 1,171 patients, 255 patients (21.8%) were diagnosed as PCA. Multivariate analyses showed that patient age, prostate volume, PSA and percent free PSA had statistically significant relationships with PCA (P < 0.05) and were used as nomogram predictor variables. The area under the (ROC) curve for all factors in a model predicting PCA was 0.759 (95% CI, 0.716-0.803).
Graphical Abstract
Keywords: Biopsy, Nomograms, Prostate, Prostatic Neoplasms
INTRODUCTION
Prostate cancer (PCA) is the fifth most common malignancy worldwide and the second most common in men (1, 2). PCA is the fifth common cancer and its prevalence is also the most rapidly increasing in Korea (3). Prostate-specific antigen (PSA) is a useful screening test for PCA. However, the confirmed diagnosis of PCA is made by a pathological examination. Prostate biopsy is usually performed when a patient has a high PSA or there are abnormal findings in either digital rectal examination or prostate imaging studies. Prostate biopsy is generally recommended for patients with a serum PSA value more than 4 ng/mL. However, it was reported that patients with PSA at the range of 2.0 to 4.0 ng/mL were also diagnosed with PCA. Additionally, the detection rate was ranged between 15% and 25% in these patients (4-7). For this reason, the PSA cut-off value remains controversial. It is reported that a lower specificity of PSA test is accounted for PCA detection rates on initial prostate biopsy ranged between 22.8% and 42.0% (8).
Various procedure-related complications including pain on biopsy sites, hematuria, hematochezia, acute urinary retention, urinary tract infection (UTI) etc. have been reported and major complications sometimes present as much more serious problems to both patients and urologists. Febrile UTIs or urosepsis frequently require hospital admission for supportive care and antibiotic treatment (8, 9).
Therefore, in this paper, a nomogram to predict the probability of PCA has been developed using available clinical data for men with serum PSA values less than 10 ng/mL.
MATERIALS AND METHODS
Prostate biopsies were obtained from 2,139 patients in our hospital from January 1998 to March 2011. Of them, 1,171 patients with a serum PSA less than 10 ng/mL were selected for this study. Patient age at the prostate biopsy, PSA, free PSA, prostate volume, PSA density (PSAD) and percent free PSA were retrospectively analyzed.
PSA and free PSA levels were measured by enzyme-linked immunoassay (EIA, R&D systems, US). Prostate volume (V) was estimated by measuring the height (H), width (W) and length (L) of the prostate on trans-rectal ultrasonography (TRUS) and by calculating on ellipsoid formula: V = 0.52 × H × W × L (10). PSAD was obtained by dividing PSA by prostate volume estimated by TRUS, and percent free PSA was obtained by dividing free PSA by total PSA.
Prostate biopsy was performed when PSA was more than 4 ng/mL or when abnormal digital rectal examination (DRE) or mass lesions on imaging studies of prostate were detected. In recent years, ultrasound-guided trans-rectal 6-12 core prostate biopsy is often performed according to prostate volumes whereas hypoechoic lesion directed biopsy in addition to sextant biopsy was commonly performed in the past. Eight-core biopsy was performed for patients with prostate volume less than 30 g; ten-core biopsy was performed for patients with prostate volume between 30-50 g; and 12-core biopsy was performed for patients with prostate volume greater than 50 g.
Relationships between the associated factors and PCA were examined by univariate and multivariate regression analyses using a Statistical Package for Social Sciences (SPSS version 18.0, Chicago, IL, USA). A nomogram was developed with significant factors using the statistical software package R, version 2.12.2 (R Development Core Team, available from: http://www.r-project.org) and its regression modeling strategies. Statistical significance was determined at the level of α=0.05.
Ethics statement
This study was reviewed and approved by the institutional review board of Pusan National University Hospital (IRB approved protocol: No. E-2012125). Since this study was performed as a retrospective study using the database and medical records, informed consent was waived by the board.
RESULTS
Of 2,139 patients who underwent prostate biopsy, 1,171 patients had a serum PSA level less than 10 ng/mL. The mean age was 63.8±9.7 (20-88) yr; the mean prostate volume was 42.1±20.3 (9.8-177.5) mL; the mean PSA was 6.20±2.15 (0.35-10.00) ng/mL; the mean PSAD was 0.177±0.105 (0.009-0.631) ng/mL2; and the mean percent free PSA was 13.7±7.4 (2.0-55.4)%. The mean number of biopsy cores was 9.1±2.2.
Among 1,171 patients, 255 patients (21.8%) had a positive biopsy result and were diagnosed with PCA accordingly. The mean ages of the positive biopsy group and the negative biopsy group were 66.2±8.7 (22-88) yr and 63.2±9.8 (20-85) (P<0.001); the mean prostate volumes of each group were 34.2±15.1 (10.5-92.6) mL and 44.5±21.1 (9.8-177.5) mL (P<0.001); the mean PSAs of each group were 6.56±2.00 (0.47-10.00) ng/mL and 6.10±2.18 (0.347-10.0) ng/mL (P=0.003); the mean PSADs of each group were 0.228±0.119 (0.009-0.631) ng/mL2 and 0.162±0.096 ng/mL2 (0.014-0.617) (P<0.001); and the mean percent free PSAs of each group were 13.7±7.4 (2.1-49.3)% and 17.2±8.3 (2.0-55.4)% (P<0.001), respectively. The mean Gleason score of the PCA group was 6.5±0.9 and the number of patients with a Gleason score less than 6, 7 and more than 8 were 157 (61.6%), 68 (26.7%) and 30 (11.8%) (Table 1).
Table 1.
Patients' characteristics and comparison between positive and negative results on prostate biopsy
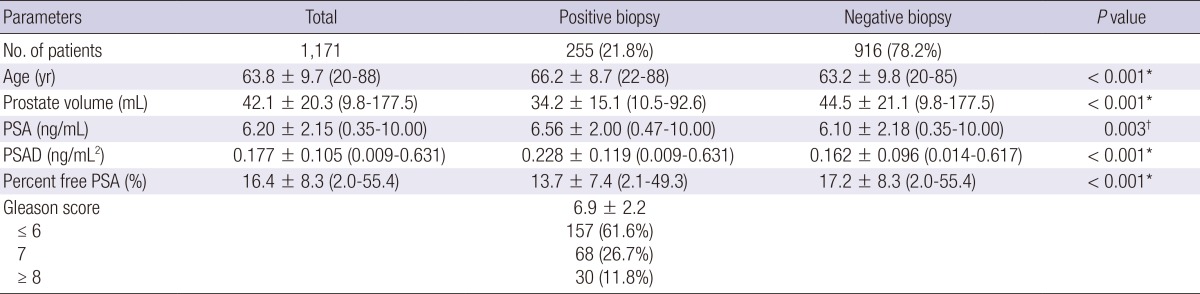
*P<0.001, †P<0.005. PSA, prostate specific antigen; PSAD, prostate specific antigen density.
Univariate analyses showed that patient age (P<0.001), prostate volume (P<0.001), PSA (P=0.003), PSAD (P<0.001) and percent free PSA (P<0.001) had statistically significant relationships with a positive prostate biopsy result. Multivariate analyses showed that patient age (P<0.001), prostate volume (P=0.007), PSA (P<0.001) and percent free PSA (P<0.001) had statistically significant relationships with positive biopsy result (Table 2).
Table 2.
Univarivate and multivariate analysis of factors associated with prostate cancer
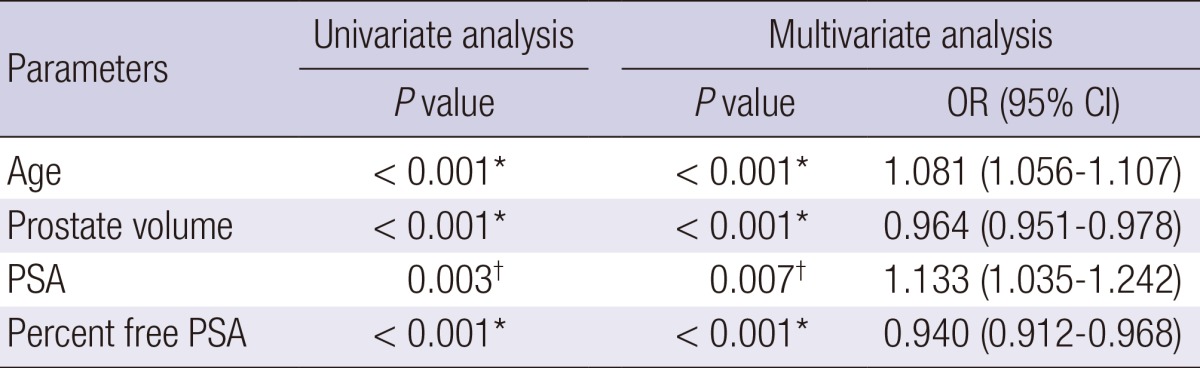
*P<0.001, †P<0.005. Adjusted for prostate volume and Gleason score. PSA, prostate specific antigen; OR, odds ratio; CI, confidence interval.
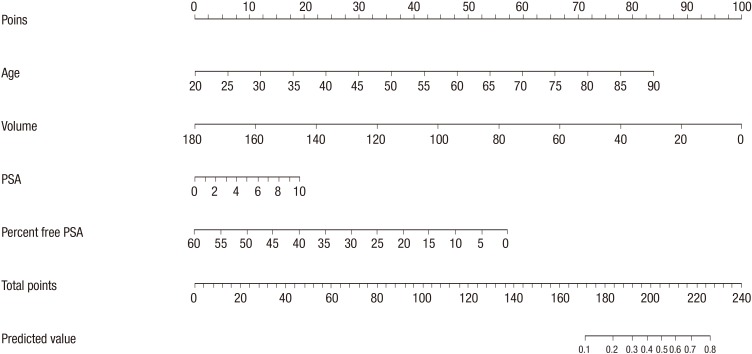
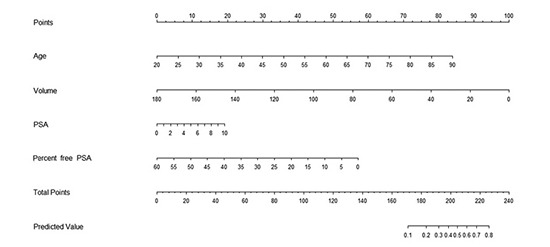
Patient age, prostate volume, PSA and percent free PSA were determined as nomogram predictor variables. Nomogram was developed with these significant factors using the statistical software package R and its regression modeling strategies (Fig. 1).
Fig. 1.
Nomogram for prediction of prostate cancer with serum prostate-specific antigen less than 10 ng/mL at prostate biopsy. PSA, prostate specific antigen.
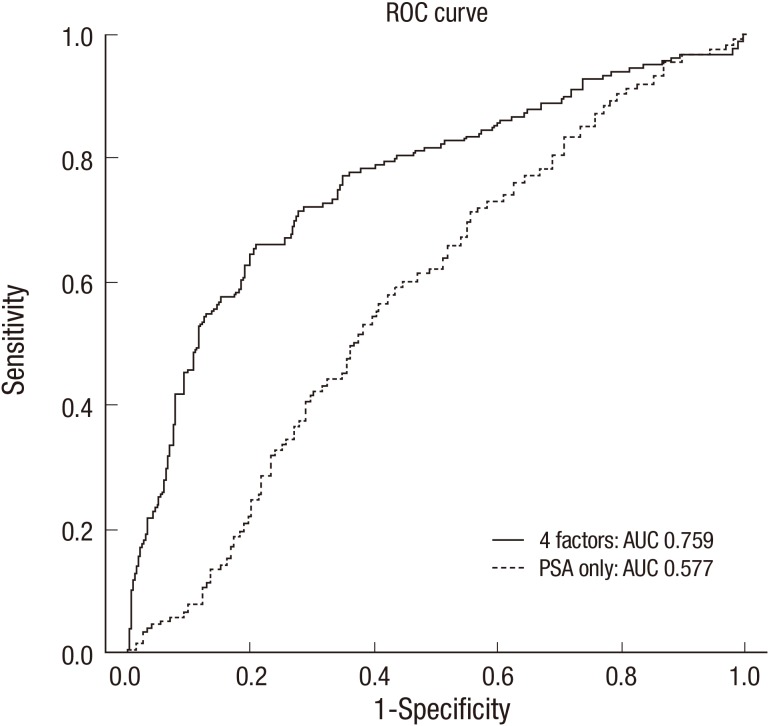
The area under the receiver operating characteristic curve (ROC) for 4 factors in a model predicting PCA was 0.759 (95% confidence interval, 0.716-0.803). The area under ROC for only PSA for predicting PCA was just 0.577 (95% confidence interval, 0.532-0.623) (Fig. 2).
Fig. 2.
Receiver operating characteristic curves for nomogram in predicting with 4 factors (Patient's age, PSA, prostate volume, percent free PSA) and only PSA. ROC, receiver operating characteristic; PSA, prostate specific antigen; AUC, area under curve.
DISCUSSION
Millions of men undergo prostate biopsy to detect PCA each year in the world (14). Although prostate biopsy is performed with caution, various procedure-related complications after prostate biopsy may occur. An increase in the incidence of infection after trans-rectal prostrate biopsy has been reported in some recent studies (14-16). Moreover, some authors reported that the cut-off value of PSA for prostate biopsy has been lowered from 4.0 ng/mL to 2.5 ng/mL (4-7). Having lowered the cut-off value of PSA for prostate biopsy, it is expected that more prostate biopsy will be performed resulting in a higher incidence of procedure-related complications and additional financial burden. In the view of this, the decision on the performance of prostate biopsy must be carefully made considering the probability of PCA and the risk of procedure-related complications, especially in patients with serum PSA less than 10 ng/mL. Nomograms to predict the probability of PCA have been developed in some parts of the world, however, there is only one study of Japanese men with serum PSA less than 10 ng/mL (17).
Carlson et al. (18) created an algorithm to predict PCA, but their model did not report a definite AUC. More importantly, the subjects of their study only included the population with a PSA value between 4 ng/mL and 10 ng/mL excluding the population with lower PSA levels which is the common clinical presentation today. Eastham et al. (19) devised a model after studying group of 700 patients whose PSA values were less than 4 ng/mL. Other studies had investigated the prostate cancer with higher than 10 ng/mL (20-22). Among Patients with a PSA value greater than 10 ng/mL, 36%-76% of them were diagnosed with PCA by prostate biopsy. It was thought that most of patients with a PSA value greater than 20 ng/mL were highly probable to have prostate cancer, therefore, these nomograms which included patients with high PSA had less worth.
In our study, patient age (P<0.001), prostate volume (P=0.007), PSA (P<0.001) and percent free PSA (P<0.001) had statistically significant relationships with positive biopsy result at multivariate analysis and used as nomogram predictor variables. In some studies, multiple values were used as a predictor of PCA, such as age, PSA, percent free PSA, family history of PCA, abnormal DRE findings and races (18-22). In some ways, DRE is a different result between individuals, so we excluded the DRE results as a predictor of PCA.
Nam et al. (16) used 6 factors including age, PSA, percent free PSA, family history of PCA, abnormal DRE findings and races to develop a predicting model for PCA and the predictive value was reached up to 74%. Karakiewicz et al. (20) developed a nomogram to evaluate the risks of PCA including age, PSA level, percent free PSA, and DRE and showed the total AUC of the model was 0.69. Optenberg et al. (21) developed a model to predict the risk of PCA on prostate biopsy using 633 patients and the total AUC of the model was 0.808. Eastham et al. (19) devised a model with a group of 700 patients whose PSA values were less than 4 ng/mL and whose DRE findings were abnormal and the AUC of the model was 0.75. Zaytoun et al. (22) conducted a study about the risk of PCA with 1,551 men who underwent prostate biopsy, using patient age, race, PSA, percent free PSA, family history of PCA, and DRE findings as prognosis factors. Univariate and multivariate logistic regression models of analysis suggested that all 6 risk factors were statically significant. Using the nomogram, the total AUC for all factors in predicting PCA was 0.73 (95% CI, 0.71-0.76), and the AUC for PSA alone was 0.55 (95% CI, 0.69-0.74). In this study, the AUC for 4 factors in the model was 0.759 (95% CI, 0.716-0.803) and the AUC for only PSA was just 0.577 (95% CI, 0.532-0.623; Fig. 2). In study with highest AUC, they included patients with a PSA value less than 20 ng/mL, therefore, higher PCA detection rates may be expected.
There are three limitations that need to be acknowledged and addressed regarding the model developed in this study. The first limitation is that this study has failed to consider the potential false-negative rate of prostate biopsy. The second limitation is that this study has conducted as a retrospective analysis without consideration of DRE which was performed several different urologists. The third limitation is that the data used in this study has been obtained from a single institution. Therefore, prostate biopsy protocol at other institutions may produce different results.
It is expected that the nomogram developed in this study will be useful to predict the risk of PCA on prostate biopsy. The ROC curve evaluated the accuracy of the predicted probability was 75.9% for the model whereas it was 57.7% when a PSA value was considered alone. The prediction rate of PCA with this nomogram was higher than that of other existing nomograms but further external validation of the nomogram will be required.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.American Cancer Society. Cancer facts and figures 2008. [accessed on 15 February 2013]. Available at http://www.cancer.org/acs/groups/content/@nho/documents/document/2008cafffinalsecuredpdf.pdf.
- 2.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Center. Cancer facts and figures 2011. [accessed on 15 February 2013]. Available at http://www.cancer.go.kr/ncic/cics_g/cics_g02/cics_g027/1647906_6065.html.
- 4.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, Petros JA, Andriole GL. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 6.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 8.Chun FK, Epstein JI, Ficarra V, Freedland SJ, Montironi R, Montorsi F, Shariat SF, Schröder FH, Scattoni V. Optimizing performance and interpretation of prostate biopsy: a critical analysis of the literature. Eur Urol. 2010;58:851–864. doi: 10.1016/j.eururo.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 9.Wagenlehner FM, van Oostrum E, Tenke P, Tandogdu Z, Çek M, Grabe M, Wullt B, Pickard R, Naber KG, Pilatz A, et al. Infective complications after prostate biopsy: outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol. 2013;63:521–527. doi: 10.1016/j.eururo.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Gancarczyk KJ, Wu H, McLeod DG, Kane C, Kusuda L, Lance R, Herring J, Foley J, Baldwin D, Bishoff JT, et al. Using the percentage of biopsy cores positive for cancer, pretreatment PSA, and highest biopsy Gleason sum to predict pathologic stage after radical prostatectomy: the Center for Prostate Disease Research nomograms. Urology. 2003;61:589–595. doi: 10.1016/s0090-4295(02)02287-2. [DOI] [PubMed] [Google Scholar]
- 11.Ilic D, O'Connor D, Green S, Wilt T. Screening for prostate cancer: a Cochrane systematic review. Cancer Causes Control. 2007;18:279–285. doi: 10.1007/s10552-006-0087-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979;17:159–163. [PubMed] [Google Scholar]
- 13.Ferro MA, Barnes I, Roberts JB, Smith PJ. Tumour markers in prostatic carcinoma: a comparison of prostate-specific antigen with acid phosphatase. Br J Urol. 1987;60:69–73. doi: 10.1111/j.1464-410x.1987.tb09137.x. [DOI] [PubMed] [Google Scholar]
- 14.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830–1834. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeb S, van den Heuvel S, Zhu X, Bangma CH, Schröder FH, Roobol MJ. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol. 2012;61:1110–1114. doi: 10.1016/j.eururo.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 16.Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH, Loblaw DA, Trachtenberg J, Stanimirovic A, Simor AE, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010;183:963–968. doi: 10.1016/j.juro.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura K, Suzuki H, Kamiya N, Imamoto T, Yano M, Miura J, Shimbo M, Suzuki N, Nakatsu H, Ichikawa T. Development of a new nomogram for predicting the probability of a positive initial prostate biopsy in Japanese patients with serum PSA levels less than 10 ng/mL. Int J Urol. 2008;15:598–603. doi: 10.1111/j.1442-2042.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- 18.Carlson GD, Calvanese CB, Partin AW. An algorithm combining age, total prostate-specific antigen (PSA), and percent free PSA to predict prostate cancer: results on 4298 cases. Urology. 1998;52:455–461. doi: 10.1016/s0090-4295(98)00205-2. [DOI] [PubMed] [Google Scholar]
- 19.Eastham JA, May R, Robertson JL, Sartor O, Kattan MW. Development of a nomogram that predicts the probability of a positive prostate biopsy in men with an abnormal digital rectal examination and a prostate-specific antigen between 0 and 4 ng/mL. Urology. 1999;54:709–713. doi: 10.1016/s0090-4295(99)00213-7. [DOI] [PubMed] [Google Scholar]
- 20.Karakiewicz PI, Benayoun S, Kattan MW, Perrotte P, Valiquette L, Scardino PT, Cagiannos I, Heinzer H, Tanguay S, Aprikian AG, et al. Development and validation of a nomogram predicting the outcome of prostate biopsy based on patient age, digital rectal examination and serum prostate specific antigen. J Urol. 2005;173:1930–1934. doi: 10.1097/01.ju.0000158039.94467.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Optenberg SA, Clark JY, Brawer MK, Thompson IM, Stein CR, Friedrichs P. Development of a decision-making tool to predict risk of prostate cancer: the Cancer of the Prostate Risk Index (CAPRI) test. Urology. 1997;50:665–672. doi: 10.1016/S0090-4295(97)00451-2. [DOI] [PubMed] [Google Scholar]
- 22.Zaytoun OM, Kattan MW, Moussa AS, Li J, Yu C, Jones JS. Development of improved nomogram for prediction of outcome of initial prostate biopsy using readily available clinical information. Urology. 2011;78:392–398. doi: 10.1016/j.urology.2011.04.042. [DOI] [PubMed] [Google Scholar]