Abstract
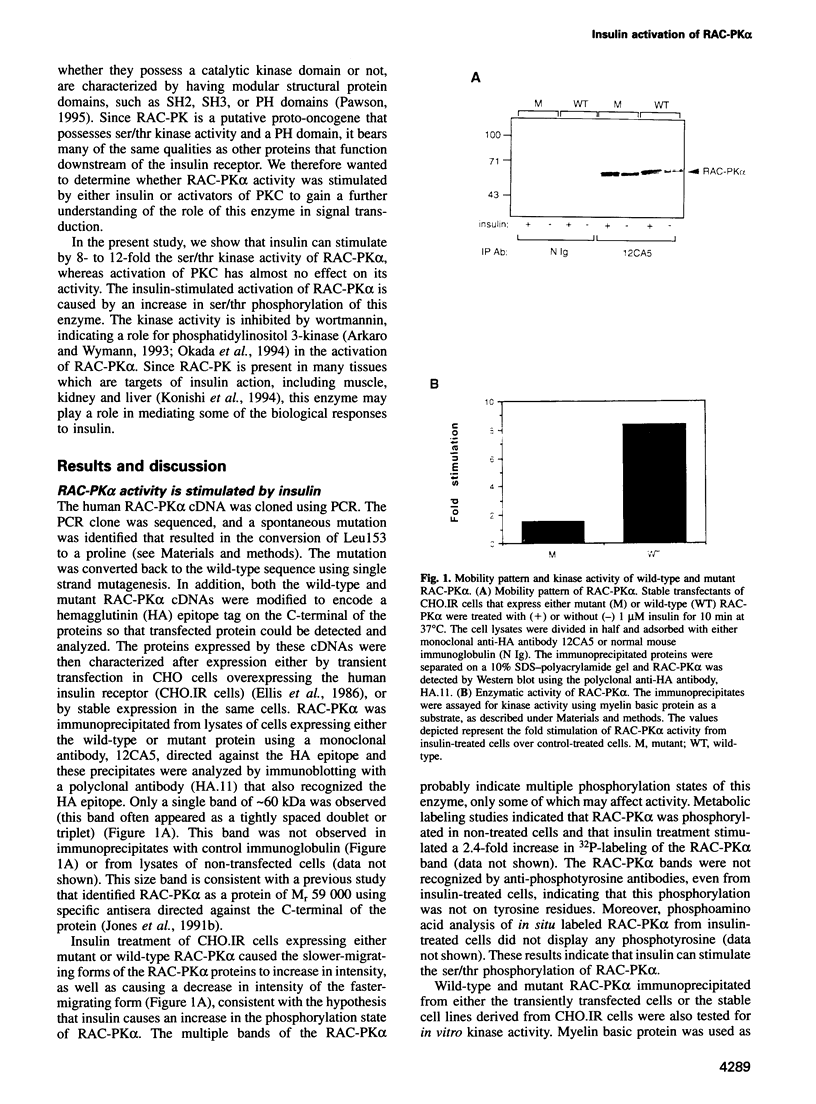
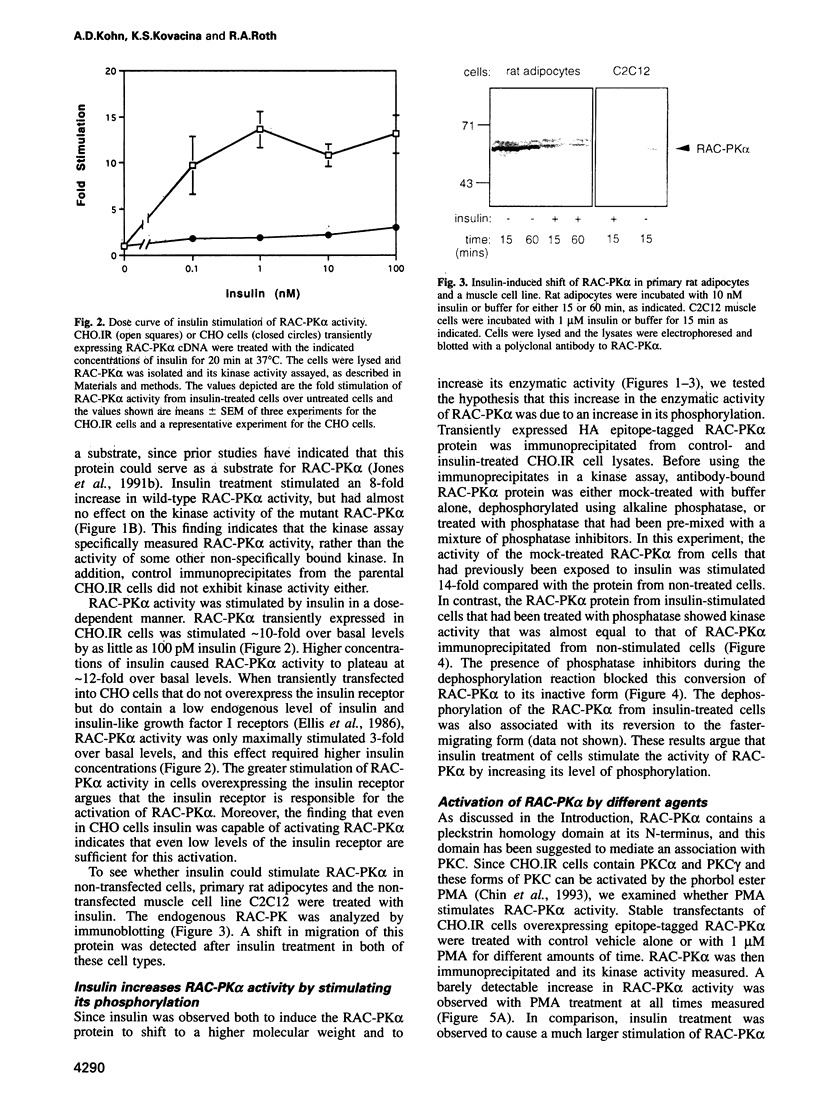
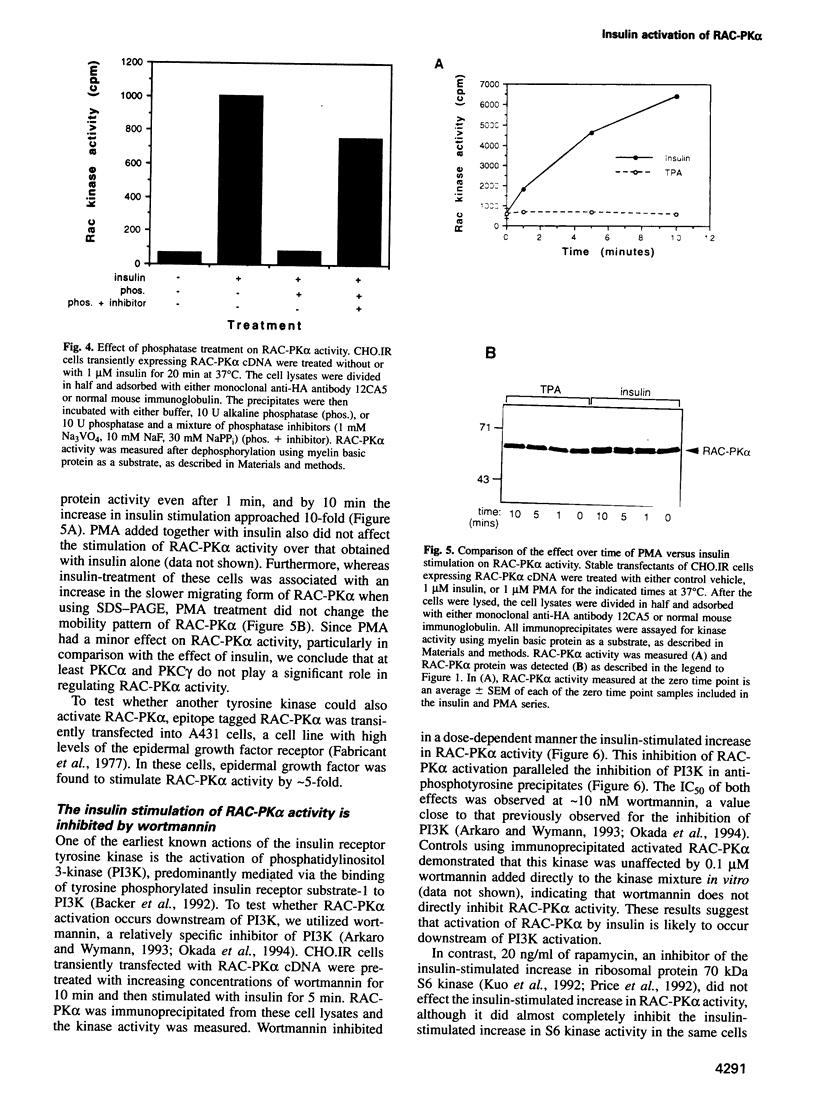
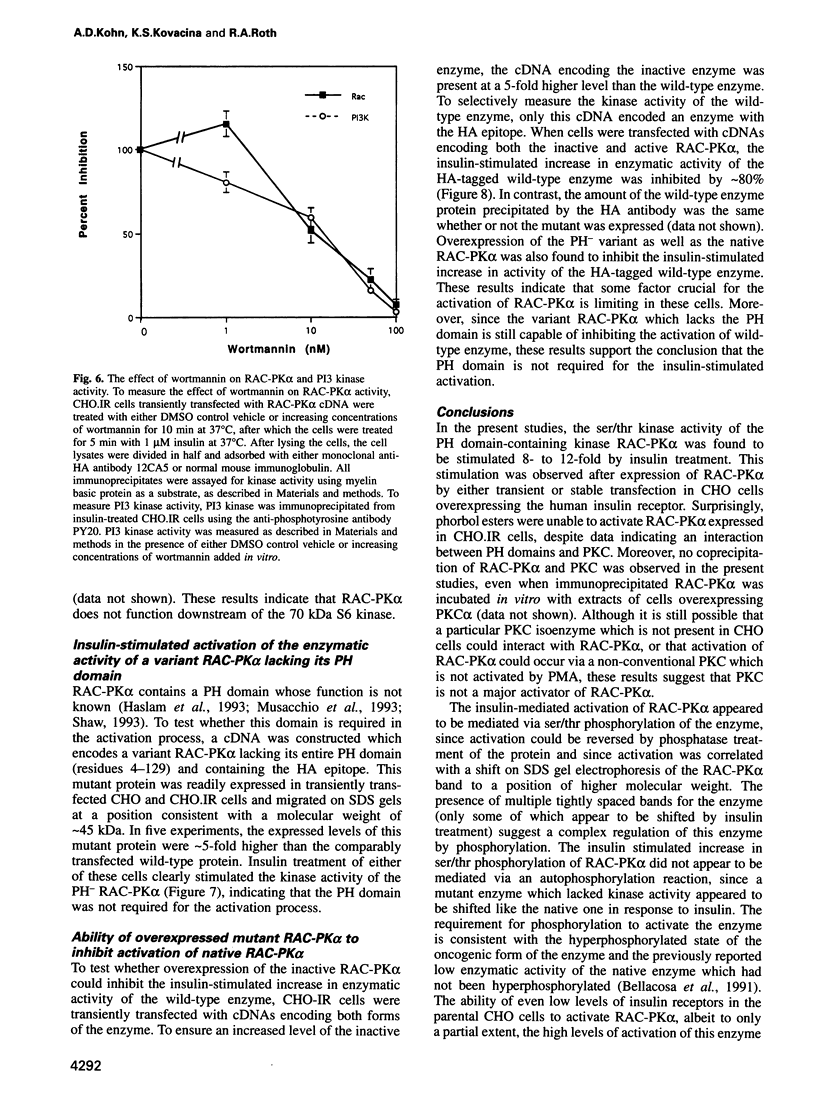
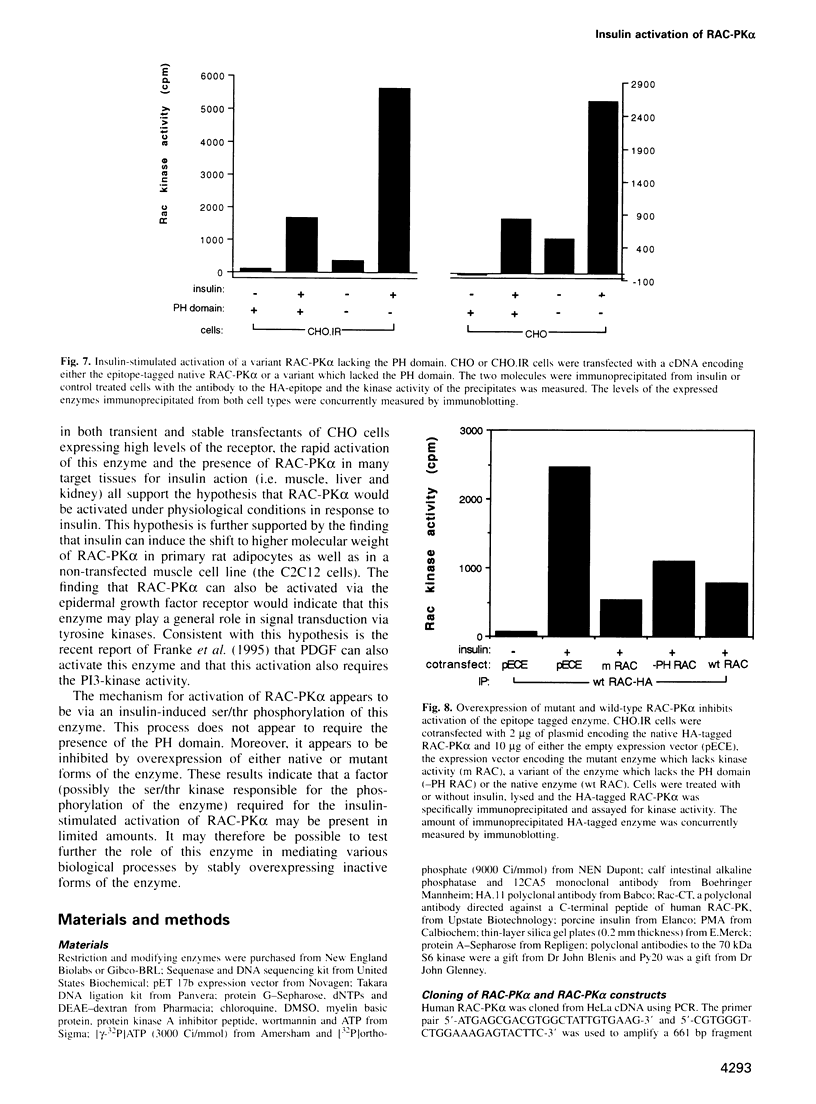
In the present study, insulin is shown to rapidly stimulate by 8- to 12-fold the enzymatic activity of RAC-PK alpha, a pleckstrin homology domain containing ser/thr kinase. In contrast, activation of protein kinase C by phorbol esters had almost no effect on the enzymatic activity of RAC-PK alpha. Insulin activation was accompanied by a shift in molecular weight of the RAC-PK alpha protein, and the activated kinase was deactivated by treatment with a phosphatase, indicating that insulin activated the enzyme by stimulating its phosphorylation. This insulin-induced shift in RAC-PK was also observed in primary rat epididymal adipocytes, as well as in a muscle cell line called C2C12 cells. The insulin-stimulated increase in RAC-PK alpha activity was inhibited by wortmannin (an inhibitor of phosphatidylinositol 3-kinase) in a dose-dependent manner with a half-maximal inhibition of 10 nM, but not by 20 ng/ml of rapamycin. Activation of RAC-PK alpha activity was also observed in a variant RAC lacking the pleckstrin homology domain. These results indicate that RAC-PK alpha activity can be regulated by the insulin receptor. RAC-PK alpha may therefore play a general role in intracellular signaling mediated by receptor tyrosine kinases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andjelković M., Jones P. F., Grossniklaus U., Cron P., Schier A. F., Dick M., Bilbe G., Hemmings B. A. Developmental regulation of expression and activity of multiple forms of the Drosophila RAC protein kinase. J Biol Chem. 1995 Feb 24;270(8):4066–4075. doi: 10.1074/jbc.270.8.4066. [DOI] [PubMed] [Google Scholar]
- Arcaro A., Wymann M. P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993 Dec 1;296(Pt 2):297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellacosa A., Testa J. R., Staal S. P., Tsichlis P. N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991 Oct 11;254(5029):274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- Cheng J. Q., Godwin A. K., Bellacosa A., Taguchi T., Franke T. F., Hamilton T. C., Tsichlis P. N., Testa J. R. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. E., Dickens M., Tavare J. M., Roth R. A. Overexpression of protein kinase C isoenzymes alpha, beta I, gamma, and epsilon in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J Biol Chem. 1993 Mar 25;268(9):6338–6347. [PubMed] [Google Scholar]
- Coffer P. J., Woodgett J. R. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991 Oct 15;201(2):475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- Cohen P., Campbell D. G., Dent P., Gomez N., Lavoinne A., Nakielny S., Stokoe D., Sutherland C., Traverse S. Dissection of the protein kinase cascades involved in insulin and nerve growth factor action. Biochem Soc Trans. 1992 Aug;20(3):671–674. doi: 10.1042/bst0200671. [DOI] [PubMed] [Google Scholar]
- Conklin B. R., Chabre O., Wong Y. H., Federman A. D., Bourne H. R. Recombinant Gq alpha. Mutational activation and coupling to receptors and phospholipase C. J Biol Chem. 1992 Jan 5;267(1):31–34. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Klarlund J. K., Yagaloff K. A., Bradford A. P., Lewis R. E. Insulin receptor signaling. Activation of multiple serine kinases. J Biol Chem. 1988 Aug 15;263(23):11017–11020. [PubMed] [Google Scholar]
- Denton R. M., Tavaré J. M., Borthwick A., Dickens M., Diggle T. A., Edgell N. J., Heesom K. J., Isaad T., Lynch D. F., Moule S. K. Insulin-activated protein kinases in fat and other cells. Biochem Soc Trans. 1992 Aug;20(3):659–664. doi: 10.1042/bst0200659. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke T. F., Yang S. I., Chan T. O., Datta K., Kazlauskas A., Morrison D. K., Kaplan D. R., Tsichlis P. N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995 Jun 2;81(5):727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Koide H. B., Hemmings B. A. Pleckstrin domain homology. Nature. 1993 May 27;363(6427):309–310. doi: 10.1038/363309b0. [DOI] [PubMed] [Google Scholar]
- Jones P. F., Jakubowicz T., Hemmings B. A. Molecular cloning of a second form of rac protein kinase. Cell Regul. 1991 Dec;2(12):1001–1009. doi: 10.1091/mbc.2.12.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. F., Jakubowicz T., Pitossi F. J., Maurer F., Hemmings B. A. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H., Shinomura T., Kuroda S., Ono Y., Kikkawa U. Molecular cloning of rat RAC protein kinase alpha and beta and their association with protein kinase C zeta. Biochem Biophys Res Commun. 1994 Nov 30;205(1):817–825. doi: 10.1006/bbrc.1994.2738. [DOI] [PubMed] [Google Scholar]
- Kovacina K. S., Yonezawa K., Brautigan D. L., Tonks N. K., Rapp U. R., Roth R. A. Insulin activates the kinase activity of the Raf-1 proto-oncogene by increasing its serine phosphorylation. J Biol Chem. 1990 Jul 25;265(21):12115–12118. [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuo C. J., Chung J., Fiorentino D. F., Flanagan W. M., Blenis J., Crabtree G. R. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992 Jul 2;358(6381):70–73. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- Musacchio A., Gibson T., Rice P., Thompson J., Saraste M. The PH domain: a common piece in the structural patchwork of signalling proteins. Trends Biochem Sci. 1993 Sep;18(9):343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- Okada T., Sakuma L., Fukui Y., Hazeki O., Ui M. Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phosphatidylinositol 3-kinase. J Biol Chem. 1994 Feb 4;269(5):3563–3567. [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995 Feb 16;373(6515):573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Price D. J., Grove J. R., Calvo V., Avruch J., Bierer B. E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992 Aug 14;257(5072):973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Rando T. A., Blau H. M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994 Jun;125(6):1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Liu F., Chin J. E. Biochemical mechanisms of insulin resistance. Horm Res. 1994;41 (Suppl 2):51–55. doi: 10.1159/000183961. [DOI] [PubMed] [Google Scholar]
- Shaw G. Identification of novel pleckstrin homology (PH) domains provides a hypothesis for PH domain function. Biochem Biophys Res Commun. 1993 Sep 15;195(2):1145–1151. doi: 10.1006/bbrc.1993.2164. [DOI] [PubMed] [Google Scholar]
- Stagsted J., Hansen T., Roth R. A., Goldstein A., Olsson L. Correlation between insulin receptor occupancy and tyrosine kinase activity at low insulin concentrations and effect of major histocompatibility complex class I-derived peptide. J Pharmacol Exp Ther. 1993 Nov;267(2):997–1001. [PubMed] [Google Scholar]
- Tanti J. F., Grémeaux T., van Obberghen E., Le Marchand-Brustel Y. Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. J Biol Chem. 1994 Feb 25;269(8):6051–6057. [PubMed] [Google Scholar]
- Thomas G. The mitogen-activated p70s6k. Biochem Soc Trans. 1992 Aug;20(3):678–681. doi: 10.1042/bst0200678. [DOI] [PubMed] [Google Scholar]
- Tyers M., Rachubinski R. A., Stewart M. I., Varrichio A. M., Shorr R. G., Haslam R. J., Harley C. B. Molecular cloning and expression of the major protein kinase C substrate of platelets. Nature. 1988 Jun 2;333(6172):470–473. doi: 10.1038/333470a0. [DOI] [PubMed] [Google Scholar]
- Yao L., Kawakami Y., Kawakami T. The pleckstrin homology domain of Bruton tyrosine kinase interacts with protein kinase C. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Luna S., Soria I., Pulido D., Ortín J., Jiménez A. Efficient transformation of mammalian cells with constructs containing a puromycin-resistance marker. Gene. 1988;62(1):121–126. doi: 10.1016/0378-1119(88)90585-9. [DOI] [PubMed] [Google Scholar]