Abstract
Parkinson’s disease (PD) is a debilitating neurodegenerative disorder that results from the loss of or damage to dopaminergic cells in the substantia nigra. Exposure to either the pesticide rotenone or the endogenous neurotoxin salsolinol has been shown to mimic this dopaminergic cell loss. In this study we first sought to determine whether combination of rotenone and salsolinol would result in an additive or synergistic toxicity. For this purpose we utilized SH-SY5Y cells, a human neuroblastoma cell line that is commonly used to model dopaminergic neurodegeneration. We then tested whether curcumin, a natural plant compound with known health benefits including potential neuroprotective properties, could also protect against rotenone and/or salsolinol induced toxicity. Moreover, since apoptotic mechanism has been implicated in toxicity of these compounds the anti-apoptotic effect of curcumin was also evaluated. Our results indicate a synergistic toxicity of low concentrations of rotenone (1 and 5 uM) and salsolinol (25 and 50 mM) that was associated with apoptosis as determined by cell flow cytometry. There was also an increase in caspase-3 levels. Pretreatment with curcumin (1-10 uM) dose-dependently attenuated rotenone and/or salsolinol induced toxicity and the associated apoptosis. These results suggest that exposure to a combination of rotenone and salsolinol may contribute to the pathology of PD, and that curcumin has a therapeutic potential in this disease.
Keywords: Curcumin, Salsolinol, Rotenone, Neuroprotection, Parkinson’s disease, SH-SY5Y cells, Cell flow cytometry, Caspase3
1.Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that causes increased debilitating symptoms resulting from loss or damage to dopaminergic cells in the substantia nigra (SN). The causes for the development and progression of neurodegenerative disorders including PD invariably involve an interaction between the person’s environment and genetic disposition. In fact, many studies link PD with exposure to various endogenous (e.g., salsolinol) and exogenous toxins (e.g., MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) (reviewed by (Tieu, 2011). Therefore, these compounds are often used to create in-vitro and in-vivo models to study PD. Salsolinol is an endogenous neuromodulator in dopaminergic cells formed during the metabolism of dopamine (Mravec, 2006). Dysregulation of salsolinol, especially its (R) enantiomer in the brain, is thought to contribute to development of PD (Dostert et al., 1988, Nagatsu, 1997, Naoi et al., 1997, Antkiewicz-Michaluk, 2002). It has been proposed that salsolinol and its derivatives (e.g. norsalsolinol, N-methyl-norsalsolinol, N-methyl- salsolinol) may serve as a marker for PD as they are increased in the cerebrospinal fluid (Maruyama et al., 1995) and the urine (Moser et al., 1996) of patients with PD. Exogenous compounds that are non-isoquinoline derivatives have also been shown to induce dopaminergic cell death in the SN. One such example is rotenone, a naturally occurring plant toxin that has been developed into a widely used pesticide and insecticide. Rotenone’s toxicity has been demonstrated in various invitro (Hartley et al., 1994, Gao et al., 2002, Freestone et al., 2009) and in-vivo (Caboni et al., 2004) studies. Moreover, it has been shown that when low doses of multiple exogenous factors are combined, a synergistic neurotoxicity may result. Thus, combination of nontoxic or minimally toxic concentrations of rotenone and lipopolysaccharide (LPS, derived from membrane of gram-negative bacteria causing inflammatory-mediated damage) can result in exaggerated or synergistic toxicity (Gao et al., 2003a). However, the effects of exposure to a combination of an endogenous compound, such as salsolinol, with an exogenous compound like rotenone are unknown.
The goal of this study was two folds: First, we sought to determine if salsolinol and rotenone administered together would have an additive or synergistic toxicity to dopaminergic cells. Second, we were curious to determine if curcumin could protect against these effects. Curcumin, an extract from the root of tumeric (Curcuma longa), has been empirically shown to have antioxidant (Sharma, 1976, Ruby et al., 1995, Sandur et al., 2007a) anti-inflammatory (Sandur et al., 2007b, Aggarwal and Harikumar, 2009, Jurenka, 2009), and anti-depressant properties (Xu et al., 2005, Kulkarni et al., 2008, Bhutani et al., 2009, Li et al., 2009, Hurley et al., 2013). Epidemiological studies have suggested that societies that widely use curcumin show reduced incidence of inflammation-influenced and cognitive function diseases such as Alzheimer’s Disease (Chandra et al., 2001, Vas et al., 2001, Ng et al., 2006, Aggarwal et al., 2007). It has also been suggested that curcumin may potentially reduce incidence of PD, as reflected in studies showing an absence of age-related changes in nigral dopaminergic neurons in Indian populations that consume large amounts of curcumin (Muthane et al., 1998, Alladi et al., 2009, Darvesh et al., 2012).
To achieve the goals of the study, we measured cell survival following exposure to various concentrations of salsolinol and rotenone, alone and in combination utilizing SH-SY5Y cells. These cells, derived from human neuroblastoma cells, are commonly used as an in-vitro model of the nigral dopaminergic cells (Copeland et al., 2007, Das and Tizabi, 2009, Xie et al., 2010, Ramlochansingh et al., 2011, Brown et al., 2013). Moreover, we evaluated possible contribution of apoptotic and/or necrotic mechanisms to the toxicity of these compounds and the protective effects of curcumin.
2. Materials and methods
2.1 Cell culture
SH-SY5Y human neuroblastoma cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). SH-SY5Y cells in passage 20-25 were grown from frozen in a humidified incubator with 5% CO2 at 37°C in a 1:1 mixture of Dulbecco’s Modified Eagle Medium and HAM’s F12 (Cellgro, Mediatech Inc. Manassas, VA) with penicillin/streptomycin (100 IU/ml), gentamicin (50 μg/ml), and 10% fetal bovine serum. Cells were grown in cell culture flasks were > 80% confluent.
2.2 Drug treatment
Once cells were confluent they were harvested and plated into 96 well plates at ~ 12,000 cells per well. The cells were allowed 24 hours to settle and adhere to the bottom before drug treatment. Then, fresh media containing various concentrations of drugs (salsolinol, rotenone, or curcumin all from Sigma-Aldrich, St. Louis, MO) were added to aspirated wells.
2.2.1 Salsolinol and rotenone
In order to determine the concentration-responses, salsolinol (25, 50, 100, 200, 400, and 800μM) or rotenone (1, 5, 25, 50, 100, and 200 μM) were added to the cell media for 24 h. Cell viability was then assessed using MTT (3,[4,5-dimethyliazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. Based on these results, non-toxic concentrations of salsolinol and rotenone were combined for the combination studies. Drugs were added to the cells at the same time for 24 hours before determining cell viability. Control wells were treated with pure media in five independent replicates of the experiment.
2.2.2 Curcumin pretreatment
In order to test the neuroprotective effects of curcumin, 1 h before addition of individual or combined toxicants, various concentrations of curcumin (1, 5, and 10 μM) were added to the wells and again, 24 h later cell viability was determined by MTT assay.
2.3 MTT assay
Cell viability was determined by MTT assay (MP Biomedical, Sana Ana, CA colorimetric assay as detailed previously (Ramlochansingh et al., 2011, Brown et al., 2013). Briefly, following the 24 h incubation, 30 μl of MTT tetrazole (0.5 mg/ml) in PBS containing 10 mM HEPES was added to each well. The plates were incubated for three additional hours at 37°C followed by aspiration. The plates were then allowed to dry in the incubator for 1 h. The incorporated dye was solubilized in 100 μl of 0.04 N HCl in isopropanol. In order to determine cell viability the absorbance was measured spectrophotometrically in a plate reader at 570 nm with a background of 630 nm. Cell viability was calculated by subtracting the test results from the background and is presented as a percentage of the control.
2.4 Cell Flow Cytometry
Cell flow cytometry as described previously (Ramlochansingh et al., 2011, Brown et al., 2013) was used to detect apoptosis vs necrosis by measuring and sorting cells by fluorescent labeling of markers on cell surface. Briefly, cells were grown and treated following the same procedure listed under drug treatment above. The cells were harvested and washed twice with cold PBS and then gently suspended in a solution that consisted of 100 ul Annexin V-Fluos labeling solution, 5 ul of fluorescein isothiocyanate labeled by Annexin V-FITC and 5 ul of propidium iodide (PI). Afterwards, the cells were incubated in the dark at room temperature for 15 minutes before adding 500 ul Annexin V-Fluos labeling solution to each well. Finally, the cells were subjected to flow cytometry using a cellometer machine (Nexcelom, Lawrence, MA) followed by analysis of apoptotic and necrotic fraction using FCS express software.
2.5 Caspase-3 Western Blot
To determine whether the apoptotic mechanism was associate with an increase in caspase levels, we quantified caspase-3 levels as described previously (Ramlochansingh et al., 2011). Briefly, following the 24 h incubation cells were removed and were incubated in cell lysis buffer (10 mM Tris-buffer, 5 mM EDTA, 150 mM NaCl, 0.5% Triton X-100 (v/v) with protease inhibitors (Sigma-Aldrich, St. Louis, MO). Protein concentration was determined using Thermo Scientific protein assay reagents. The protein was loaded at 30 μg per well, as verified by -actin and separated on a 12% SDS-polyacrylamide gel and then transferred to PVDF membrane (Immobilon-P: Millipore Corporation, Bedford, MA). After a 1/2 hour block in Blocking Reagent, 5% nonfat milk in TBST buffer (TBS buffer with 2% Tween-20) the membranes were incubated with primary antibody (1:800) in TBST buffer overnight at 4°C. The following day the membranes were rinsed 5 times in fresh TBST and incubated for 1 hour at room temperature in secondary antibody (1:1000). The membranes were then rinsed 5 times in fresh TBST and relative intensity of the bands was visualized and recorded using chemiluminescence.
2.6 Statistical analysis
Statistical differences between groups were determined by one-way ANOVA and the Tukey post-hoc using Graphpad Prisim 3 (Graphpad Software Inc, San Diego, CA) where significance was set a priori at p<0.05
3. Results
3.1 Rotenone only toxicity
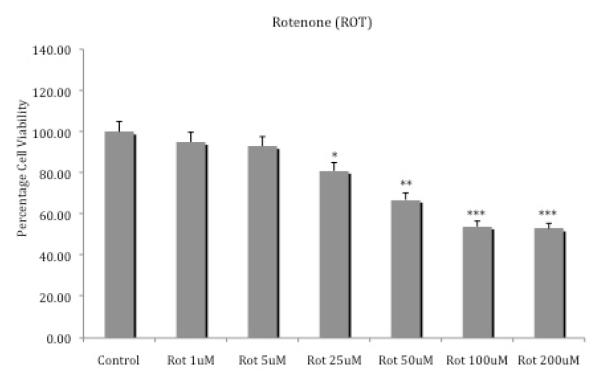
Figure 1 depicts the effects of various concentrations of rotenone on SH-SY5Y cell viability. There was a clear dose-response effect, with higher concentrations of rotenone resulting in greater cell toxicity. Maximum toxicity (about 50%) was achieved with the 100 uM rotenone (p<0.001). Thus, this concentration of rotenone was chosen to test the potential protective effects of curcumin. Rotenone at low concentration (1 or 5 μM) did not result in any toxicity, so these low concentrations were selected for the combination studies.
Fig. 1.
Effects of various concentrations of rotenone on SH-SY5Y cell viability. Cells were exposed for 24 h and cell viability was determined by MTT assay. Values represent mean ± SEM of five independent experiments, *p <0.05, **p<0.01, ***p<0.001 compared to control.
3.2 Salsolinol only toxicity
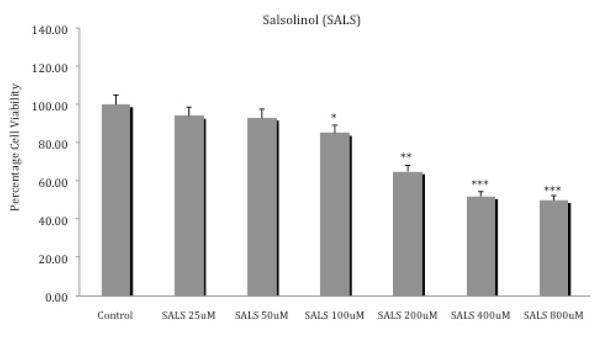
Figure 2 depicts the effects of various concentrations of salsolinol on SH-SY5Y cell viability. There was a clear dose-response effect, with higher concentrations of salsolinol resulting in greater cell toxicity. Maximum toxicity (about 50%) was achieved with the 400 uM salsolinol (p<0.001). Thus, this concentration of salsolinol was chosen to test the potential protective effects of curcumin. Salsolinol at low concentration (25 or 50 μM) did not result in any toxicity, so these low concentrations were selected for the combination studies.
Fig. 2.
Effects of various concentrations of salsolinol on SH-SY5Y cell viability. Cells were exposed for 24 h and cell viability was determined by MTT assay. Values represent mean ± SEM of five independent experiments, *p <0.05, **p<0.01, ***p<0.001 compared to control.
3.3 Rotenone + Salsolinol toxicity
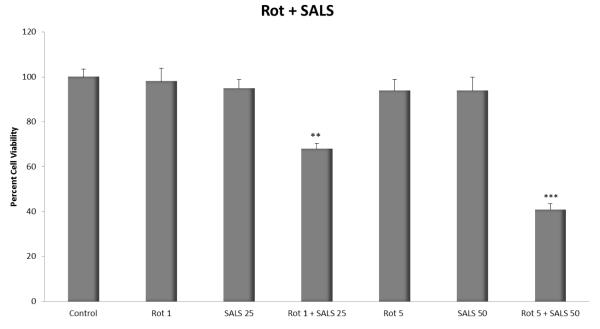
Figure 3 depicts the effects of rotenone and salsolinol combination on cell viability. Combination of very low and ineffective concentration of rotenone (1 μM) with salsolinol (25 μM) resulted in significant toxicity (approx. 32%, p<0.01). Combination of higher ineffective concentrations of rotenone (5 μM) with salsolinol (50 μM) resulted in significantly higher toxicity (approx. 59%, p<0.001). Thus, a synergistic toxicity was observed with rotenonesalsolinol combination. These concentrations were therefore used to assess curcumin’s protective effects.
Fig. 3.
Effects of various combinations of low concentrations of rotenone and salsolinol on SHSY5Y cell viability. Cells were exposed for 24 h and cell viability was determined by MTT assay. Values represent mean ± SEM of five independent experiments, *p <0.05, **p<0.01, ***p<0.001 compared to control.
3.4 Curcumin protection against rotenone toxicity
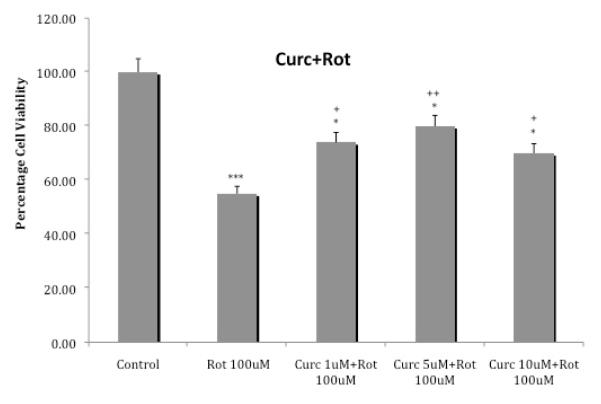
Figure 4 depicts the effects of various concentrations of curcumin on cellular toxicity induced by 100 uM rotenone. Curcumin at the lowest concentration of 1 uM reduced rotenone’s toxicity by approx. 34% (p<0.05) and at 5uM reduced the toxicity by approx. 45% (p<0.01). The higher concentration of 10 uM curcumin, resulted in approx. 27% reduction in toxicity (p<0.05). Thus, maximal protection was provided by the middle concentration of 5 uM curcumin.
Fig. 4.
Effects of various concentrations of curcumin on cellular toxicity induced by 100 uM rotenone. Cells were exposed for 24 h and cell viability was determined by MTT assay. Values represent mean ± SEM of five independent experiments, *p<0.05, ***p<0.001 compared to control. +p<0.05, ++p<0.01 compared to rotenone.
3.5 Curcumin protection against salsolinol toxicity
Figure 5 depicts the effects of various concentrations of curcumin on cellular toxicity induced by 400 uM salsolinol. Curcumin at the lowest concentration of 1 uM reduced salsolinol toxicity by approx. 41% (p<0.01) and at 5uM reduced the toxicity by approx. 45% (p<0.01). The higher concentration of 10 uM curcumin, resulted in approx. 22% (p<0.05). Thus, similar to what was observed with rotenone, maximal protection against salsolinol was provided by the middle concentration of 5 uM curcumin.
Fig. 5.
Effects of various concentrations of curcumin on cellular toxicity induced by 400 uM salsolinol. Cells were exposed for 24 h and cell viability was determined by MTT assay. Values represent mean ± SEM of five independent experiments, **p<0.01, ***p<0.001 compared to control. +p<0.05, ++p<0.01 compared to salsolinol.
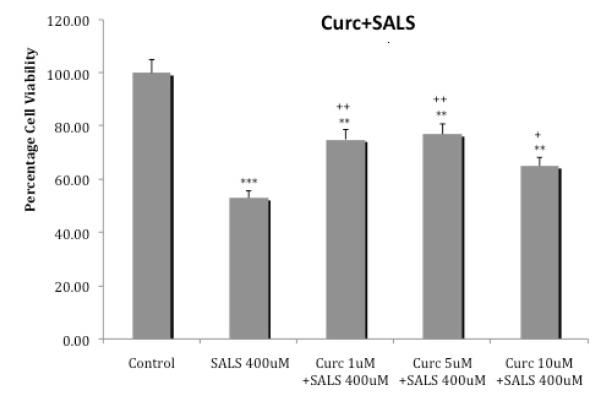
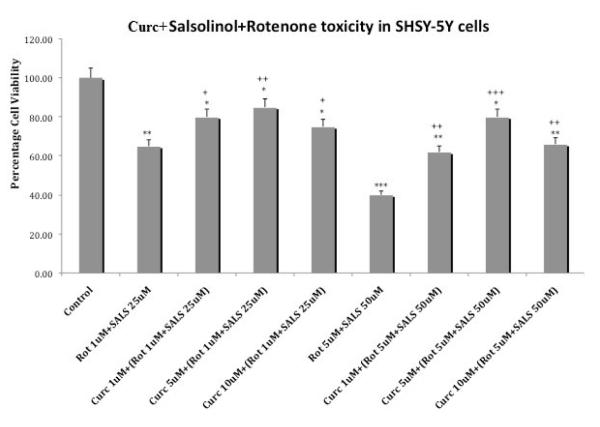
3.5 Curcumin protection against rotenone + salsolinol toxicity
Figure 6 depicts the effects of various concentrations of curcumin on cellular toxicity induced by the combination of either 1 uM rotenone + 25 uM salsolinol or 5 uM rotenone + 50 uM salsolinol. Curcumin at the lowest concentration of 1 uM reduced the toxicity of rotenone 1 uM+ salsolinol 25 uM by approx. 23% (p<0.05) and at 5 uM reduced the toxicity by approx. 30% (p<0.01). The higher concentration of 10 uM curcumin, resulted in approx. 15% (p<0.05) reduction in toxicity. Similar results were obtained with curcumin against toxicity induced by the higher combination of rotenone 5 uM + salsolinol 50 uM, where maximum protection was provided by 5 uM curcumin. Hence in this case also maximal protection against both combinations of rotenone + salsolinol was provided by the middle concentration of 5 uM curcumin.
Fig. 6.
Effects of various concentrations of curcumin on cellular toxicity induced by combination of rotenone and salsolinol. Cells were exposed for 24 h and cell viability was determined by MTT assay. Values represent mean ± SEM of five independent experiments, *p<0.05, **p<0.01, ***p<0.001 compared to control. +p<0.05, ++p<0.01, +++p<0.001 compared to combination of rotenone and salsolinol.
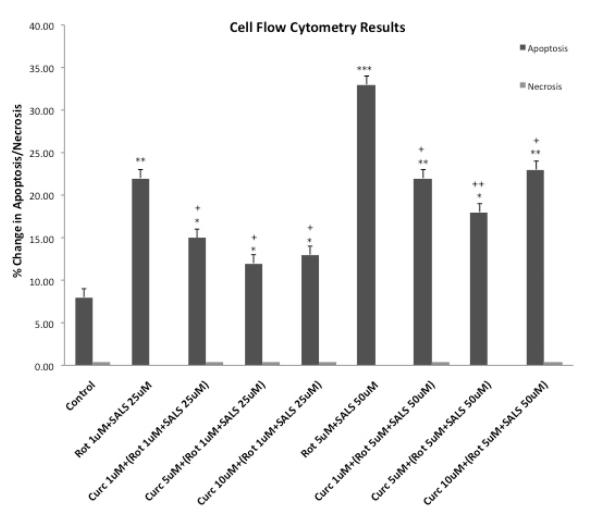
3.6 Cell Flow Cytometry
Figure 7 depicts the percentage of apoptosis and necrosis following combination treatments of rotenone and salsolinol as well as pretreatment with various concentrations of curcumin. As evident, cellular toxicity was principally mediated by apoptosis. Here also, curcumin dose-dependently reduced apoptosis with maximum reduction caused by 5 uM curcumin.
Fig. 7.
Effects of rotenone, salsolinol and their combination, as well as curcumin pretreatment on percentage of apoptosis and necrosis as determined by cell flow cytometry. Cells were exposed for 24 h. Values represent mean ± SEM of five independent experiments, *p<0.05, **p<0.01, ***p<0.001 compared to control. +p<0.05, ++p<0.01 compared to combination of rotenone and salsolinol.
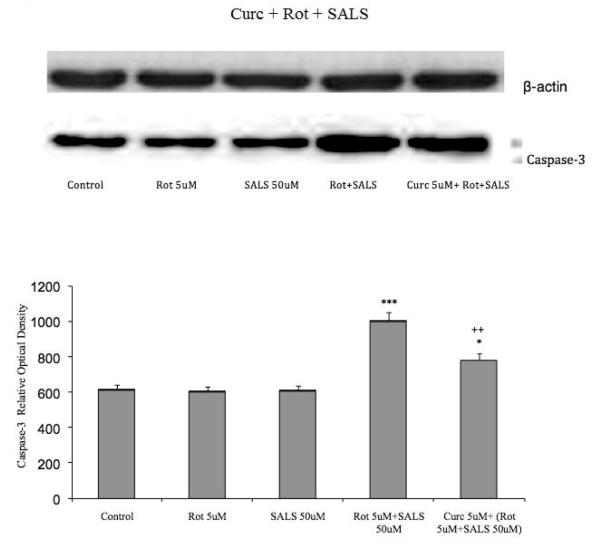
3.7 Caspase 3
Figure 8 depicts the levels of caspase3, a mediator of apoptosis following treatments with rotenone 5uM, salsolinol 50 uM and their combination as well as pretreatment with 5 uM curcumin. Whereas neither rotenone nor salsolinol individually had any effect on caspase3 levels their combination resulted in a significant increase (approx. 58%, p<0.001) in caspase3, which was reduced by approximately 24% following curcumin pretreatment (p<0.01).
Fig. 8.
Effects of rotenone, salsolinol and their combination, as well as curcumin pretreatment on caspase3 levels as determined by Western blot. Cells were exposed for 24 h. Values represent mean ± SEM of five independent experiments, *p<0.05, ***p<0.001 compared to control, ++p<0.01 compared to combination of rotenone and salsolinol.
4. Discussion
The results of the current study not only confirm that rotenone and salsolinol can induce toxicity in SH-SY5Y cells model of dopaminergic neurons (Copeland et al., 2007, Das and Tizabi, 2009, Ramlochansingh et al., 2011, Song et al., 2012, Brown et al., 2013), but also indicate that when the two are combined in concentrations that do not induce any toxicity on their own would result in significant toxicity. This is an important extension of the current knowledge, since it implies that the two agents might interact synergistically to induce Parkinson like symptoms in susceptible individuals. Moreover, the results suggest therapeutic potential for curcumin in PD. This contention is based on the findings that curcumin at relatively low concentrations could at least partially protect against the cellular toxicity induced by rotenone, salsolinol and their combination.
The pathways by which salsolinol and rotenone cause neurotoxicity have some similarities, but also include key differences. It has been suggested that salsolinol leads to neurotoxicity through inhibition of mitochondrial complex II (succinate-Q reductase) activity, and/or by initiating apoptotic processes by generating free radicals (Maruyama et al., 1995, Storch et al., 2000). Rotenone’s effect has been attributed to inhibition of mitochondrial complex I (Betarbet et al., 2000, Alam and Schmidt, 2002, Hoglinger et al., 2003), the release of NADPH oxidase-derived superoxide from activated microglia (Gao et al., 2002) and possibly alteration of glutamate transmission (Leng et al., 2003, Moussa et al., 2008). Both drugs might also cause inflammation, which appears to contribute to the formation of PD (Gao et al., 2003a,b, Zhang et al., 2011, Chinta et al., 2013). Our results indicate that the toxicity induced by the combination of rotenone and salsolinol is also mediated through apoptotic process that is likely caspase-dependent, although this latter contention needs to be verified by further analysis of other caspases as well as the caspase-3 activity.
Curcumin’s protective effects appear to be mediated by inhibition of apoptosis, as there was a dose-dependent reduction in apoptosis and a parallel decrease in caspase3 levels. However, it is important to note that even at the highest effective concentration, curcumin only partially blocked the toxicity induced by rotenone and/or salsolinol. Although this effect of curcumin was highly significant, it would be of interest to determine whether curcumin might have synergistic protection with other drugs (e.g. nicotine) that has been shown to be protective in this model (Copeland et al., 2005, Copeland et al., 2007, Ramlochansingh et al., 2011). Moreover, it would be of relevance to determine the effect of curcumin alone or in combination with other drugs in in-vivo models.
It is also of relevance to note that the varied effects of curcumin, including its anti-oxidant (Sharma, 1976, Ruby et al., 1995, Sandur et al., 2007b) and anti-inflammatory (Sandur et al., 2007b, Aggarwal and Harikumar, 2009, Jurenka, 2009) properties may provide further benefits in treatment of PD with distinct etiologies. Moreover, the mood regulating effects of curcumin as documented by various preclinical and some clinical studies (Xu et al., 2005, Kulkarni et al., 2008, Bhutani et al., 2009, Li et al., 2009, Hurley et al., 2013) can be an added advantage in PD, since epidemiological studies suggest significant co-morbidity between PD and depression (Loas et al., 2012; Starkstein et al., 2012; Worth, 2013)
In summary, the results indicate that combination of salsolinol (a potential endogenous toxin) and rotenone (a potential exogenous toxin) at concentrations that alone do not cause any toxicity can create a one-two-punch effect, and significantly decreases survivability of SH-SY5Y cells. Pre-treatment with curcumin provided significant, but not total, neuroprotection against high concentrations of these toxicants as well as their combination. Although further in-vivo experiments into protection and mechanism of curcumin protection is necessary, the results support therapeutic potential of curcumin in PD.
Acknowledgment
Supported by NIH/NIGMS 2 SO6 GM08016-39, NIMH-COR (MH 16580-28) and Howard University College of Medicine Bridge Grant
References
- Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. The international journal of biochemistry & cell biology. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Advances in experimental medicine and biology. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Alam M, Schmidt WJ. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behavioural brain research. 2002;136:317–324. doi: 10.1016/s0166-4328(02)00180-8. [DOI] [PubMed] [Google Scholar]
- Alladi PA, Mahadevan A, Yasha TC, Raju TR, Shankar SK, Muthane U. Absence of age-related changes in nigral dopaminergic neurons of Asian Indians: relevance to lower incidence of Parkinson’s disease. Neuroscience. 2009;159:236–245. doi: 10.1016/j.neuroscience.2008.11.051. [DOI] [PubMed] [Google Scholar]
- Antkiewicz-Michaluk L. Endogenous risk factors in Parkinson’s disease: dopamine and tetrahydroisoquinolines. Polish journal of pharmacology. 2002;54:567–572. [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bhutani MK, Bishnoi M, Kulkarni SK. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacology, biochemistry, and behavior. 2009;92:39–43. doi: 10.1016/j.pbb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Brown D, Tamas A, Reglodi D, Tizabi Y. PACAP protects against salsolinol-induced toxicity in dopaminergic SH-SY5Y cells: implication for Parkinson’s disease. Journal of molecular neuroscience. 2013;50:600–607. doi: 10.1007/s12031-013-0015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caboni P, Sherer TB, Zhang N, Taylor G, Na HM, Greenamyre JT, Casida JE. Rotenone, deguelin, their metabolites, and the rat model of Parkinson’s disease. Chem Res Toxicol. 2004;17:1540–1548. doi: 10.1021/tx049867r. [DOI] [PubMed] [Google Scholar]
- Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, Ganguli M. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology. 2001;57:985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Ganesan A, Reis-Rodrigues P, Lithgow GJ, Andersen JK. Anti-inflammatory role of the isoflavone diadzein in lipopolysaccharide-stimulated microglia: implications for Parkinson’s disease. Neurotox Res. 2013;23:145–53. doi: 10.1007/s12640-012-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland RL, Jr., Das JR, Kanaan YM, Taylor RE, Tizabi Y. Antiapoptotic effects of nicotine in its protection against salsolinol-induced cytotoxicity. Neurotoxicity research. 2007;12:61–69. doi: 10.1007/BF03033901. [DOI] [PubMed] [Google Scholar]
- Copeland RL, Jr., Leggett YA, Kanaan YM, Taylor RE, Tizabi Y. Neuroprotective effects of nicotine against salsolinol-induced cytotoxicity: implications for Parkinson’s disease. Neurotoxicity research. 2005;8:289–293. doi: 10.1007/BF03033982. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Carroll RT, Bishayee A, Novotny NA, Geldenhuys WJ, Van der Schyf CJ. Curcumin and neurodegenerative diseases: a perspective. Expert Opin Investig Drugs. 2012;21:1123–1140. doi: 10.1517/13543784.2012.693479. [DOI] [PubMed] [Google Scholar]
- Das JR, Tizabi Y. Additive protective effects of donepezil and nicotine against salsolinol-induced cytotoxicity in SH-SY5Y cells. Neurotoxicity research. 2009;16:194–204. doi: 10.1007/s12640-009-9040-2. [DOI] [PubMed] [Google Scholar]
- Dostert P, Strolin Benedetti M, Dordain G. Dopamine-derived alkaloids in alcoholism and in Parkinson’s and Huntington’s diseases. Journal of neural transmission. 1988;74:61–74. doi: 10.1007/BF01245140. [DOI] [PubMed] [Google Scholar]
- Freestone PS, Chung KK, Guatteo E, Mercuri NB, Nicholson LF, Lipski J. Acute action of rotenone on nigral dopaminergic neurons--involvement of reactive oxygen species and disruption of Ca2+ homeostasis. Eur J Neurosci. 2009;30:1849–1859. doi: 10.1111/j.1460-9568.2009.06990.x. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003a;23:1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003b;23:6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley A, Stone JM, Heron C, Cooper JM, Schapira AH. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson’s disease. Journal of neurochemistry. 1994;63:1987–1990. doi: 10.1046/j.1471-4159.1994.63051987.x. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Feger J, Prigent A, Michel PP, Parain K, Champy P, Ruberg M, Oertel WH, Hirsch EC. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. Journal of neurochemistry. 2003;84:491–502. doi: 10.1046/j.1471-4159.2003.01533.x. [DOI] [PubMed] [Google Scholar]
- Hurley LL, Akinfiresoye L, Nwulia E, Kamiya A, Kulkarni AA, Tizabi Y. Antidepressant-like effects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF. Behavioural brain research. 2013;239:27–30. doi: 10.1016/j.bbr.2012.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Alternative medicine review : a journal of clinical therapeutic. 2009;14:141–153. [PubMed] [Google Scholar]
- Kulkarni SK, Bhutani MK, Bishnoi M. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology. 2008;201:435–442. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- Leng A, Feldon J, Ferger B. Rotenone increases glutamate-induced dopamine release but does not affect hydroxyl-free radical formation in rat striatum. Synapse. 2003;50:240–250. doi: 10.1002/syn.10260. [DOI] [PubMed] [Google Scholar]
- Li YC, Wang FM, Pan Y, Qiang LQ, Cheng G, Zhang WY, Kong LD. Antidepressant-like effects of curcumin on serotonergic receptor-coupled AC-cAMP pathway in chronic unpredictable mild stress of rats. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:435–449. doi: 10.1016/j.pnpbp.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Loas G, Krystkowiak P, Godefroy O. Anhedonia in Parkinson’s disease: an overview. J Neuropsychiatry Clin Neurosci. 2012;24:444–451. doi: 10.1176/appi.neuropsych.11110332. [DOI] [PubMed] [Google Scholar]
- Maruyama W, Dostert P, Matsubara K, Naoi M. N-methyl(R)salsolinol produces hydroxyl radicals: involvement to neurotoxicity. Free radical biology & medicine. 1995;19:67–75. doi: 10.1016/0891-5849(95)00013-n. [DOI] [PubMed] [Google Scholar]
- Moser A, Siebecker F, Vieregge P, Jaskowski P, Kompf D. Salsolinol, catecholamine metabolites, and visual hallucinations in L-dopa treated patients with Parkinson’s disease. Journal of neural transmission. 1996;103:421–432. doi: 10.1007/BF01276418. [DOI] [PubMed] [Google Scholar]
- Moussa CE, Rusnak M, Hailu A, Sidhu A, Fricke ST. Alterations of striatal glutamate transmission in rotenone-treated mice: MRI/MRS in vivo studies. Exp Neurol. 2008;209:224–233. doi: 10.1016/j.expneurol.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec B. Salsolinol, a derivate of dopamine, is a possible modulator of catecholaminergic transmission: a review of recent developments. Physiological research / Academia Scientiarum Bohemoslovaca. 2006;55:353–364. doi: 10.33549/physiolres.930810. [DOI] [PubMed] [Google Scholar]
- Muthane U, Yasha TC, Shankar SK. Low numbers and no loss of melanized nigral neurons with increasing age in normal human brains from India. Ann Neurol. 1998;43:283–287. doi: 10.1002/ana.410430304. [DOI] [PubMed] [Google Scholar]
- Nagatsu T. Isoquinoline neurotoxins in the brain and Parkinson’s disease. Neurosci Res. 1997;29:99–111. doi: 10.1016/s0168-0102(97)00083-7. [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W, Matsubara K, Hashizume Y. A neutral N-methyltransferase activity in the striatum determines the level of an endogenous MPP+-like neurotoxin, 1,2-dimethyl-6,7-dihydroxyisoquinolinium ion, in the substantia nigra of human brains. Neuroscience letters. 1997;235:81–84. doi: 10.1016/s0304-3940(97)00723-4. [DOI] [PubMed] [Google Scholar]
- Ng TP, Chiam PC, Lee T, Chua HC, Lim L, Kua EH. Curry consumption and cognitive function in the elderly. American journal of epidemiology. 2006;164:898–906. doi: 10.1093/aje/kwj267. [DOI] [PubMed] [Google Scholar]
- Ramlochansingh C, Taylor RE, Tizabi Y. Toxic effects of low alcohol and nicotine combinations in SH-SY5Y cells are apoptotically mediated. Neurotoxicity research. 2011;20:263–269. doi: 10.1007/s12640-011-9239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer letters. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloylmethane) Free radical biology & medicine. 2007a;43:568–580. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, Limtrakul P, Badmaev V, Aggarwal BB. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007b;28:1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- Sharma OP. Antioxidant activity of curcumin and related compounds. Biochemical pharmacology. 1976;25:1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- Song JX, Shaw PC, Wong NS, Sze CW, Yao XS, Tang CW, Tong Y, Zhang YB. Chrysotoxine, a novel bibenzyl compound selectively antagonizes MPP(+), but not rotenone, neurotoxicity in dopaminergic SH-SY5Y cells. Neuroscience letters. 2012;521:76–81. doi: 10.1016/j.neulet.2012.05.063. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Brockman S, Hayhow BD. Psychiatric syndromes in Parkinson’s disease. Curr Opin Psychiatry. 2012;25:468–472. doi: 10.1097/YCO.0b013e3283577ed1. [DOI] [PubMed] [Google Scholar]
- Storch A, Kaftan A, Burkhardt K, Schwarz J. 1-Methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (salsolinol) is toxic to dopaminergic neuroblastoma SH-SY5Y cells via impairment of cellular energy metabolism. Brain Res. 2000;855:67–75. doi: 10.1016/s0006-8993(99)02272-6. [DOI] [PubMed] [Google Scholar]
- Tieu K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harbor perspectives in medicine. 2011;1:a009316. doi: 10.1101/cshperspect.a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, Sachdeva S. Prevalence of dementia in an urban Indian population. International psychogeriatrics / IPA. 2001;13:439–450. doi: 10.1017/s1041610201007852. [DOI] [PubMed] [Google Scholar]
- Xie HR, Hu LS, Li GY. SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chinese medical journal. 2010;123:1086–1092. [PubMed] [Google Scholar]
- Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacology, biochemistry, and behavior. 2005;82:200–206. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Worth PF. How to treat Parkinson’s disease in 2013. Clin Med. 2013;13:93–96. doi: 10.7861/clinmedicine.13-1-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang L, Mu X, Duan J, Zhu Y, Qing H, Li Y, Xiao S, Deng Y. Assessment of salsolinol N-methyltransferase activity in rat peripheral lymphocytes by liquid chromatography-electrospray time-of-flight mass spectrometry. Analytical and bioanalytical chemistry. 2011;399:3541–3545. doi: 10.1007/s00216-011-4683-2. [DOI] [PubMed] [Google Scholar]