Abstract
Glycogen synthase kinase (GSK) -3β is a multifunctional protein that has been implicated in the pathological characteristics of Alzheimer’s disease (AD), including the heightened levels of neurofibrillary tangles, amyloid-beta (Aβ) and neurodegeneration. In this study we used 12 month old SAMP8 mice, an AD model, to examine the effects GSK-3β may cause regarding the cognitive impairment and oxidative stress associated with AD. To suppress the level of GSK-3β, SAMP8 mice were treated with an antisense oligonucleotide (GAO) directed at this kinase. We measured a decreased level of GSK-3β in the cortex of the mice, indicating the success of the antisense treatment. Learning and memory assessments of the SAMP8 mice were tested post-antisense treatment using an aversive T-maze and object recognition test, both of which observably improved. In cortex samples of the SAMP8 mice, decreased levels of protein carbonyl and protein-bound HNE were measured indicating decreased oxidative stress. Nuclear factor erythroid -2-related factor 2 (Nrf2) is a transcription factor known to increase the level of many antioxidants, including glutathione-S transferase (GST), and is negatively regulated by the activity of GSK-3β. Our results indicated the increased nuclear localization of Nrf2 and level of GST, suggesting the increased activity of the transcription factor as a result of GSK-3β suppression, consistent with the decreased oxidative stress observed. Consistent with the improved learning and memory, and consistent with GSK-3b being a tau kinase, we observed decreased tau phosphorylation in brain of GAO-treated SAMP8 mice compared to that of RAO-treated SAMP8 mice. Lastly, we examined the ability of GAO to cross the blood-brain barrier and determined it to be possible. The results presented in this study demonstrate that reducing GSK-3 with a phosphorothionated antisense against GSK-3 improves learning and memory, reduces oxidative stress, possibly coincident with increased levels of the antioxidant transcriptional activity of Nrf2, and decreases tau phosphorylation. Our study supports the notion of GAO as a possible treatment for AD.
Keywords: Alzheimer’s disease (AD), glycogen synthase kinase-3β (GSK-3β), nuclear factor-E2-related factor 2 (Nrf2), antisense, SAMP8 mice, oxidative stress
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease that, according to the Alzheimer’s Association website [1], affects 5.4 million Americans today, costing an estimated $200 billion in 2012 to care for these individuals. Pathologically, AD is characterized by the accumulation of neurofibrillary tangles (NFTs) and amyloid-beta (Aβ)-plaques, two primary hallmarks of the disease, as well as a heightened oxidative environment in the brain and subsequent neurodegeneration. Clinically, individuals affected by AD experience a progressive cognitive decline in learning and memory which eventually leads to a highly compromised quality of life. Aβ-oligomers and NFTs are associated with the cognitive decline characteristic of the disorder [2]. To date there is no treatment which can stop or reverse the dysfunctions produced by the disorder.
Glycogen synthase kinase (GSK)-3β is a pleiotropic enzyme involved in a variety of cell activities, and has been postulated as a therapeutic target for AD due to its multiple connections to the pathology of the disease [3, 4]. Brains from Alzheimer’s subjects reportedly have increased GSK-3β-associated NFTs, tau phosphorylation and neurodegeneration [5–12]. There are two isoforms of GSK-3, α and β, both of which reportedly elicit an increase in Aβ [8, 13–17]. However, the involvement of GSK-3 in the phosphorylation of presenilin-1 (PS-1) which leads to increased production of Aβ is unclear [18]. In the brain, GSK-3β is the predominant kinase that phosphorylates tau, resulting in the hyperphosphorylation and related NFT generation of AD [19–23].
Antioxidant transcription factor nuclear factor-E2-related factor 2 (Nrf2) is among the many substrates negatively regulated by GSK-3β and is thought to have neuroprotective effects [24–27]. The main role of Nrf2 is to protect the cell against increased oxidative insults and thought to be regulated by cellular localization. In the absence of oxidative stress Nrf2 is bound to the chaperone protein Keap1, which sequesters the transcription factor in the cytosol [28–30]. During increased oxidative insults, Nrf2 disassociates from Keap1 and translocates to the nucleus where it up-regulates the transcription of over a hundred antioxidant genes, mainly phase II enzymes such as glutathione S-transferase (GST), γ-glutamylcysteine ligase, heme oxygenase-1, and glutathione peroxidase [31, 32].
The SAMP8 mouse is a model of AD that develops deficits in learning and memory by 8 months of age [33, 34]. Correlated with the cognitive impairments, SAMP8 mice exhibit an age-related increase in Aβ, Tau phosphorylation, and oxidative stress [35–38].The cognitive deficits can be reversed by lowering Aβ with antisense directed at APP [35, 39]. More recently, treatments thar reduce GSK have been found to improve learning and memory, and decrease oxidative stress in SAMP8 mice [40].
We have developed an antisense oligonucleotide that targets GSK-3β to determine if disrupting the activity of GSK-3β will improve learning and memory in the SAMP8 mouse, a model of AD. We determined the antisense could improve learning and memory when administered intracerebroventricularly (ICV). At the completion of the learning and memory testing, the cortex was collected and analyzed for GSK levels and oxidative stress. Upon cellular fractionalization, we measured nuclear and cytosolic levels of Nrf2 to examine the possible effects its GSK-3β-induced inhibition may have on the increased oxidative status associated with this model of AD. Transcriptional activity of Nrf2 was assessed through analyzing the level of GST in the homogenized samples. Finally, we examined the ability of the antisense to cross the blood brain-barrier (BBB).
2. Materials and Methods
2.1 Animal Subjects
At the start of treatment, the subjects for the experiments were 11 month old SAMP8 mice from our breeding colony. Sentinels from the facility were tested regularly to ensure our facility is virus- and pathogen- free. Food (Richland 5001) and water were available on an ad libitem basis and the rooms had a 12 hour light-dark cycle with lights on at 0600 hours. Behavioral experiments were conducted between 0730 and 1100 hours.
2.2 Antisense
GSK antisense oligonucleotide (GAO) [sequence: 5΄ (P=S) GGTTACCTTGCTGCCATCTT-3΄], or random antisense (RAO) (sequence: GATCACGTACACATCGACACCAGTCGCCATGACTGAGCTT) (Midland Certified Reagent Company, Midland, TX) were synthesized. Mice received 3 treatments of the respective antisenses dissolved in saline at 1 week intervals ICV.
2.3 Surgery and Drug Administration
Forty-eight hours prior to training, mice were anesthetized with 4% isoflurane, placed in a stereotaxic instrument, the scalp was deflected and a hole drilled through the skull over the injection site. The injection coordinates for the ICV injections were 0.5 mm posterior to the Bregma and 1.0 mm to the right of the sagittal suture. The injection depth was 2.0 mm. As noted, mice were injected 3 times at one week intervals. After ICV injection, the scalp was closed and the mice were returned to their cages.
In this study, cortical brain regions were collected from SAMP8 mice treated with GAO (n = 9) and RAO (n = 7), the latter serving as the control.
2.4 Chemicals and Materials
All chemicals were of the highest purity and purchased from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise. Nitrocellulose membranes, polyacrylamide gels, XT MES electrophoresis running buffer, and Precision Plus Protein™, and all Blue Standards were purchased from Biorad (Hercules, CA).
2.5 Behavioral Testing
2.5.1 T-Maze training and testing procedures
Acquisition was tested 5 days after the third injection in an aversive T-maze. The T-maze is both a learning task based on working-memory and a reference-memory task. The T-maze consisted of a black plastic alley with a start box at one end and two goal boxes at the other. The start box was separated from the alley by a plastic guillotine door that prevented movement down the alley until raised at the onset of training. An electrifiable floor of stainless steel rods ran throughout the maze to deliver a mild scrambled foot-shock.
Mice were not permitted to explore the maze prior to training. A block of training trials began when a mouse was placed into the start box. The guillotine door was raised and a cue buzzer sounded simultaneously; 5 sec later foot-shock was applied. The arm of the maze entered on the first trial was designated “incorrect” and the mild foot-shock was continued until the mouse entered the other goal box, which in all subsequent trials was designated as “correct” for the particular mouse. At the end of each trial, the mouse was returned to its home cage until the next trial.
Mice were trained until they made 1 avoidance. Training used an intertrial interval of 35 sec, the buzzer was of the door-bell type sounded at 55 dB, and shock was set at 0.35 mA (Coulbourn Instruments scrambled grid floor shocker model E13-08). Retention was tested one week later by continuing training until mice reached the criterion of 5 avoidances in 6 consecutive trials. The results were reported as the number of trials to criterion for the retention test.
2.5.2 Object-Place Recognition
Object recognition was tested the three days following retention testing. Object-place recognition is a declarative memory task that involves the hippocampus when, as performed here, the retention interval is 24 hours after initial exposure to the objects [41]. Mice were habituated to an empty apparatus for 5 minutes a day for 3 days prior to entry of the objects. During the training session, the mouse was exposed to two similar objects (plastic frogs) which it was allowed to examine for 5 minutes. The apparatus and the objects were cleaned between each mouse. Twenty-four hours later, the mouse was exposed to one of the original objects and a new novel object in a new location and the percent of time spent examining the new object was recorded. The novel object was made out of the same material as the original object and of the same size, but a different shape. This eliminated to possibility of smell associated with a particular object being a factor. The underlying concept of the task is based on the tendency of mice to spend more time exploring new, novel objects than familiar objects. Thus, the greater the retention/memory at 24 hours, the more time spent with the new object.
2.6 Sample preparation for GSK-3β and oxidative stress measurements
Brain samples were briefly homogenized with a Wheaton tissue homogenizer in an ice-cold lysis buffer (pH 7.4) containing 320 mM sucrose, 1% mM Tris-HCl (pH 8.8), 0.098 mM MgCl2, 0.076 mM EDTA, and proteinase inhibitors leupeptin (0.5 mg/mL), pepstatin (0.7 μg/mL), aprotinin (0.5 mg/mL) and PMSF (40 μg/mL) and a phosphatase inhibitor cocktail. The homogenized samples were then diluted 2X with lysis buffer. After homogenization, a small aliquot of homogenized samples were sonicated for 10 seconds at 20% power with a Fisher 550 Sonic Dismembrator (Pittsburgh, PA, USA) and frozen. The remaining homogenate was centrifuged at 3000 g for 5 min and the supernatant cytosolic and membranous fractions were transferred out into another set of tubes. Following the addition of 400 μL of lysis buffer, the remaining pellet nuclear fraction was centrifuged at 3,000 g for 5 min and supernatant removed. The pellet was suspended in 20 μL of lysis buffer and inhibitor. The supernatant cytosolic and membranous fractions were centrifuged at 10,000 g for 10 min, and the resulting supernatant cytosolic fraction was transferred out into another set of tubes leaving the pellet membranous fraction. All sonicated samples and fractions were stored at −70 °C until used for further experiments. Protein concentrations were measured through Pierce Bicinchoninic Acid (BCA) method [42].
2.7 Slot blot analysis
2.7.1. Protein carbonyl detection
Protein carbonyls are an index of protein oxidation [37]. For protein carbonyl detection, slot blot analysis of the 2,4-dinitrophenyl hydrazone (DNP) schiff-base adduct of the carbonyls was employed. Sample aliquots of 5 μl were incubated at room temperature with 5 μl of 12% sodium dodecyl sulfate and 10 μl of 2,4-dinitrophenylhydrazine (from OxyBlottm Protein oxidation kit, Chemicon-millipore, Billerica, MA, USA) for 20 min, followed by the addition of 7.5 μl of neutralization solution containing Tris (2 M) in 30% glycerol to each sample. Following derivatization samples were diluted to 1 μg/mL using 1× phosphate buffer solution (PBS) containing sodium chloride, mono, and dibasic sodium phosphate. The corresponding sample solutions (250 μl) were rapidly loaded as duplicates onto a nitrocellulose membrane through water vacuum pressure. The resulting protein-bound nitrocellulose membrane was then blocked with fresh blocking solution containing 750mg of bovine serum albumin (BSA) in 25ml of wash blot containing 35.2 g sodium chloride, 1.77 g monobasic sodium phosphate, 9.61 g dibasic sodium phosphate and 1.6 mL TWEEN, diluted to 4 L with deionized water for 90 min. The membrane was then incubated with polyclonal RbxDNP (from OxyBlottm Protein oxidation kit, Chemicon-millipore, Billerica, MA, USA, dilution 1:100) in wash blot for 2 h. After three 5 min washes with fresh wash blot, the membrane was then incubated with polyclonal anti-rabbit IgG alkaline phosphatase (Chemicon, Temecula, CA, USA, dilution 1:8000) for 1 hour and washed with fresh wash blot in three increments of 5, 10 and 10 min. After washing, the membrane was developed colorimetrically using a 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium reagent solution for alkaline phosphatase secondary antibody. After development, blots were dried and scanned on a CanoScan8800F (Canon) scanner using Adobe Photoshop and analyzed using Scion Image software (Scion Corporation).
2.7.2 Protein-bound HNE detection
Protein-bound HNE is an index of lipid peroxidation [43]. For slot blot analysis of protein-bound HNE detection, sample aliquots of 5 μl were incubated at room temperature with 5 μl of 12% sodium dodecyl sulfate and 10 μl of Laemmli buffer for 20 min, followed by dilution to 1 μg/mL using 1× phosphate buffer solution (PBS) containing sodium chloride, mono, and dibasic sodium phosphate. The corresponding sample solutions (250 μl) were rapidly loaded as duplicates onto a nitrocellulose membrane through water vacuum pressure. The resulting protein-bound nitrocellulose membrane was then blocked with fresh blocking solution for 90 min. The membrane was then incubated with polyclonal anti-HNE (Alpha diagnostic, San Antonio, TX, USA, dilution 1:5000) in wash blot for 2 h. After three 5 min washes with fresh wash blot, the membrane was then incubated with polyclonal anti-rabbit IgG alkaline phosphatase (Chemicon, Temecula, CA, USA, dilution 1:8000) for 1 hour and washed with fresh wash blot in three increments of 5, 10 and 10 min. After washing, the membrane was developed colorimetrically using a 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium reagent solution for alkaline phosphatase secondary antibody. After development, blots were dried and scanned on a CanoScan8800F (Canon) scanner using Adobe Photoshop and analyzed using Scion Image software (Scion Corporation).
2.8 Western blot analysis
The Western blot technique was used to measure protein levels of GSK-3β, Nrf2, phospho-tau, and GST. For Western blot, 30 μg or 50 μg of protein were combined with loading buffer containing 0.5 M Tris (pH 6.8), 40% glycerol, 8% SDS, 20% β-mercaptoethanol and 0.01% Bromophenol Blue and denatured in boiling water for 5 min, then cooled on ice. Sample proteins were resolved on a 4–12% bis-tris polyacrylamide gel at room temperature using a Criterion Cell™ vertical electrophoresis buffer tank filled with 1X XT MES running buffer. During the electrophoretic run, the voltage was initially set at 80 V for ~10 min, to ensure proper protein stacking, and then increased to 120 V for ~130 min. The voltage of the phospho-tau measurement was 170V for 80 min. The separated proteins from the gel were then transferred to nitrocellulose membrane using a Trans-Blot® Turbo™ transfer system SD semi-Dry Transfer Cell (Bio-Rad) at 1.0 A/gel for 30 min, while 80 min was applied for phospho-tau transference. The protein transfer from the gel to the membrane was checked using the reversible protein stain, ponceau S.
The subsequent protein-bound membranes were incubated for 90 min in fresh blocking buffer, and then incubated for 3 h in dilutions of primary anti-GSK-3β (rabbit, Cell Signaling, Danvers, MA, USA, dilution 1:2000), anti-Nrf2 (rabbit, Enzo Life Sciences, Farmingdale, NY, USA, dilution 1:1000), AT180 (Pierce, Rockford, IL, USA, dilution 1:1000) and anti-GST (rabbit, Epitomics, Burlingame, CA, USA, dilution 1:1000) prepared in fresh wash blot. Subsequent membranes were then washed three times with fresh wash blot and incubated for 1 h in a dark room with the ECL Plex CyDye conjugated secondary antibodies (GE Healthcare, Pittsburgh, PA, USA), Cy5 (anti-mouse) and Cy3 (anti-rabbit). Membranes were washed again with fresh wash blot three times. Bands were visualized using a fluorescent laser Typhoon™ FLA9500 (Cy5: λEX=633 nm λEM=67, Cy3: λEX=532 nm λEM=570, GE Healthcare, Pittsburgh, PA, USA) scanner and quantified using Scion Image software (Scion Corporation). The membrane incubated with AT180 primary antibody for phospho-tau was subsequently incubated with anti-mouse HRP secondary antibody (Pierce, Rockford, IL, USA, dilution 1:20000) for 1 h. Bands were visualized using SuperSignal West Dura Chemiluminescent Substrate (Pierce, Rockford, IL, USA) and exposed to X-Ray film. Bands were quantified using ImageJ software. For loading control, the blots were probed with anti-β-actin (dilution 1:2000), anti-GAPDH (Abcam, Cambridge, MA, USA, dilution 1:1000) or anti-histone 2B (EMD Millipore, Billerica, MA, USA, dilution 1:1000) raised in mouse, followed by incubation with anti-mouse secondary antibody (Cy3).
2.9 Blood-Brain-Barrier Influx
2.9.1 Labeling of GAO
The anti-GSK was labeled with 32-P as previously described [44]. Briefly, GAO anti-GSK was end-labeled by mixing 5μg of GAO with 3μl of 10× kinase buffer, 1.5μl of T4 polynucleotide kinase and 3μl of [γ32P] ATP. Following incubation and subsequent kinase heat-inactivation, the labeled 32-P- GAO (P-GAO) was removed from the reaction mixture by ethanol precipitation followed by centrifugation. The pellet containing the P- GAO went through three cycles of washing with ethanol and centrifugation, and then the pellet was air dried in a vacuum centrifuge and re-suspended in 100μl water.
2.9.2 Clearance from Serum
Male CD-1 mice 4 months of age were anesthetized with an ip injection of 0.2ml urethane. The jugular vein and carotid artery were exposed. Each mouse was given a 0.2ml injection into the jugular vein of saline solution containing 1% bovine serum albumin(S-BSA) and 3 × 105 CPM P-GAO. At time points of 1, 2, 3, 4, 5, 7.5, 10, 15, 20, 30, 60, or 120 min post-injection, carotid arterial blood was collected, the mouse immediately decapitated and the brain removed and weighed. Arterial blood was centrifuged and 3600 rpm for 15 min and the serum was collected. The level of radioactivity in serum and brain was determined with a scintillation counter. The rate of clearance of P-GAO P-GSK from the serum was determined by expressing the results as the percent of the injected dose in each milliliter of serum (%Inj/ml) and plotting these values against time (min). The %Inj/ml was determined by the equation:
%Inj/ml = (CPM/ml serum)/(mean CPM/injection)100.
2.9.3 Blood to Brain Passage of P- GAO
Multiple-time regression analysis was used to determine the rate of uptake from blood to brain for P- GAO. The brain/serum ratios (B/S) were calculated for each time point ranging from 1 to 120min after iv injection of P- GAO for the extended curve (n=1/time point) and 1 to 20 min after iv injection for the short curve (n=1–2/time point). These ratios were plotted against their respective exposure times (Expt). From this graph, the slope of the linear portion of the line represents the unidirectional influx rate (Ki) and the y-intercept represents the initial volume of distribution (Vi). The B/S was calculated using the formula:
B/S = (Brain CPM/g of brain)/(serum CPM/ μl of serum).
Expt was calculated using the formula:
Expt =[ ∫ot Cp(t)dt] /Cpt, where t is time, Cp is the level of radioactivity in the serum and Cpt is the level of radioactivity in the serum at time t; units = min.
The percentage of the injected dose taken up by each gram of brain tissue (%Inj/g) was calculated as:
%Inj/g = (B/S – Vi)(%Inj/ml)/1000.
2.9.4 Capillary Depletion of P- GAO
This study was performed to determine the distribution of the P- GAO between the brain tissue and the brain capillaries. The procedure has been previously described [45]. Mice (n=7) were anesthetized with 40% urethane and then received and iv injection of 1 × 106 CPM of P-GAO in 0.2ml S- BSA into the jugular vein. Ten minutes post-injection the abdomen was opened and blood collected from the abdominal aorta. The thorax was then opened through a midline sternal incision, the descending aorta clamped, both jugular veins severed and washout of the vascular space was performed by injecting 20ml of saline into the left ventricle of the heart over a 1 min period. This procedure washed out the vascular space of the brain, by removing any substances that were intravascular or loosely adhered to the capillary lumen of the brain microvasculature. The brain was then removed and placed in a glass homogenizer containing 0.8ml of physiologic buffer. After 10 strokes with the pestle, 1.6ml of the physiologic buffer containing 26% dextran was added to the homogenate. The homogenate was homogenized a second time (3 strokes). The homogenate was centrifuged at 4000 × g for 30 min at 4°C in a swinging bucket rotor. The supernatant (brain parenchyma) was separated from the pellet (brain microvasculature) and the levels of radioactivity determined. The results were expressed as the volume of distribution in μl/g in tissue (parenchyma or capillary)/serum rations with the formula:
μl/g = (CPM/gram of tissue)/(CPM/ml of serum).
2.10 Statistical Analysis
Results from the T-maze were analyzed by a T-test. Results from the GSK and oxidative stress measurements were compared by the Mann-Whitney test using Prism 5.0 statistical package software to assess statistical significance in comparing protein carbonyl, protein-bound HNE, GSK-3β, Nrf2 and GST levels in protein samples between control and experimental data sets. Significant differences were set at P < 0.05.
3. Results
3.1 Effects of GAO on Learning and Memory
Seventy-two hours after GAO treatment mice were tested in T-maze foot shock avoidance acquisition. One week later, the mice were tested for retention. The t-test for trial to first avoidance during acquisition in the T-maze showed a significant effect t(15)=2.092, P<0.05. The mice that received GAO took significantly fewer trials to reach their first avoidance (8.75±1.22) than the mice that received random antisense (11.67±0.745) (Figure 1A). The t-test for trials to criterion on the retention test showed a significant effect t(14)=2.945, P<0.01 (Figure 1B). The mice that received GAO (6.714 ± 0.42) took significantly fewer trials to reach criterion than the mice that received RAO (12.89±1.806).
Figure 1.
Antisense oligonucleotide treatment led to improved cognition in 12 month old SAMP8 mice compared to SAMP8 mice treated with random antisense oligonucleotide. Specifically: The 12month old SAMP8 mice which received GAO (N=9) took significantly fewer number of trials to make their first avoidance during acquisition (A) and significantly fewer number of trials to make 5 avoidances in 6 consecutive trials on the one week retention test (B) in T-maze foot shock avoidance compared to the 12 month old SAMP8 mice which received RAO. In the novel object recognition task with a 24 hour retention delay the 12 month old SAM P8 mice that received GAO spent significantly greater percent of the total exploration time exploring the novel object during the retention test compared to the 12 month old SAMP8 mice that received RAO (N=8) (C), * P<0.05; ** P<0.01.
The t-test for time exploring the novel object during the 24 hour retention test was significant t(15)=2.373, P<0.03. The mice that received GAO (59.33±5.16) spent a significantly greater amount of time exploring the novel object than the mice that received random antisense (44.75±2.93) (Figure 1C).
3.2 Measurement of Nuclear and Cytosolic GSK-3β
Immunochemical methods were used to determine if the antisense treatment successfully decreased the level of GSK-3β in SAMP8 mice compared to the control. GSK-3β was successfully down regulated in the nuclear and cytosolic fractions by the antisense treatment (Figures 2A-B). Immunoblot analysis of the GSK-3β levels in aliquots of nuclear (30 μl) and cytosolic (50 μl) fractions indicate a significant decrease of 36.9% (P<0.04) and 16.9% (P<0.05), respectively, from SAMP8 GAO mice compared to the control.
Figure 2.
(A) Nuclear GSK-3β level in GAO compared to RAO SAMP8 mice. Level of GSK-3β decreased in the nuclear fraction of SAMP8 mice treated with GAO compared to that of SAMP8 mice treated with RAO. Data are represented as % control, and shown as mean ± SEM with *P<0.04. Total number of animals used in each group are: GAO=5 and RAO=5. (B) Cytosolic GSK-3β level in GAO compared to RAO SAMP8 mice. Level of GSK-3β decreased in the cytosolic fraction of SAMP8 mice treated with GAO compared to that of SAMP8 mice treated with RAO. Data are represented as % control, and shown as mean ± SEM with *P<0.05. Total number of animals used in each group are: GAO=8 and RAO=7.
3.3 Analysis of Protein Carbonyls
Sensitive immunochemical methods were used to determine if suppression of GSK-3β in SAMP8 mice had any effect on protein carbonyl levels. The results indicate a decrease in brain protein oxidation as a result of the suppressed GSK-3β level in SAMP8 mice. Immunoblot analysis of homogenized brain samples indicates a significant 26.3 percent decrease (P<0.02) in the protein carbonyl level from SAMP8 GAO mice compared to the control (Figure 3A).
Figure 3.
(A) Protein carbonyl level in GAO compared to RAO SAMP8 mice. Protein Carbonyl level decreased in SAMP8 mice treated with AO directed at GSK-3β (N=9) compared to that of SAMP8 mice treated with random AO (N=7). Data are represented as % control, and shown as mean ± SEM with *P<0.02. (B) Protein-bound HNE level in GAO compared to RAO SAMP8 mice. Protein-bound HNE level decreased in SAMP8 mice treated with AO directed at GSK-3β (N=9) compared to that of SAMP8 mice treated with random AO (N=6). Data are represented as % control, and shown as mean ± SEM with *P<0.0008.
3.4 Analysis of Protein-Bound HNE
Our results indicate a decreased lipid peroxidation as a result of the suppressed GSK-3β level in SAMP8 mice brain. Immunoblot analysis of homogenized samples show a significant 20.3 percent decrease (P<0.0008) in SAMP8 GAO mice compared to the control (Figure 3B).
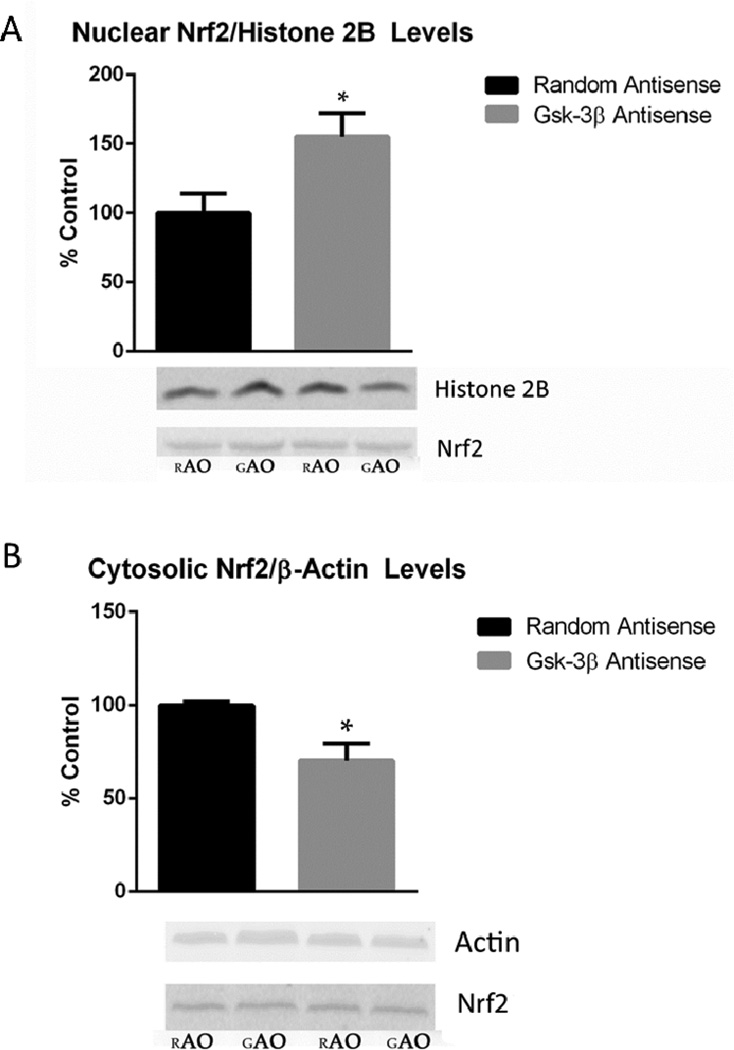
3.5 Measurement of Nuclear and Cytosolic Nrf2
To determine if the suppression of GSK-3β in SAMP8 mice had an effect on the nuclear translocation of Nrf2, nuclear and cytosolic levels of this redox sensitive transcription factor were measured. Nrf2 band intensities of the nuclear and cytosolic fractions were normalized to histone-2B and β-actin, respectively, each serving as a loading control. Our results suggest a possible increase in the translocation of Nrf2 from the cytosol to the nucleus as a result of the suppressed GSK-3β level in SAMP8 mice. Immunoblot analysis of the Nrf2 levels in aliquots of nuclear and cytosolic fractions indicate a significant 69.4 percent increase (P<0.04) and 29.5 percent increase (P<0.02), respectively, from SAMP8 GAO mice compared to the control (Figure 4A-B).
Figure 4.
(A) Nuclear Nrf2 level in GAO compared to RAO SAMP8 mice. The level of Nrf2 increased in the nuclear fraction of SAMP8 mice treated with GAO compared to that of SAMP8 mice treated with RAO. Data are represented as % control, and shown as mean ± SEM with *P<0.04. Total number of animals used in each group are: GAO=5 and RAO=5. (B) Cytosolic Nrf2 level in GAO compared to RAO SAMP8 mice. Reduced expression of Nrf2 in the cytosolic fraction of SAMP8 mice treated with GAO compared to that of SAMP8 mice treated with RAO. Data are represented as % control, and shown as mean ± SEM with *P<0.02. Total number of animals used in each group are: GAO=9 and RAO=5.
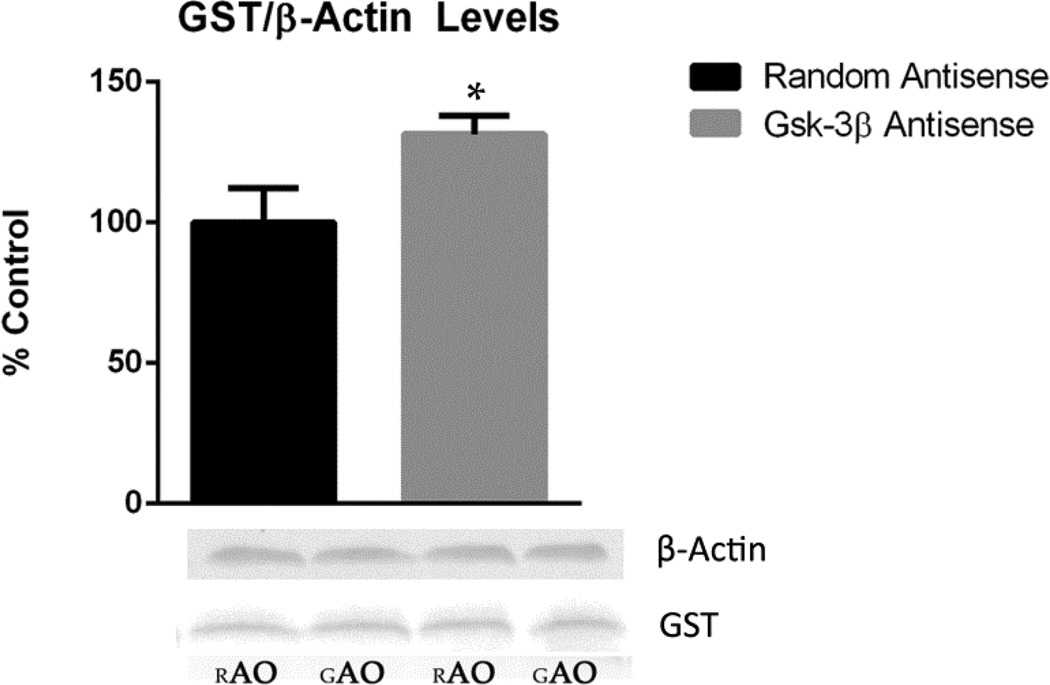
3.6 Measurement of GST
GST is one of several antioxidant enzymes up-regulated by Nrf2 transcriptional activity. As a means of determining Nrf2 transcriptional activity, the level of GST was measured. GST band intensities of the homogenized samples were normalized to β-actin, a loading control. Our results suggest a possible increase in Nrf2 transcriptional activity as a result of the suppressed GSK-3β level in SAMP8 mice. Immunoblot analysis of the GST level in 30 μl aliquots of protein sample show a significant 31.5 percent increase (P<0.03), from SAMP8 GAO mice compared to the control (Figure 5).
Figure 5.
GST level in GAO compared to RAO SAMP8 mice. The level of GST increased in the homogenized samples of SAMP8 mice treated with GAO compared to that of SAMP8 mice treated with RAO. Data are represented as % control, and shown as mean ± SEM with *P<0.03. Total number of animals used in each group are: GAO=7 and RAO=6.
3.7 Measurement of Phospho-tau
Given that: (1) GSK-3β is a kinase for tau; (2) tau hyperphosphorylation is highly detrimental to neurons; and (3) GAO treatment led to improved learning and memory in SAMP8 mice (Fig. 1), we tested the hypothesis that GAO treatment would result in lower levels of phospho-tau in brain of SAMP8 mice compared to brain from RAO-treated SAMP8 mice. Figure 6 shows that this hypothesis was confirmed (p<0.01).
Figure 6.
Phospho-tau (AT180) level in brain of GAO-treated SAMP8 mice compared to RAO-treated SAMP8 mice. The level of phospho-tau (AT180) decreased in the homogenized samples of SAMP8 mice treated with GAO compared to that of SAMP8 mice treated with RAO. Data are represented as % control, and shown as mean ± SEM with *P<0.01. Total number of animals used in each group are: GAO=9 and RAO=7. Shown are two representative Western blots of samples from each group and of GAPDH (loading control).
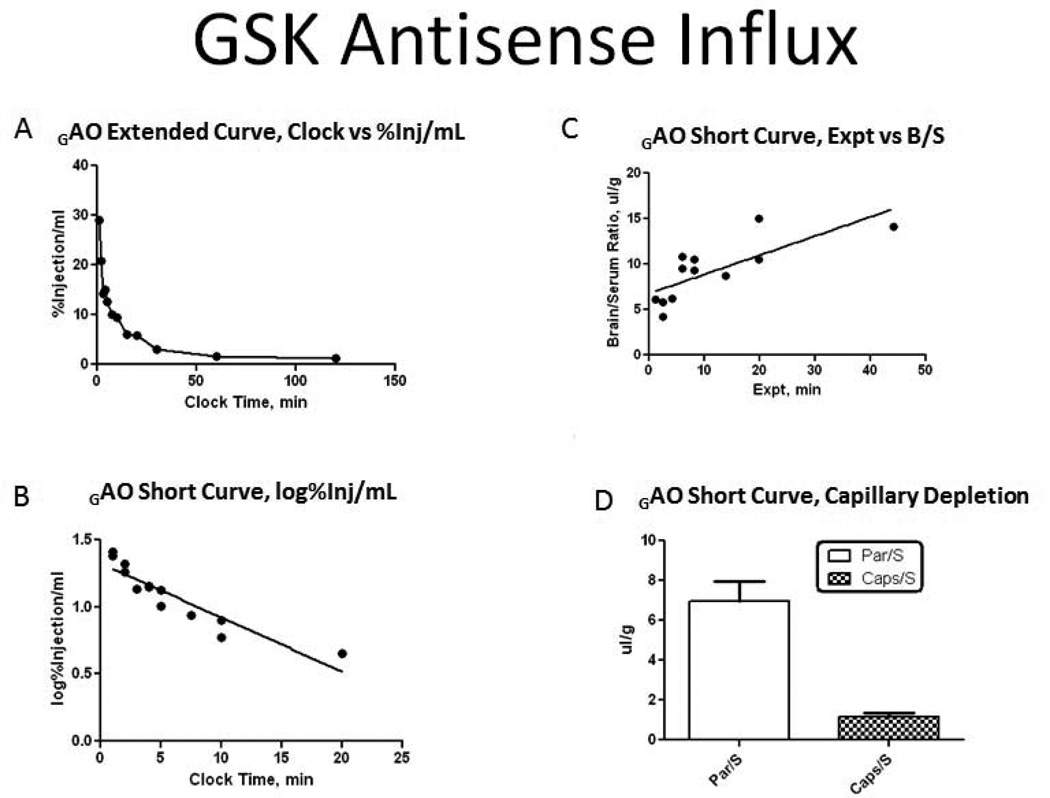
3.7 GAO Influx Across the Blood Brain-Barrier
The %Inj/ml demonstrated that there was an early decline of P- GAO from serum. Serum levels reached a steady state by 30 min after IV injection. The early distribution phase of clearance from serum was linear out to 20 min, with a significant correlation between log[%Inj/ml] and time. The half-time disappearance rate from serum was −7.47min (r=0.9205, p<0.0001). The linear relation between the brain/serum ratios (B/S) and exposure time (Expt) during the first 20 min demonstrated P- GAO influx into the brain. The unidirectional rate of influx (Ki) from blood to brain was 0.2108±0.0523 μl/g-min (r=0.7722, p=0.0020). The volume of distribution at time zero (Vi) was 6.78±0.816 μl/g. When plotted against time, the percentage of the iv injected dose of P- GAO taken up by the brain was 0.033%Inj/g at 20 min post-injection. Capillary depletion studies produced a mean brain parenchyma/serum ratio of 6.956±0.995ul/g and a mean capillary/serum ration of 1.183±0.163ul/g. The parenchyma/serum ratio was almost 6 times greater than the capillary/serum ratio indicating the P- GAO completely crossed the BBB to enter the brain parenchymal space (Figure 7).
Figure 7.
Kinetics of blood-to-brain transport of 32P-GAO after IV administration. (A) Clearance of 32P-GAO from blood. (B) The initial distribution phase was linear out to 20 min. (C) Slope of the line with multiple-time regression analysis demonstrated unidirectional influx of 32P-GAO with a Ki of 0.2108±0.0523 ul/g-min and a Vi of 6.78±0.0816ul/g. (D) Capillary depletion demonstrated 32P-GAO crossed the blood-brain barrier to enter the brain parenchyma.
4. Discussion
The regulatory kinase GSK-3β has been implicated in AD through its various contributions including hyperphosphorylated tau formation and neurodegeneration [6, 9, 22, 23, 46]. High levels of GSK-3β have been reported in AD brain, further supporting a connection between the kinase and the pathogenesis of the neurodegenerative disease that remains to be elucidated [47, 48]. In this current study, we examined the possible effects GSK-3β may have on cognitive deficits and brain oxidative stress observed in a mouse model of AD through the antisense-mediated suppression of the kinase in SAMP8 mice.
The SAMP8 mice used in this study were treated with antisense oligonucleotide directed at GSK-3β and random antisense oligonucleotide, the latter serving as the control. The GSK-3β antisense had a sequence that corresponds to 94–113 nucleotides downstream from the initiation codon of GSK-mRNA. This is an internal sequence with high probability of being located away from any loop formation in the mRNA. As an internal site, it should not block 100% of GSK mRNA. This is important as GSK-3 is essential for intracellular signaling pathways such as cell proliferation, cellular migration, glucose regulation, inflammatory responses and apoptosis [49]. Analysis of cortical tissue showed a suppression of GSK-3β, indicating the success of the administered antisense treatment directed at the kinase.
ICV administration of GAO improved learning and memory in T-maze foot shock avoidance and object recognition in the SAMP8 mouse model of AD, consonant with the notion that this kinase is implicated in the cognitive deficits associated with the disorder. This improved learning and memory was associated with decreased markers of protein oxidation and lipid peroxidation (protein carbonyls and protein-bound HNE, respectively) in brain and was associated with decreased phosphorylation of tau. We measured the levels of protein carbonyl and protein-bound HNE, parameters of protein oxidation and lipid peroxidation, respectively. Both protein carbonyl and protein-bound HNE significantly decreased in brain of GAO treated SAMP8 mice compared to the control, consistent with the notion that this kinase plays a role in the elevated oxidative status characteristic of AD brain. The observed reduction in oxidative stress may be a consequence of the increased antioxidant transcriptional activity of Nrf2, resulting from its decreased inhibition by GSK-3β.
The neuroprotective transcription factor Nrf2 is one of the many proteins negatively regulated by the activity of GSK-3β and this transcription factor plays an important role in the cellular defense against oxidative stress through inducing the expression of antioxidant phase II genes, including, among others, heme oxygenase-1, glutamate-cysteine ligase, and glutathione S-transferase [24, 50, 51]. To determine if the suppression of GSK-3β in SAMP8 led to the nuclear re-localization of Nrf2, we measured the nuclear and cytosolic levels of this transcription factor. Significantly increased nuclear and decreased cytosolic Nrf2 levels measured supports the increased nuclear localization of the transcription factor. As a means of determining increased transcriptional activity, we measured the level of GST, which is an antioxidant enzyme up-regulated by Nrf2, responsible for the conjugation of HNE to glutathione for export from the brain. The level of GST significantly increased in SAMP8 GAO mice compared to the control, in agreement with the observed decrease in protein-bound HNE, suggesting the increased transcriptional activity of Nrf2. These results support the idea that the activity of GSK-3β, and its associated inhibition of Nrf2-mediated antioxidant transcription, play major roles in the loss of tolerance to an oxidative environment observed in AD. As mentioned, GAO had a neuroprotective effect of reducing phosphorylation of tau compared to treatment with RAO (Fig. 6). Elevated tau phosphorylation is a cardinal hallmark of AD pathology and neurodegeneration. If the activity of GSK-3β does play a prominent role in the pathogenesis of AD, then inhibitors of the kinase may be an effective therapeutic treatment of the disorder.
Given the critical cellular functions of GSK-3β, antisense treatment may be an effective way to control the over-activity of the kinase without completely blocking its functions. Currently, there is increasing interest in the use of antisenses for the treatment of diseases. Working through the Watson-Crick mechanism, antisenses bind to and induce the cleavage of homologous stretches of mRNA sequences resulting in the targeted destruction of mRNA [52]. Antisenses are currently in various stages of testing for such conditions as cancer, hypercholesterolemia, Ebola virus infection, type 2 diabetes, HIV infection and ocular disease, and may be a feasible treatment for AD as well [52, 53].
Although the behavioral and oxidative stress studies presented here followed ICV treatment with GAO or RAO, we investigated the possibility of peripherally administered GAO crossing the BBB, a prerequisite to development of an effective therapy. By examining influx of GAO at the blood-brain barrier, we were able to show that peripherally injected GSK antisense crosses the blood-brain barrier and enters the CNS. These findings with GAO are similar to the findings of an antisense we previously developed directed at the C-terminal portion of the APP peptide (OL-1). We found that administration of OL-1 improved learning and memory, decreased oxidative stress and crossed the BBB [54–57]. Based on our previous results with APP antisense and our results here with GSK antisense, AD is an additional condition for which antisense likely would be an effective treatment. The current findings in this study suggest that peripheral administration of GAO is feasible and may improve learning and memory and reverse oxidative stress in AD brain.
In conclusion, this paper provides evidence that the inhibition of GSK-3β with antisense improves cognition and indices of oxidative stress in a mouse model of Alzheimer’s disease. In addition, the reduction in Nrf2 provides an additional potential mechanism through which GSK-3β over-activity contributes to the oxidative damage associated with AD. The ability of GAO to cross the BBB suggests that peripheral administration is possible and that GAO should be investigated further as a potential treatment for AD.
Highlights.
Glycogen synthase kinase-3β is implicated in pathological aspects of AD.
Antisense oligonucleotide against GSK-3β was used in brain of SAMP8 mice.
Improved learning/memory, less oxidative stress and less tau phosphorylation found
Increased Nrf-2 translocation to nucleus contributed to decreased oxidative stress.
Results are consistent with therapeutic strategies to reduce GSK-3β for AD.
Acknowledgements
This work was supported by a NIH grant to D.A.B [AG-05119] and grants to S.A.F. [VA Merit Review] and Edunn Biotechnology, St. Louis, MO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Association As. 2013 Alzheimer's disease facts and figures. Alzheimer's Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclen: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30:7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondragon-Rodriguez S, Perry G, Zhu X, Moreira PI, Williams S. Glycogen synthase kinase 3: a point of integration in Alzheimer's disease and a therapeutic target. International Journal of Alzheimer's Disease. 2012;2012:1–4. doi: 10.1155/2012/276803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodgett JR. Judging a protein by more than its name: GSK-3. Sci STKE. 2001;2001:re12. doi: 10.1126/stke.2001.100.re12. [DOI] [PubMed] [Google Scholar]
- 5.Cho JH, Johnson GV. Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3beta (GSK3beta) plays a critical role in regulating tau's ability to bind and stabilize microtubules. Journal of neurochemistry. 2004;88:349–358. doi: 10.1111/j.1471-4159.2004.02155.x. [DOI] [PubMed] [Google Scholar]
- 6.Engel T, Goni-Oliver P, Lucas JJ, Avila J, Hernandez F. Chronic lithium administration to FTDP-17 tau and GSK-3beta overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. Journal of neurochemistry. 2006;99:1445–1455. doi: 10.1111/j.1471-4159.2006.04139.x. [DOI] [PubMed] [Google Scholar]
- 7.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. Journal of neurochemistry. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurtado DE, Molina-Porcel L, Carroll JC, Macdonald C, Aboagye AK, Trojanowski JQ, Lee VM. Selectively silencing GSK-3 isoforms reduces plaques and tangles in mouse models of Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:7392–7402. doi: 10.1523/JNEUROSCI.0889-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. The EMBO journal. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pei JJ, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. Journal of neuropathology and experimental neurology. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Sereno L, Coma M, Rodriguez M, Sanchez-Ferrer P, Sanchez MB, Gich I, Agullo JM, Perez M, Avila J, Guardia-Laguarta C, Clarimon J, Lleo A, Gomez-Isla T. A novel GSK- 3beta inhibitor reduces Alzheimer's pathology and rescues neuronal loss in vivo. Neurobiology of disease. 2009;35:359–367. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, Diaz-Nido J. Lithium protects cultured neurons against beta-amyloid-induced neurodegeneration. FEBS letters. 1999;453:260–264. doi: 10.1016/s0014-5793(99)00685-7. [DOI] [PubMed] [Google Scholar]
- 13.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ly PT, Wu Y, Zou H, Wang R, Zhou W, Kinoshita A, Zhang M, Yang Y, Cai F, Woodgett J, Song W. Inhibition of GSK3beta-mediated BACE1 expression reduces Alzheimerassociated phenotypes. The Journal of clinical investigation. 2013;123:224–235. doi: 10.1172/JCI64516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Hernandez JI, Gomez-Villafuertes R, Leon-Otegui M, Hontecillas-Prieto L, Del Puerto A, Trejo JL, Lucas JJ, Garrido JJ, Gualix J, Miras-Portugal MT, Diaz-Hernandez M. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer's disease through GSK3beta and secretases. Neurobiology of aging. 2012;33:1816–1828. doi: 10.1016/j.neurobiolaging.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Ryder J, Su Y, Liu F, Li B, Zhou Y, Ni B. Divergent roles of GSK3 and CDK5 in APP processing. Biochemical and biophysical research communications. 2003;312:922–929. doi: 10.1016/j.bbrc.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 18.Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloi-beta peptides. Nature. 2003;423:435. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 19.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2006;9:309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- 20.Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–426. doi: 10.1016/j.neuron.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanger DP, Hughes K, Woodgett JR, Brion JP, Anderton BH. Glycogen synthase kinase-3 induces Alzheimer's disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neuroscience letters. 1992;147:58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- 22.Lovestone S, Reynolds CH, Latimer D, Davis DR, Anderton BH, Gallo JM, Hanger D, Mulot S, Marquardt B, Stabel S, et al. Alzheimer's disease-like phosphorylation of the microtubule-associated protein tau by glycogen synthase kinase-3 in transfected mammalian cells. Current biology : CB. 1994;4:1077–1086. doi: 10.1016/s0960-9822(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 23.Hong M, Chen DC, Klein PS, Lee VM. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. The Journal of biological chemistry. 1997;272:25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- 24.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calabrese V, Ravagna A, Colombrita C, Scapagnini G, Guagliano E, Calvani M, Butterfield DA, Giuffrida Stella AM. Acetylcarnitine induces heme oxygenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. Journal of neuroscience research. 2005;79:509–521. doi: 10.1002/jnr.20386. [DOI] [PubMed] [Google Scholar]
- 27.Williamson TP, Johnson DA, Johnson JA. Activation of the Nrf2-ARE pathway by siRNA knockdown of Keap1 reduces oxidative stress and provides partial protection from MPTPmediated neurotoxicity. Neurotoxicology. 2012;33:272–279. doi: 10.1016/j.neuro.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo SC, Li X, Henzl MT, Beamer LJ, Hannink M. Structure of the Keap1:Nrf2 interface provides mechanistic insight into Nrf2 signaling. The EMBO journal. 2006;25:3605–3617. doi: 10.1038/sj.emboj.7601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes & development. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoh K, Ishii T, Wakabayashi N, Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free radical research. 1999;31:319–324. doi: 10.1080/10715769900300881. [DOI] [PubMed] [Google Scholar]
- 31.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1- dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Molecular and cellular biology. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yagi H, Katoh S, Akiguchi I, Takeda T. Afe-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in recent memory. Neurosci Biobehav Rev. 1988;474:86–93. doi: 10.1016/0006-8993(88)90671-3. [DOI] [PubMed] [Google Scholar]
- 34.Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev. 1998;22:1–20. doi: 10.1016/s0149-7634(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 35.Morley JE, Kumar VB, Bernardo AI, Farr SA, Uezu K, Tumosa N, Flood JF. Beta-amyloid precursor polypeptide in SAMP8 mice affects learning and memory. Peptides. 2000;21:1761–1767. doi: 10.1016/s0196-9781(00)00342-9. [DOI] [PubMed] [Google Scholar]
- 36.Pallas M, Camins A, Smith MA, Perry G, Lee HG, Casadesus G. From aging to Alzheimer's disease: unveiling "the switch" with the senescence-accelerated mouse model (SAMP8) J Alzheimers Dis. 2008;15:615–624. doi: 10.3233/jad-2008-15408. [DOI] [PubMed] [Google Scholar]
- 37.Butterfield DA, Stadtman ER. Protein Oxidation Processes in Aging Brain. Adv Cell Aging Gerontol. 1997:161–191. [Google Scholar]
- 38.Poon HF, Castegna A, Farr SA, Thongboonkerd V, Lynn BC, Banks WA, Morley JE, Klein JB, Butterfield DA. Quantitative proteomics analysis of specific protein expression and oxidative modification in aged senescence-accelerated-prone 8 mice brain. Neuroscience. 2004;126:915–926. doi: 10.1016/j.neuroscience.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 39.Erickson MA, Niehoff ML, Farr SA, Morley JE, Dillman LA, Lynch KM, Banks WA. Peripheral admininstration of antisense oligonucleotides targeting the amyloid- Β protein precursor reverses AΒPP and LRP-1 overexpression in the aged SAMP8 mouse brain. J Alzheimers Dis. 2012;28:951–960. doi: 10.3233/JAD-2011-111517. [DOI] [PubMed] [Google Scholar]
- 40.Tajes M, Yeste-Velasco M, Zhu X, Chou SP, Smith MA, Pallas M, Camins A, Casadesus G. Activation of Akt by lithium: pro-survival pathways in aging. Mech Ageing Dev. 2009;130:253–261. doi: 10.1016/j.mad.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicichoninic acid. Analytical Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 43.Sultana R, Perluigi M, Butterfield DA. Lipid peroxidation triggers neurodegeneration: A redox prloteomics view into the Alzheimer disease brain. Free Radic Biol Med. 2012 doi: 10.1016/j.freeradbiomed.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banks WA, Jaeger LB, Urayama A, Kumar VB, Hileman SM, Gaskin FS, Llanza NV, Farr SA, Morley JE. Preproenkephalin targeted antisenses cross the bloodbrain barrier to reduce brain methionine enkephalin levels and increase voluntary ethanol drinking. Peptides. 2006;27:784–796. doi: 10.1016/j.peptides.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Jaeger LB, Dohgu S, Hwang MC, Farr SA, Murphy MP, Fleegal-DeMotta MA, Lynch JL, Robinson SM, Niehoff ML, Johnson SN, Kumar VB, Banks WA. Testing the neurovascular hypothesis of Alzheimer's disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. Journal of Alzheimer's disease : JAD. 2009;17:553–570. doi: 10.3233/JAD-2009-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi H, Ishiguro K, Uchida T, Takashima A, Lemere CA, Imahori K. Preferential labeling of Alzheimer neurofibrillary tangles with antisera for tau protein kinase (TPK) I/glycogen synthase kinase-3 beta and cyclin-dependent kinase 5, a component of TPK II. Acta neuropathologica. 1996;92:232–241. doi: 10.1007/s004010050513. [DOI] [PubMed] [Google Scholar]
- 47.Pei JJ, Tanaka T, Tung YC, Braak E, Iqbal K, Grundke-Iqbal I. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. Journal of neuropathology and experimental neurology. 1997;56:70–78. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Leroy K, Yilmaz Z, Brion JP. Increased level of active GSK-3beta in Alzheimer's disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathology and applied neurobiology. 2007;33:43–55. doi: 10.1111/j.1365-2990.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 49.Jope RS, Yukaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): Inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. The Journal of biological chemistry. 1999;274:33627–33636. doi: 10.1074/jbc.274.47.33627. [DOI] [PubMed] [Google Scholar]
- 51.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochemical and biophysical research communications. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 52.Malik R, Roy I. Making sense of therapeutics using antisense technology. Expert opinion on drug discovery. 2011;6:507–526. doi: 10.1517/17460441.2011.565744. [DOI] [PubMed] [Google Scholar]
- 53.Tamm I. AEG-35156, an antisense oligonucleotide against X-linked inhibitor of apoptosis for potential tretment of cancer. Curr Opin Investig Drugs. 2008;9:638–646. [PubMed] [Google Scholar]
- 54.Petursdottir AL, Farr SA, Morley JE, Banks WA, Skuladottir GV. Lipid peroxidation in brain during aging in the senescence-accelerated mouse (SAM) Neurbiol Aging. 2006;28:1170–1178. doi: 10.1016/j.neurobiolaging.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 55.Poon HF, Joshi G, Sultana R, Farr SA, Banks WA, Morley JE, Calabrese V, Butterfield DA. Antisense directed at the Abeta region of the APP decreases brain oxidative maarkers in aged senescence accelerated mice. Brain Res. 2004;1018:86–96. doi: 10.1016/j.brainres.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 56.Banks WA, Farr SA, Butt W, Kumar VB, Franko MW, Morley JE. Delivery across the blood-brain barrier of antisense directed against amyloid beta: reversal of learning and memory deficits in mice overexpressing amyloid precursor protein. J Pharmacol Exp Ther. 2001;297:1113–1121. [PubMed] [Google Scholar]
- 57.Kumar VB, Farr SA, Flood JF, Kamlesh V, Franko M, Banks WA, Morley JE. Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice. Peptides. 2000;21:1769–1775. doi: 10.1016/s0196-9781(00)00339-9. [DOI] [PubMed] [Google Scholar]