Abstract
Rationale
Psychoactive substituted phenethylamines 2,5-dimethoxy-4-chlorophenethylamine (2C-C); 2,5-dimethoxy-4-methylphenethylamine (2C-D); 2,5-dimethoxy-4-ethylphenethylamine (2C-E); 2,5-dimethoxy-4-iodophenethylamine (2C-I); 2,5-dimethoxy-4-ethylthiophenethylamine (2C-T-2) and 2,5-dimethoxy-4-chloroamphetamine (DOC) are used recreationally and may have deleterious side effects.
Objectives
This study compares behavioral effects and mechanisms of action of these substituted phenethylamines with those of hallucinogens and a stimulant.
Methods
The effects of these compounds on mouse locomotor activity and in rats trained to discriminate dimethyltryptamine, (−)DOM, (+)LSD, (±)MDMA and (S+)methamphetamine were assessed. Binding and functional activity of the phenethylamines at 5-HT1A, 5-HT2A, 5-HT2C receptors and monoamine transporters were assessed using cells heterologously expressing these proteins.
Results
The phenethylamines depressed mouse locomotor activity, although 2C-D and 2C-E stimulated activity at low doses. The phenethylamines except 2C-T-2 fully substituted for at least one hallucinogenic training compound but none fully substituted for (+)-methamphetamine. At 5-HT1A receptors, only 2C-T-2 and 2C-I were partial-to-full very low potency agonists. In 5-HT2A arachidonic acid release assays, the phenethylamines were partial to full agonists except 2C-I which was an antagonist. All compounds were full agonists at 5-HT2A and 5-HT2C receptor inositol phosphate assays. Only 2C-I had moderate affinity for, and very low potency at, the serotonin transporter.
Conclusions
The discriminative stimulus effects of 2C-C, 2C-D, 2C-E, 2C-I and DOC were similar to those of several hallucinogens but not methamphetamine. Additionally, the substituted phenethylamines were full agonists at 5-HT2A and 5-HT2C receptors, but for 2C-T-2, this was not sufficient to produce hallucinogenlike discriminative stimulus effects. Additionally, the 5-HT2A inositol phosphate pathway may be important in 2C-I’s psychoactive properties.
Keywords: Substituted phenethylamines; Drug discrimination; Serotonin receptor; Locomotor activity; Lysergic acid diethylamide (LSD); (-)-2,5-dimethoxy-4-methylamphetamine; Drug abuse
Introduction
Synthetic “designer” hallucinogens are psychoactive compounds derived from phenalkylamines such as mescaline and amphetamine, from tryptamines such as N,N,-dimethyltryptamine (DMT), or ergolines such as lysergic acid diethylamide (LSD) (Nichols 2004). These psychoactive drugs do not produce any clear withdrawal syndrome (Shulgin and Shulgin 1991), but psychosis in predisposed individuals following LSD ingestion has occurred (reviewed in Cohen 1967;Nichols 2004). The United States Drug Enforcement Agency has categorized some hallucinogenic compounds, including LSD, DMT and substituted phenethylamines 2,5-dimethoxy-4-chlorophenethylamine (2C-C); 2,5-dimethoxy-4-methylphenethylamine (2C-D); 2,5-dimethoxy-4-ethylphenethylamine (2C-E); and 2,5-dimethoxy-4-iodophenethylamine (2C-I); 2,5-dimethoxy-4-ethylthiophenethylamine (2C-T-2) (Fig. 1), as Schedule 1 substances, a category having abuse liability and no recognized therapeutic uses (DEA 2013). 2,5-Dimethoxy-4-chloroamphetamine (DOC) is regulated by the Federal Analog Act. The synthesis and psychoactive properties of the phenethylamines in humans have been described (Shulgin and Shulgin 1991).
Fig. 1.
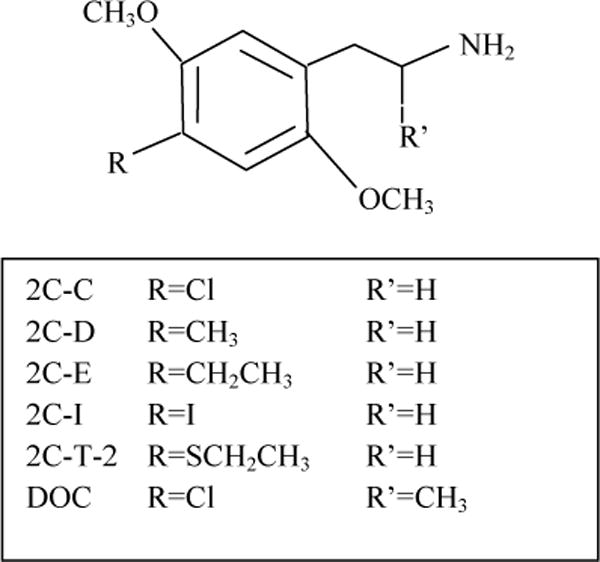
Structures of 2C-C, 2C-D, 2C-E, 2C-I, 2C-T-2 and DOC
The reported effects of these compounds are dose-dependent, with a combination of stimulant and hallucinogenic effects (reviewed in Dean et al. 2013). Generally, stimulation and increased visual, auditory and tactile sensation are seen with low doses, hallucinations with moderate doses, and unpleasant hallucinations, tachycardia, hypertension and excited delirium with higher doses (Dean et al. 2013). While there is a paucity of clinical data, some case reports of adverse side effects include stroke and quadriplegia following ingestion of 2C-I with 3,4-methylenedioxyamphetamine (Drees et al. 2009) and seizures and rhabdomyolysis following ingestion of DOC, MDMA and ethanol (Ovaska et al. 2008).
Although there is agreement that the neuronal serotonergic system is involved in the discriminative stimulus effects of hallucinogenic compounds (Glennon et al. 1984;Winter 2009), debate continues regarding which receptor subtypes are involved and whether the compounds are agonists, partial agonists or antagonists (reviewed in Halberstadt and Geyer 2011;Nichols 2004). Possible biochemical targets of these compounds have been investigated in several systems (Berg et al. 1998;Kurrasch-Orbaugh et al. 2003;Moya et al. 2007). Using antagonists with differential affinity for 5-HT2A and 5-HT2C receptors in rats trained to discriminate LSD and 2,5-dimethoxy-4-methylamphetamine (DOM) from water, Fiorella et al. (1995) found that affinities of antagonists at 5-HT2A, but not 5-HT2C, receptors correlated with IC50 values for blocking LSD and DOM behavioral effects. 5-HT2A receptor antagonists also decreased rhesus monkeys’ responding to the phenethylamines DOM and 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) (Li et al. 2010). Thus, the stimulus effects of some substituted ergolines and phenethylamines may be elicited by 5-HT2A receptor agonist activity. 2C-C, 2C-E and 2C-I partially stimulated guanosine 5′-O-[gammathio]triphosphate (GTPγS) binding in cortical membranes, an effect blocked by methiothepin, an antagonist for 5-HT1,6,&7 receptors (Nonaka et al. 2007). In rat brain synaptosomes, 2C-C, 2C-E and 2C-I inhibited serotonin and norepinephrine uptake at mid-micromolar concentrations but had no effect on dopamine uptake or neurotransmitter release via the transporters (Nagai et al. 2007).
The goal of this study was to assess behavioral effects and mechanisms of action of these substituted phenethylamines. First, drug-induced locomotor changes were characterized in mice to estimate the effective dose range and time course of behavioral effects. Next, the ability of these compounds to produce discriminative stimulus effects similar to those of a range of known drugs of abuse was tested in rats. Because phenethylamines can produce either psychostimulant or hallucinogenic effects, a number of compounds with a range of stimulant and/or hallucinogenic effects were used to screen for psychoactive effects. In addition, the ability of these compounds to bind to and activate pharmacological targets of known abused drugs was examined to confirm and extend the biochemical data available for these substituted phenethylamines. The 4-substituents on the phenyl ring can differentially influence the biochemical activity of phenethylamines (Nichols 1986b). 5-HT1A, 5-HT2A and 5-HT2C receptors are primary pharmacological targets for hallucinogens such as DMT and LSD, and the methylated phenethylamine, amphetamine, exerts its initial effects via the dopamine, serotonin and norepinephrine transporters, leading to effects at neurotransmitter (dopamine) receptors. For these reasons drug effects on these systems were characterized.
Materials and Methods
Subjects
Male Sprague-Dawley rats were obtained from Harlan-Sprague Dawley (Indianapolis, IN). All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320–350 g by limiting food to 15 g/day which included the food received during operant sessions. Water was freely available. Male Swiss–Webster mice were obtained from Harlan (Indianapolis, IN) at approximately 8 weeks of age and tested at approximately 10 weeks of age. Mice were group housed in cages on a 12:12-h light/dark cycle and were allowed free access to food and water. All housing and procedures were in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Locomotor Activity
The study was conducted using 40 Digiscan (model RXYZCM, Omnitech Electronics, Columbus, OH) locomotor activity testing chambers (40.5 × 40.5 × 30.5 cm) housed in sets of two, within sound-attenuating chambers as previously described (Gatch et al. 2011). Separate groups of 8 mice were injected intraperitoneally with either vehicle (0.9% saline), 2C-C (1, 3, 10, 30 and 100 mg/kg), 2C-D (1, 3, 10 and 30 mg/kg), 2C-E (0.03, 0.1, 0.3, 1, 3, 10 and 30 mg/kg), 2C-I (0.3, 1, 3, 10 and 30 mg/kg), 2C-T-2 (0.1, 0.3, 1, 3 and 10 mg/kg), or DOC (0.1, 0.3, 1, 3 and 10 mg/kg), immediately prior to locomotor activity testing. In all studies, horizontal activity (interruption of photocell beams) was measured for 8 h within 10-min periods, beginning at 0800 h (2 h after lights on).
Discrimination Procedures
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA) were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). The computers were programmed in MED-PC IV (Med Associates, East Fairfield, VT) for the operation of the chambers and collection of data.
Using a two-lever choice methodology, separate groups comprised of 15 to 32 rats were trained to discriminate one of five compounds from saline: METH (1 mg/kg), MDMA (1.5 mg/kg), LSD (0.1 mg/kg), DOM (0.5 mg/kg)and DMT (5 mg/kg) as previously described (Gatch et al. 2009;Gatch et al. 2011). Rats were injected i.p. with either saline or drug and then placed in the operant chambers, where food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available under a fixed-ratio 10 schedule of reinforcement. Each training session lasted a maximum of 10 min, and rats could earn up to 20 food pellets. Pretreatment times were 5 min for DMT, 10 min for METH, 15 min for LSD and MDMA, and 30 min for DOM.
The substitution test sessions lasted for a maximum of 20 min. In contrast with training sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until 20 reinforcers were obtained, or for a maximum of 20 min. 2C-C, 2C-D, 2C-E, 2C-I, 2C-T-2, or DOC were tested for substitution in subsets of 6 rats from each training drug group. Doses of these compounds were presented incrementally in separate sessions, using a repeated measures design (i.e., each of 6 rats was tested at all doses). All compounds were tested 15 min after i.p. injection, based on the earliest time a locomotor effect was observed. DOC and 2C-D were also tested 60 and 70 min, respectively, after injection in separate groups of rats to investigate whether stimulus control was different during the stimulant phase.
5-HT receptors, 5-HT1A receptor
Human embryonic kidney (HEK) cells expressing the human 5-HT1A receptor (HEK-5-HT1A) were used; the cell culture, membrane preparation, [3H]8-hydroxy-N,N-dipropyl-2-aminotetralin ([3H]8-OH-DPAT) binding assay and [35S]GTPγS binding assay were conducted as described previously (Gatch et al. 2011;Newman-Tancredi et al. 1998). 5-HT2A and 5-HT2C receptors HEK cells expressing the human 5-HT2A receptor (HEK-5-HT2A cells) or the human 5-HT2C receptor (HEK-5-HT2C cells) were used. [125I]2,5-dimethoxy-4-iodoamphetamine ([125I]DOI) binding, accumulation of inositol monophosphate using the IP-1 Elisa kit (Cisbio, Bedford, MA), and drug-induced facilitation of release of [3H]AA from HEK-5-HT2A cells were conducted as previously described (Eshleman et al. 2013;Gatch et al. 2011;Knight et al. 2004;Kurrasch-Orbaugh et al. 2003)
Human dopamine (hDAT), serotonin (hSERT) and norepinephrine (hNET) transporters, Binding, Uptake, and Release
[125I]RTI-55 binding, [3H]neurotransmitter uptake, and neurotransmitter release assays were conducted as previously described (Eshleman et al. 1999;Eshleman et al. 2013;Gatch et al. 2011). HEK cells expressing recombinant hDAT, hSERT, or hNET were used.
Dopamine D1, D2 and D3 receptors, [3H]SCH-23390 and [3H]YM-09151-2 binding
Mouse fibroblast cells expressing the human dopamine D1 receptor, and chinese hamster ovary (CHO) cells expressing the human dopamine D2 or D3 receptor were obtained from Stanford Research Institute (SRI, Menlo Park, CA). The dopamine receptor assays were conducted as described previously (Toll et al. 1998).
Drugs
For behavioral assays, all drugs were dissolved in 0.9% saline. Hydrochloride salts of 2C-C, 2C-D, 2C-E, 2C-I, 2C-T-2, DOC, (−)-cocaine, (S+)-METH, (±) and (+)-3,4-methylenedioxymethamphetamine (MDMA), and (−)DOM, N,N-DMT fumarate and (+)-LSD(+)tartrate were provided by the National Institute on Drug Abuse Drug Supply Program. [125I]DOI, [3H]8-OH-DPAT, [125I]RTI-55, [3H]dopamine, [3H]serotonin, [3H]norepinephrine, [3H]AA and [35S]GTPγS were purchased from Perkin Elmer (Boston, MA). Most other chemicals were purchased from Sigma (St. Louis, MO).
Data Analysis
Locomotor activity data were expressed as the mean number of photocell counts in the horizontal plane during each 10-min testing period. A 30-min period, beginning when maximal stimulation of locomotor activity first appeared as a function of dose, was used for analysis of dose-response data and calculation of ED50 values. from the ascending linear portion of the dose response curve. A one-way analysis of variance was conducted on horizontal activity counts for the 30-min period of maximal effect, and planned comparisons were conducted for each dose against saline control using single degree-of-freedom F tests. Drug discrimination data were expressed as the mean percentage of training-drug-appropriate responses occurring in each substitution test. Response rates were expressed as the number of responses made, divided by total session time. Percent training-drug-appropriate responding was not calculated if a rat failed to complete at least 10 responses on one of the levers, and doses for which fewer than three rats met this criterion were not considered in the discrimination data analysis. Full substitution was defined as ≥80% drug-appropriate responding and not statistically different from the training drug, and partial substitution as ≥40% and <80% drug-appropriate responding and not statistically different from the training drug. The ED50 values were calculated by fitting straight lines to the dose-response data for each compound by means of TableCurve 2D (Jandel Scientific, San Rafael, CA). Response rate data were analyzed by one-way repeated measures ANOVA. Effects of individual doses were compared to the appropriate control value using single degree-of-freedom F tests. Criterion for significance was set at p<0.05.
For binding and functional assays, IC50 or EC50 values were calculated with GraphPad Prism. Binding IC50 values were converted to Ki values using the Cheng-Prusoff correction (Cheng and Prusoff 1973), Kd values used are listed in (Eshleman et al. 2013). Fractional release was defined as the amount of radioactivity in a fraction divided by the total radioactivity remaining in the sample. For serotonin receptor functional assays, drug effect each day was normalized to maximal serotonin effect. One-way ANOVAs were conducted using the logarithms of Ki, IC50, or EC50 values followed by Tukey’s posthoc analysis. For release assays, area under the curve (AUC) was calculated using GraphPad Prism. Criterion for significance was set at p<0.05.
Results
Locomotor Activity
Figure 2 shows time course data for the test compounds. Because of the large amount of data, only doses which produced peak depressant or stimulant effects are shown, along with vehicle data for comparison. Treatment with 2C-C resulted in time- and dose-dependent depression of locomotor activity following 30 and 100 mg/kg [F(5,41)=32.94, p<.001]; these effects occurred within 10 min following injection and lasted 30 to 120 min. Convulsions were observed in 2/8 mice and tremors in 6/8 mice at 30 min following 100 mg/kg 2C-C. Lethality occurred in 1/8 mice within 120 min following 100 mg/kg. Treatment with 2C-I resulted in time- and dose-dependent depression of locomotor activity following 3–30 mg/kg [F(5,42)= 20.90, p<0.001]; depressant effects occurred within 10 min following injection and lasted 30–60 min (Fig 1). Treatment with 2C-T-2 resulted in time- and dose-dependent depression of locomotor activity following 3 and 10 mg/kg [F(5,42)=19.88, p<0.001]; depressant effects occurred within 20 min following injection and lasted 30–50 min. Time- and dose-dependent depression of locomotor activity following 3 and 10 mg/kg DOC occurred within 10 min following injection [F(5,42)=44.58, p<.001] and lasted 30–80 min.
Fig. 2.
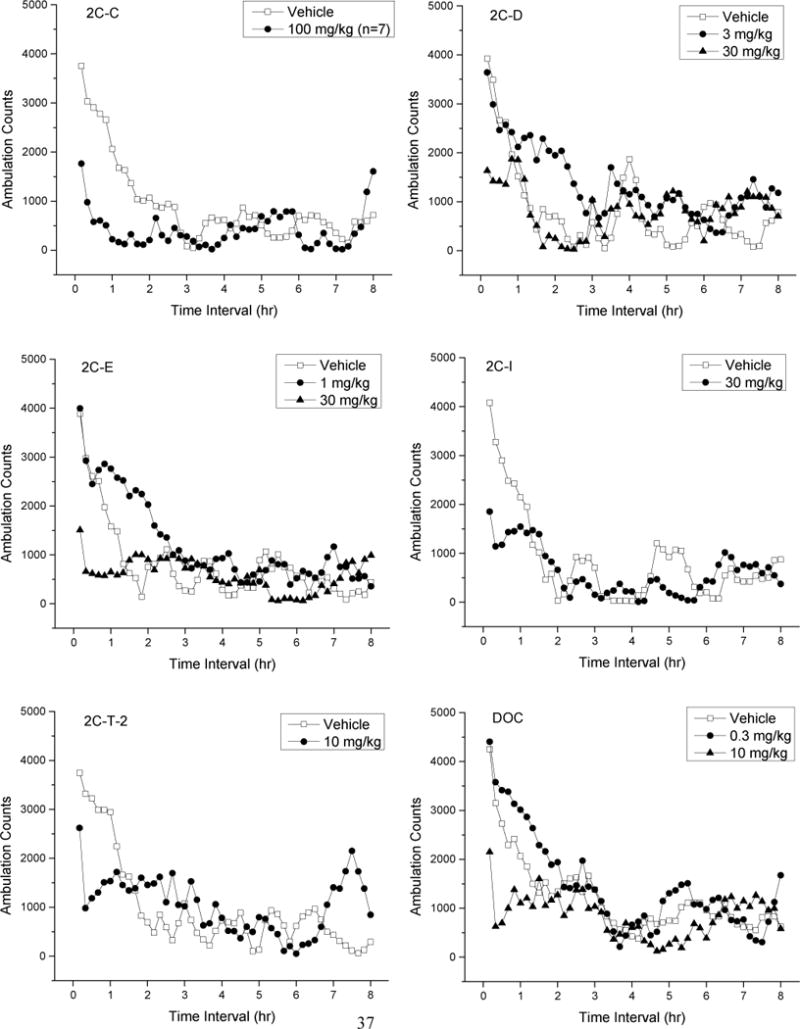
Average horizontal activity counts/10 min (ambulation counts) as a function of time (0–8 hr) and dose of test compound. Data for the vehicle and the dose which produced peak depressant effects are shown in each panel. 2C-D and DOC also produced stimulant effects, and data for the dose which produced peak stimulant effects are also shown. N=8 for each treatment.
Treatment with 2C-D resulted in both stimulation and depression of locomotor activity. Depressant effects of 10 and 30 mg/kg 2C-D [F(4,35)=11.62, p<.001] occurred within 10 min following injection and lasted 20–40 min. Stimulant effects of 3 mg/kg 2C-D [F(4,35)=1.46, p=.236] occurred within 50 min following injection and lasted 180 min. Lethality occurred in 8/8 mice within 30 min following 100 mg/kg 2C-D. 2C-E stimulated locomotor activity at low doses and depressed locomotor activity at higher doses. Depressant effects of 10 and 30 mg/kg 2C-E [F(5,42)=23.56, p<.001] occurred within 10 min following injection and lasted 50–70 min. Stimulant effects of 0.3 and 1 mg/kg 2C-E [F(7,56)=2.71, p=.017] occurred within 50 min after injection and lasted 70–100 min.
Drug Discrimination
Table 1 shows summary data for the drug discrimination studies. 2C-C fully substituted for the discriminative stimulus effects of DOM (ED50=0.95± 0.09 mg/kg) and MDMA (ED50=1.48±0.15 mg/kg). 2C-C (5 mg/kg) produced a maximum of 75% drug-appropriate responding in both DMT- and LSD-trained rats, whereas 2C-C (10 mg/kg) produced minimal drug-appropriate responding in METH-trained rats. 2C-C decreased response rates following 2.5 mg/kg in MDMA-trained rats [F(4,20)=6.18, p=.002], 10 mg/kg in DMT-trained rats [F(5,25)=6.41, p=.001] and LSD-trained rats [F(6,30)=5.51, p=.001], and 25 mg/kg in METH-trained rats [F(4,8)=5.98, p=.016]. Adverse effects were observed following 25 mg/kg 2C-C, including reddening of the extremities (3/3 rats) and salivation (1/3 rats), and this dose was not tested in DMT- or LSD-trained rats.
Table 1.
Maximum percent drug-appropriate responding (DAR) of each test compound in each of the training groups and effects on rate of responding at that dose. 1st rfr
| Test Compound | Training Drug | Dose (mg/kg) | N Test | %DAR | Rate (resp/s) |
|---|---|---|---|---|---|
| 2C-C | DMT | 5 | 4/6 | 75±25† 1 | 0.235±0.098 |
| 2C-C | DOM | 2.5 | 6/6 | 82±16† | 0.366±0.070 |
| 2C-C | LSD | 5 | 4/6 | 75±25† 2 | 0.200±0.079 |
| 2C-C | MDMA | 2.5 | 5/6 | 89±11† | 0.244±0.086 |
| 2C-C | Meth | 10 | 3/6 | 33±332 | 0.156±0.096 |
|
| |||||
| 2C-D 15 min | DMT | 10 | 3/6 | 100±0† | 0.154±0.070 |
| 2C-D | DOM | 2.5 | 6/6 | 100±0† | 0.383±0.038 |
| 2C-D | LSD | 2.5 | 6/6 | 83±17† | 0.385±0.060 |
| 2C-D | MDMA | 2.5 | 6/6 | 61±20† 2 | 0.402±0.073 |
| 2C-D | Meth | 5 | 4/6 | 15±152 | 0.190±0.086 |
|
| |||||
| 2C-D 70 min | DMT | 10 | 6/6 | 83±17† | 0.332±0.067 |
| 2C-D | DOM | 10 | 6/6 | 76±17† | 0.338±0.187 |
| 2C-D | LSD | 5 | 6/6 | 83±17† | 0.379±0.054 |
| 2C-D | MDMA | 10 | 6/6 | 50±222 | 0.547±0.127 |
| 2C-D | Meth | 25 | 5/6 | 44±222 | 0.072±0.032 |
|
| |||||
| 2C-E | DMT | 5 | 6/6 | 80±16† | 0.466±0.166 |
| 2C-E | DOM | 2.5 | 6/6 | 97±2† | 0.576±0.087 |
| 2C-E | LSD | 2.5 | 6/6 | 97±3† | 0.413±0.061 |
| 2C-E | MDMA | 10 | 3/6 | 100±0† | 0.053±0.036 |
| 2C-E | Meth | 5 | 5/6 | 23±161 | 0.228±0.024 |
|
| |||||
| 2C-I | DMT | 2.5 | 6/6 | 93±4† | 0.480±0.092 |
| 2C-I | DOM | NT | |||
| 2C-I | LSD | 5 | 5/6 | 80±20† | 0.233±0.075 |
| 2C-I | MDMA | 2.5 | 4/6 | 65±21† 1 | 0.453±0.169 |
| 2C-I | Meth | 1 | 4/6 | 38±171 | 0.447±0.058 |
|
| |||||
| 2C-T-2 | DMT | 2.5 | 4/6 | 73±24† 1 | 0.170±0.106 |
| 2C-T-2 | DOM | NT | |||
| 2C-T-2 | LSD | 2.5 | 3/6 | 33±332 | 0.208±0.117 |
| 2C-T-2 | MDMA | 1 | 6/6 | 12±72 | 0.448±0.130 |
| 2C-T-2 | Meth | 0.5 | 6/6 | 7±52 | 0.748±0.109 |
|
| |||||
| DOC 15 min | DMT | 1 | 5/6 | 65±202 | 0.354±0.125 |
| DOC | DOM | 0.5 | 6/6 | 83±17† | 0.708±0.072 |
| DOC | LSD | 1 | 6/6 | 81±16† | 0.402±0.049 |
| DOC | MDMA | 0.5 | 6/6 | 44±191 | 0.449±0.104 |
| DOC | Meth | 0.5 | 6/6 | 24±161 | 0.876±0.330 |
|
| |||||
| DOC 60 min | DMT | 1 | 6/6 | 83±17† | 0.777±0.095 |
| DOC | DOM | 1 | 6/6 | 87±13† | 0.493±0.058 |
| DOC | LSD | 1 | 5/6 | 95±3† | 0.410±0.139 |
| DOC | MDMA | 1 | 5/6 | 60±24† 2 | 0.356±0.141 |
| DOC | Meth | 2.5 | 4/6 | 0±02 | 0.276±0.098 |
N Test = number of rats which completed the first fixed ratio/total number tested.
resp/s = responses per second.
Higher dose(s) produced less drug-appropriate responding.
A higher dose suppressed response rate.
Indicates significantly different from vehicle control/not different from drug control
2C-D was tested at two time points, 15 and 70 min, which corresponded with peak depressant and peak stimulant locomotor activity effects, respectively. At 15 min after administration, 2C-D fully substituted for the discriminative stimulus effects of DMT (ED50=3.14±0.15 mg/kg), DOM (ED50=0.77±0.10 mg/kg) and LSD (ED50=0.71±0.12 mg/kg). 2C-D (2.5 mg/kg) produced 61% drug-appropriate responding in MDMA-trained rats, and 10 mg/kg produced 15% METH-appropriate responding (Table 1). Response rate was decreased following 1 and 2.5 mg/kg in DOM-trained rats [F(4,20)=6.23, p=.002], 5 mg/kg in MDMA-trained rats [F(5,25)=9.04, p<.001], 10 mg/kg in DMT-trained rats [F(4,20)=5.13, p=.005], and 5 and 10 mg/kg in METH-trained rats [F(4,20)=6.86, p=.001]. In METH-trained rats, 2/6 rats exhibited reddening of the extremities following 10 mg/kg 2C-D, and 4/6 rats failed to complete the first fixed ratio. At 70 min after administration, 2C-D fully substituted for the discriminative stimulus effects of DMT (ED50=2.99±0.13 mg/kg) and LSD (ED50=3.04±0.13 mg/kg). 2C-D (10 mg/kg) produced partial substitution of drug-appropriate responding in DOM-trained rats and MDMA-trained rats, and 25 mg/kg 2C-D partially substituted in (+)-METH-trained rats. Response rate was decreased following 10 mg/kg in DOM-trained rats [F(4,20)=4.34, p=.011], 25 mg/kg in MDMA-trained rats [F(5,25)=4.18, p=.007], and 25 and 50 mg/kg in (+)-METH-trained rats [F(6,12)=5.49, p=.006]. Two of three rats receiving 50 mg/kg 2C-D exhibited salivation and failed to complete the first fixed ratio.
2C-E fully substituted for the discriminative stimulus effects of DMT (ED50= 0.95±0.20 mg/kg), DOM (ED50= 0.84±0.08 mg/kg), LSD (ED50= 0.62±0.10 mg/kg), and MDMA (ED50= 2.48±0.10 mg/kg), but produced minimal METH-appropriate responding. Response rate was decreased following 2.5, 5, and 10 mg/kg in MDMA-trained rats [F(9,45)=10.25, p<.001] and 5 and 25 mg/kg in METH-trained rats [F(10,50)=4.01, p<.001]. Loss of muscle tone was observed at 10 mg/kg in MDMA-trained rats, and 25 mg/kg 2C-E completely suppressed responding.
2C-I fully substituted for the discriminative stimulus effects of DMT (ED50=0.68±0.11 mg/kg) and LSD (ED50= 1.66 mg/kg±0.12). In MDMA-trained rats, 2.5–10 mg/kg 2C-I produced a maximal 65% drug-appropriate responding, and 1 mg/kg 2C-I produced only 38% drug-appropriate responding in METH-trained rats (Table 1). Response rate was decreased with doses of 2.5–10 mg/kg 2C-I in MDMA-trained rats [F(4,20)=3.66, p=.022] and with 1 and 5 mg/kg in METH-trained [F(4,20)=5.08, p=.005].
2C-T-2 produced 73% drug-appropriate responding following 2.5 mg/kg in DMT-trained rats. A 10 mg/kg dose of 2C-T-2 elicited hind limb paralysis, salivation and loss of muscle tone and was not tested further. 2C-T-2 failed to substitute for LSD, MDMA, or (+)-METH. 2C-T-2 substantially decreased response rates following 2.5 and 5 mg/kg in rats trained to DMT [F(5,25)=6.66, p<.001], LSD [F(4,20)=4.60, p=.008], MDMA [F(3,15) =3.46, p=.043], and (+)-METH [F(3,15)=14.75, p<.001]. In each case, 5/6 rats failed to complete the first fixed ratio at the highest dose tested. Decreased muscle tone was observed in 3/6 rats following 5 mg/kg 2C-T-2 in LSD-trained rats.
DOC was tested at two time points, 15 and 60 min, which corresponded with the peak depressant and peak stimulant locomotor activity effects, respectively. At 15 min following administration, DOC fully substituted for the discriminative stimulus effects of DOM (ED50=0.13±0.16 mg/kg) and LSD (ED50=0.39±0.33 mg/kg). DOC produced 65% DMT-appropriate responding following 1 mg/kg, and less than 50% drug-appropriate responding in MDMA-trained and METH-trained rats. Response rate was decreased following 2.5 mg/kg DOC in rats trained to LSD [F(7,35)=3.86, p=.003], MDMA [F(5,25)=3.81, p=.011], and METH [F(5,25)=3.86, p=.010]. With 2.5 mg/kg DOC, substantial rate suppression was observed, such that 4/6 or 5/6 rats tested in each case failed to respond and decreased muscle tone was observed in 12/24 rats. At 60 min, DOC fully substituted for the discriminative stimulus effects of DMT (ED50=0.61±0.19 mg/kg), DOM (ED50=0.26±0.19 mg/kg), and LSD (ED50= 0.23±0.10 mg/kg). In MDMA-trained rats, DOC produced 60% drug-appropriate responding following 1 mg/kg, and no drug-appropriate responding at any dose in METH-trained rats. Doses of 1 mg/kg and higher decreased response rates in rats trained to DMT [F(5,25)=3.66, p=.013], LSD [F(5,25)=2.96, p=.031], MDMA [F(6,30)=3.96, p=.005], and METH [F(6,30)=4.07, p=.004]. Substantial rate suppression and failure to complete the first fixed ratio was observed following 2.5 mg/kg DOC in MDMA-trained rats (4/6 rats) and 5 mg/kg in METH-trained rats (5/6 rats).
In Vitro Pharmacology, Interaction with Serotonin Receptors
In HEK-h5-HT1A cells, the phenethylamines were tested for their affinities for the [3H]8-OH-DPAT binding site and their effect on 5-HT1A function (Table 2). The agonist [3H]8-OH-DPAT binds to the 5-HT1A high affinity state and agonist activation of the receptor results in increased binding of [35S]GTPγS to the Gαi/o subunit of G proteins and reflects receptor function. All six compounds had lower affinities (high nanomolar to low micromolar) for the [3H]8-OH-DPAT binding site than serotonin and LSD (ps<0.001, one way ANOVA followed by Tukey’s multiple comparison test). 2C-C, 2C-I, and 2C-T-2 had higher affinities than DOM, DOC, MDMA and METH (ps<0.05); 2C-E had higher affinity than MDMA and METH (ps<0.05); and DOC had lower affinity than DMT (p<0.001). In the [35S]GTPγS functional assay, serotonin and LSD had very high potency, higher than all other compounds (ps<0.001). 2C-I and 2C-T-2 had similar low micromolar potencies and 2C-T-2 was more potent than MDMA and METH (ps<0.001). The four other phenethylamines had minimal efficacy (<25%). 2C-I and 2C-T-2 had similar efficacies to serotonin and LSD and 2C-I had higher efficacy than DOM, MDMA and METH (p<0.05).
Table 2.
Pharmacology of 2C-C, 2C-D, 2C-E, 2C-I, 2C-T-2 and DOC at 5-HT1A, 5-HT2A and 5-HT2C receptors: Effects on binding and function.
| 5-HT1A | 5-HT2A | 5-HT2C | |||||
|---|---|---|---|---|---|---|---|
| Drug | [3H]8-OH-DPAT binding Ki (nM) ± sem | Function [35S]GTPγS Binding EC50 (nM) EC50 (nM) ± sem % maximal effecta | [125I]DOI binding Ki (nM) ± sem | Function [3H]AA release EC50 (nM) ± sem % maximal effecta | Function IP-1 formation EC50 (nM) ± sem % maximal effecta | [125I]DOI binding Ki (nM) ± sem | Function IP-formation EC50 (nM) ± sem % maximal effecta |
| 2C-C | 740 ± 170 | >10 μM* | 5.47 ± 0.68 | 13.9 ± 6.1 | 40.1 ± 4.7 | 5.4 ± 1.2 | 24.2 ± 5.6 |
| <25% | 64.2 ± 2.2% | 102.1 ± 5.5% | 93.5 ± 6.5% | ||||
| 2C-D | 1630 ± 150 | >10 μM* | 23.9 ± 3.9 | 153 ± 28 | 145 ± 39 | 12.7 ± 1.3 | 71.1 ± 8.0 |
| <25% | 81.1 ± 9.7% | 93.0 ± 6.7% | 100 ± 14% | ||||
| 2C-E | 1190 ± 250 | >10 μM* | 4.50 ± 0.71 | 7.1 ± 3.3 | 84 ± 16 | 5.4 ± 1.1 | 18.0 ± 5.9 |
| <20% | 65 ± 11% | 125 ± 13% | 98 ± 16% | ||||
| 2C-I | 970 ± 430 | 4900 ± 2000 | 9.3 ± 3.2 | ND | 7.9 ± 2.2 | 10.2 ± 1.7 | 2.8 ± 1.0 |
| 102.1 ± 4.7% | 32.5 ± 1.5% | 82.4 ± 6.4% | 79 ± 16 | ||||
| 2C-T-2 | 1740 ± 660 | 3000 ± 1300 | 12.5 ± 4.4 | 2.30 ± 0.69 | 7.9 ± 2.0 | 14.2 ± 3.6 | 3.8 ± 1.1 |
| 76 ± 10% | 106.6 ± 6.3% | 87.4 ± 6.5% | 93.0 ± 1.8% | ||||
| DOC | >9200 | >10 μM* | 4.00 ± 0.45 | 2.91 ± 0.66 | 10.5 ± 2.6 | 3.57 ± 0.56 | 14.6 ± 3.0 |
| <10% | 81 ± 16% | 102.4 ± 4.2% | 97 ± 19% | ||||
| Serotonin | 4.3 ± 1.0 | 15.7 ± 5.4 | 25.8 ± 4.6 | 7.8 ± 2.0 | 43 ± 10 | 3.74 ± 0.71 | 1.94 ± 0.49 |
| 105.3 ± 2.2% | 100% | 99.7 ± 0.9% | 93.9 ± 2.0% | ||||
| LSD | 2.50 ± 0.95 | 6.4 ± 1.8 | 0.47 ± 0.12 | 1.01 ± 0.31 | 0.264 ± 0.052 | 3.22 ± 0.57 | 1.14 ± 0.36 |
| 109.6 ± 4.5% | 87.8 ± 3.4% | 80.3 ± 4.9% | 74.6 ± 9.1% | ||||
| DOM | 11900 ± 5600 | 13900 ± 1500 | 8.1 ± 2.2 | 31.2 ± 9.6 | 25.9 ± 4.2 | 8.4 ± 3.0 | |
| 53.8 ± 9.7% | 106 ± 11% | 94 ± 14% | |||||
| DMT | 400 ± 190 | 1490 ± 570 | 201 ± 48 | 260 ± 120 | 269 ± 61 | 111 ± 34 | 114 ± 35 |
| 100.4 ± 5.2% | 105.1 ± 3.0% | 39.0 ± 4.6% | 99 ± 11% | ||||
| MDMA | 11900 ± 1200 | 36000 ± 16000 | 20900 ± 4600 | 5.5 ± 2.3 mM | 9600 ± 2400 | 9100 ± 1500 | |
| 64.3 ± 9.4% | 40.2 ± 7.1% | 92.2 ± 8.0% | |||||
| METH | 9700 ± 1800 | 23600 ± 8100 | 41900 ± 9600 | >1 mMa | 10100 ± 3200 | 74000 ± 16000 | |
| 68 ± 11% | <3% | 52 ± 10% | |||||
For test compounds, n=3–11 for each assay. Data are expressed as mean ± S.E.M. Hill slopes for 5-HT1A [3H]8-OH-DPAT binding ranged from −0.73 to −1.04, for 5-HT2A [125I]DOI binding ranged from −0.53 to −1.10 and for 5-HT2C [125I]DOI binding ranged from −0.65 to −1.32.
ND- the EC50 value was not determined. 2C-I was an antagonist at the 5-HT2A [3H]AA release functional assay, with an IC50 of 2060 ± 860 nM and 84 ± 11% inhibition, compared to the maximal inhibition by ketanserin, of 100 nM serotonin stimulation.
These compounds also had minimal effect as antagonists at the 5-HT1A receptor, with maximal inhibition <30% of the maximal inhibition by WAY 100,635.
Maximal effect of each drug was normalized to the maximal effect of serotonin.
In HEK-h5-HT2A cells, the phenethylamines were tested for their affinity for the [125I]DOI binding site and effects on 5-HT2A signaling pathways. Agonist binding to 5-HT2A receptors can activate both phospholipase A2 increasing AA release from plasma membranes and phospholipase C increasing the inositol phosphate cascade (IP-1 assay). Binding affinities of the drugs were significantly different (p<0.0001, one way ANOVA, Table 2). LSD had higher affinity than all other tested compounds (ps<0.001). The phenethylamines had high affinities, and their Ki values did not differ from each other or from serotonin or DOM (p>0.05), and were higher than DMT, MDMA and METH (ps<0.001).
In the 5-HT2A [3H]AA release assay, 2C-C, 2C-D, 2C-E, 2C-T-2, DOC, LSD and 5-HT were agonists with similar efficacies (p=0.26, one way ANOVA, Table 2) while potencies differed significantly (p<0.0001, one way ANOVA). LSD and serotonin had potencies in the low nanomolar range, with LSD having higher potency (p<0.01). 2C-T-2, 2C-E, and DOC were very potent with EC50 values that did not differ from serotonin and LSD. 2C-T-2, 2C-C, 2C-E, DOC, LSD and serotonin were more potent than 2C-D and DMT (ps<0.05 to 0.001). In contrast, 2C-I minimally stimulated release (Fig 3A) and no EC50 was determined. This finding was unexpected, since 2C-I substituted for the discriminative effects of LSD and DMT, which stimulated [3H]AA release (Table 2 and Fig 3A). 2C-I fully inhibited serotonin-stimulated [3H]AA release with low potency, similar to ketanserin (p>0.05, two-tailed t-test, Fig 3B). To confirm that the same compound was used in behavioral and biochemical assays, an aliquot of 2C-I from the behavioral assays was tested and confirmed 2C-I antagonism of serotonin-mediated [3H]AA release.
Fig. 3.
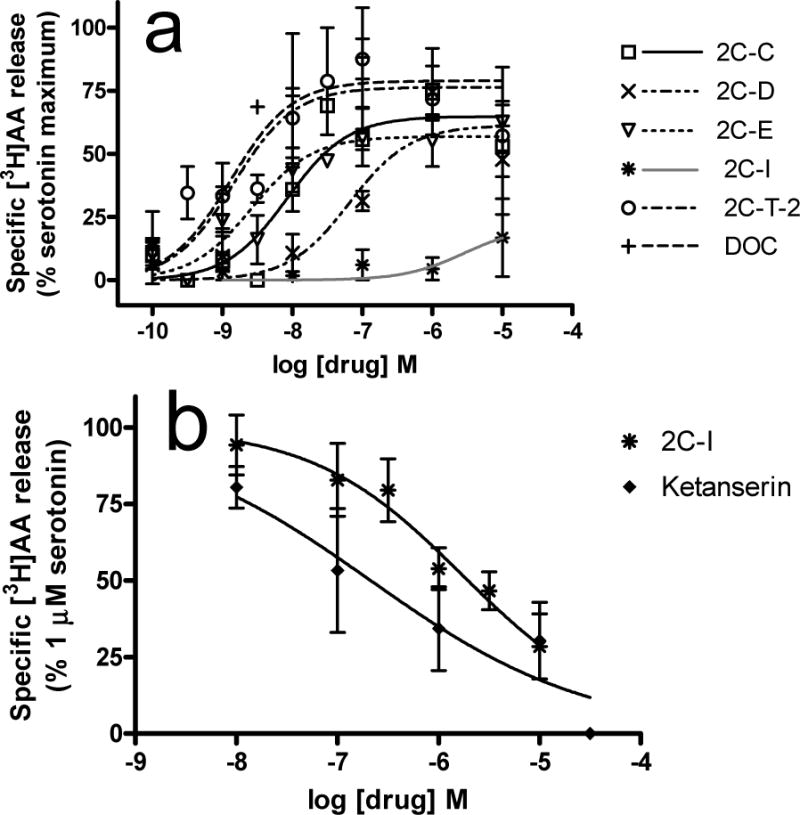
[3H]AA release from HEK-5-HT2A cells. Experiments were conducted as described in methods. Data presented are means ± sem. (A) Agonist assay. Basal activity is subtracted, and data are normalized to the maximal stimulation by serotonin on each experimental day. n=3–5 except n=2 for 2C-I. (B) Antagonist assay. Nonspecific release, measured in the presence of 30 μM ketanserin, is subtracted from all data and data are normalized to the maximal release stimulated by serotonin. n=3–4.
In the 5-HT2A IP-1 functional assay, all compounds tested were agonists (except METH with no measurable efficacy), with significantly different potencies (p<0.001, one way ANOVA, Table 2, Fig 4A). LSD had higher potency (ps<0.001) while MDMA had lower potency (ps<0.001) than all other compounds. 2C-I and 2-C-T-2 were the most potent phenethylamines. 2C-I had higher potency than serotonin, 2C-D, and 2C-E (ps<0.05 to 0.001). 2C-T-2 had higher potency than serotonin, DOM, 2C-D and 2C-E (ps<0.05 to 0.001). 2C-C, 2C-D, 2C-E, DOC, DOM and serotonin had similar potencies. Efficacies differed significantly (p <0.0001, one way ANOVA). 2C-E had higher efficacy than LSD, 2C-I and 2C-T-2 (ps<0.05). 2C-C, 2C-D, 2C-I, 2C-T-2, DOC, serotonin, LSD and DOM had similar efficacies. DMT and MDMA had similar efficacy that was lower than all other compounds (ps<0.01).
Fig. 4.
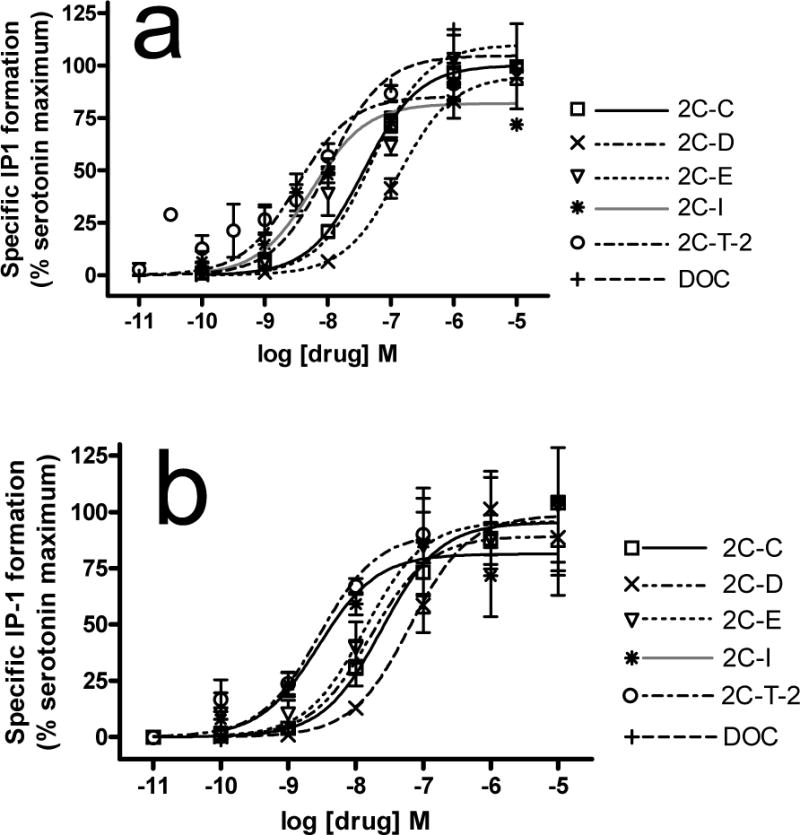
Stimulation of IP-1 formation in HEK-5-HT2A and HEK-5-HT2C cells. Experiments were conducted as described in methods. A. HEK-5-HT2A cells. All compounds are full or partial agonists. The average maximal stimulation by serotonin was 565 ± 46 nM IP1. n=3–8. B. HEK-5-HT2C cells. All compounds are full agonists. The average maximal stimulation by serotonin was 1390 ± 180 nM. n=4–7.
In HEK-h5-HT2C cells, the phenethylamines were tested for their affinities for the [125I]DOI binding site and effects on 5-HT2C-mediated IP-1 turnover. In the [125I]DOI binding assay, there were significant differences in affinities (p<0.0001, one way ANOVA). 2C-C, 2C-D, 2C-E, 2C-I, 2C-T-2, DOC, serotonin and LSD had similar, low nanomolar, affinities (Table 2). DOM had lower affinity than serotonin and LSD (p <0.01) but was similar to the phenethylamines. DMT had similar affinity to DOM. MDMA and METH had similar affinities which were lower than all other compounds (p<0.001).
All compounds were 5-HT2C agonists, activating the phospholipase C-inositol phosphate cascade, with significant differences in potencies (p<0.001, one way ANOVA, Table 2, Fig 4B). The highest potency drugs were LSD, serotonin, 2C-I, 2C-T-2 and DOM. 2C-I was more potent than 2C-D, DMT, MDMA and METH (ps<0.01). 2C-T-2 was more potent than DMT, MDMA and METH (ps<0.001). 2C-C, 2C-D, 2C-E and DOC had similar low-to-mid nanomolar potencies and were less potent than serotonin (ps<0.05) and LSD (except DOC). DMT, MDMA, and METH had very low potencies. Efficacies did not differ (p =0.28, one way ANOVA).
In Vitro Pharmacology, Interaction with hDAT, hSERT and hNET and dopamine receptors
In the transporter assays, the phenethylamines had no measurable or very low affinity for hDAT and hNET in the binding assays, and very low potency (at least micromolar) in the [3H]dopamine and [3H]norepinephrine uptake assays (Table 3). Only 2C-I had measurable affinity (high nanomolar) for hSERT and very low potency in the [3H]serotonin uptake assay. In release assays, the compounds had no releasing efficacy, while METH elicited robust release in all cell lines. The phenethylamines also had no measurable affinity for the dopamine D1, D2 and D3 receptors (data not shown). Thus, the substituted phenethylamines have minimal interaction with hDAT and hNET and dopamine receptors and very low potency at hSERT.
Table 3.
Pharmacology of 2C-C, 2C-D, 2C-E, 2C-I, 2C-T-2 and DOC at DAT, SERT and NET: Effects on binding and function.
| HEK-hDAT cells | HEK-hSERT cells | HEK-hNET cells | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug | [125I]RTI-55 binding Ki, nM | [3H]Dopamine uptake IC50, nM | [3H]Dopamine release EC50, nM % METH maximal effect | [125I]RTI-55 binding Ki, nM | [3H]Serotonin uptake IC50, nM | [3H]Serotonin release EC50, nM % PCA maximal effect | [125I]RTI-55 binding Ki, nM | [3H]NE uptake IC50, nM | [3H]NE release EC50, nM % METH maximal effect |
| 2C-C | >10 μM | >10 μM | ND Minimal |
>10 μM | >9600# | ND Minimal |
>7600# | >9500 | ND Minimal |
| 2C-D | >10 μM | >10 μM | ND Minimal |
>10 μM | >8400# | ND Minimal |
>8500# | >9200 | ND Minimal |
| 2C-E | >10 μM | >10 μM | ND Minimal |
>10 μM | >7500# | ND Minimal |
>7600# | >9800 | ND Minimal |
| 2C-I | >10 μM | >9600# | ND Minimal |
950 ± 440 | 5600 ± 2100 | ND Minimal |
>10 μM | >8200# | ND Minimal |
| 2C-T-2 | >10 μM | >10 μM | ND Minimal |
>9400# | >8600# | ND Minimal |
>10 μM | >7200# | ND Minimal |
| DOC | >10 μM | >10 μM | ND Minimal |
>10 μM | >8600# | ND Minimal |
>7700# | >8400# | ND Minimal |
| Cocaine | 462 ± 97 | 374 ± 36 | ND Minimal |
321 ± 26 | 383 ± 49 | ND Minimal |
1280 ± 150 | 332 ± 29 | ND Minimal |
| METH | 4750 ± 530 | 56 ± 15 | 970 ± 250a 100% |
188000 ± 14000 | 4100 ± 1300 | 27800 ± 7700a | 1480 ± 130 | 23.0 ± 5.5 | 67 ± 18 a 100% |
| LSD | >10 μM | >10 μM | >10 μM | >10 μM | 5600 ± 260 | >10 μM | |||
Abbreviations: NE, norepinephrine, PCA. p–chloroamphetamine. Hill slopes for [125I]RTI-55 binding ranged from −0.97 to −1.05 for hDAT, −0.70 to −1.15 for hSERT and −0.99 to −2.13 for hNET.
For test compounds, n=2 (when >10 μM) to 7. Data are expressed as mean ± S.E.M. The standard compounds were, for purposes of comparison, cocaine for the binding and uptake assays, methamphetamine for [3H]dopamine and [3H]norepinephrine release and PCA for [3H]serotonin release. The EC50 value for PCA for stimulating [3H]serotonin release was 1350 ± 420 nM.
If some experiments yielded IC50 or Ki values less than 10 μM and other experiments yielded IC50 or Ki values greater than 10 μM, the latter experiments were assigned a value of 10 μM and averages calculated (n≥3). The actual value is greater than that average and no standard error is reported.
ND- The EC50 value could not be determined.
Values from Gatch et al., 2011.
Discussion
Discriminative stimulus effects of six substituted phenethylamines were tested in separate groups of rats trained to discriminate DMT, DOM, LSD, MDMA, or METH from saline. Previously, we reported that these training compounds produced non-identical patterns of cross substitution: LSD fully substituted for all training compounds except METH; MDMA fully substituted for all compounds except partial substitution for LSD; DOM substituted fully for DMT and LSD, partially for MDMA but not for METH; DMT substituted fully for DOM, partially for LSD and MDMA but not for METH; while METH only substituted for MDMA (Gatch et al. 2009).
In the current study, five of the six phenethylamines produced full substitution to at least one of the three prototypic hallucinogens, LSD (ergoline), DMT (tryptamine) and DOM (phenalkylamine). In LSD-trained rats, 2C-D, 2C-E, 2C-I and DOC fully substituted (≥ 80% DAR), whereas 2C-C produced 75% LSD-appropriate responding. The pattern of substitution for DMT-trained rats was similar, with the addition of partial substitution by 2C-T-2. The four compounds tested in DOM-trained rats (2C-C, 2C-D, 2C-E, and DOC) all fully substituted, suggesting that these phenethylamines have very similar discriminative stimuli to DOM, consistent with their structural similarities. In contrast, 2C-T-2 partially substituted only for DMT, indicating that it does not share discriminative stimulus effects with most serotonergic hallucinogens or with psychostimulants.
For the MDMA-trained rats, 2C-C and 2C-E fully substituted, 2C-D and 2C-I and DOC produced 60 to 65% MDMA-appropriate responding, but DOC produced only 44%. These findings are similar to those of earlier studies in which MDMA, an entactogen that induces feelings of empathy and emotional closeness to others (Nichols 1986a), did not consistently cross-substitute for the classic serotonergic hallucinogens (Baker et al. 1995;Gatch et al. 2009;Oberlender and Nichols 1988;Schechter 1998).
In agreement with very low potencies of 2C-C, 2C-E, and 2C-I for inhibition of uptake and minimal stimulation of release in rat brain synaptosomes (Nagai et al. 2007), the phenethylamines minimally substituted for METH, consistent with their low to negligible affinity and potency at the transporters. This suggests that the compounds have minimal psychostimulant properties and agrees with the general depression of locomotor activity by other hallucinogens (Krebs and Geyer 1994;Krebs-Thomson et al. 1998), although lower doses of 2C-D, 2C-E, and DOC had a delayed stimulant activity.
Many studies have demonstrated the involvement of G-protein-coupled 5-HT2 receptors in the actions of hallucinogenic drugs including LSD, DOM, DOI, and 2,5-dimethoxy-4-bromophenethylamine (2C-B) (Egan et al. 2000;Fantegrossi et al. 2008;Glennon et al. 1984;Kurrasch-Orbaugh et al. 2003;Titeler et al. 1988). More specifically, 5-HT2A/2C receptors, which have high sequence homology and similarity in second messenger signaling, are important targets of hallucinogenic compounds (reviewed in Fantegrossi et al. 2008;Halberstadt and Geyer 2011;Nichols 2004;Winter 2009). Both 5-HT2A/2C receptors activate phospholipase A2 via Gα12/13, liberating AA from membrane phospholipids (reviewed in Raymond et al. 2001) and activate phospholipase C via Gq/11, generating the second messengers inositol triphosphate and diacylglycerol with subsequent release of intracellular stores of calcium and protein kinase C activation (Backstrom et al. 1999). Among the training compounds, LSD and DOM were full agonists at the 5-HT2A/2C receptors although in rat brain membranes and NIH3T3-5HT2A cells, these compounds are partial agonists (Kurrasch-Orbaugh et al. 2003;Sanders-Bush et al. 1988). In contrast, at the 5-HT2A receptor, DMT and MDMA were very low potency partial agonists, and METH had no efficacy, whereas at the 5-HT2C receptor, DMT and MDMA were low to very low potency full agonists and METH was a very low potency partial agonist. Activation of 5-HT1A receptors may not play a role in the discriminative stimulus (Nichols 2004); while LSD was a very potent full 5-HT1A agonist, all other psychoactive exogenous compounds tested had potencies in the micromolar range, and only 2C-I and 2C-T-2 had partial to full efficacy among the phenethylamines. These results partly agree with the partial stimulation of GTPγS binding in brain preparations by 2C-C, 2C-E and 2C-I (Nonaka et al. 2007)
In CHO cells expressing the 5-HT2A or 5-HT2C receptors, 2C-D, 2C-I and 2C-B were partial, low potency agonists for the phospholipase C or A2 pathways. (Moya et al. 2007). Herein, 2C-C and DOC had similar affinities for 5-HT2A/2C receptors, and each was a full agonist with similar potencies at both receptors, similar to LSD and DOM. 2C-D had higher affinity for, and was a full agonist with slightly higher potency at, the 5-HT2C compared to the 5-HT2A receptor. 2C-E had similar affinity for the 5-HT2A/2C receptors and was a partial agonist in the 5-HT2A AA assay, but a full agonist in stimulating the inositol phosphate pathway by both 5-HT2A/2C receptors. Although 2C-T-2 substituted partially only for DMT, it was a full high potency agonist at the 5-HT2A/2C receptors, similar to LSD. A structurally similar compound, 2,5-dimethoxy-4-propylthiophenethylamine (2C-T-7), partially substitutes for LSD and serves as a discriminative stimulus, an effect blocked by a 5-HT2A antagonist (Fantegrossi et al. 2005). 2C-T-2 has been sold on the internet and in Europe (de Boer and Bosman 2004) and anecdotal evidence (Erowid.com) suggests that it is psychoactive.
In contrast, 2C-I had differential effect on 5-HT2A-mediated pathways. 2C-I had high affinity for both 5-HT2A/2C receptors, consistent with Ki and Kd values obtained using stable HEK-5HT2A cells (Parrish et al. 2005) and [125I]2C-I in rat frontal cortex, which did not discriminate between 5-HT2A and 5-HT2C receptors (Johnson et al. 1990). 2C-I fully antagonized serotonin’s activation of the phospholipase A2/[3H]AA release pathway. However, 2C-I was a full agonist, with efficacy similar to LSD, in 5-HT2A/2C receptor-mediated IP-1 formation. Because 2C-I fully substituted for DMT and LSD and stimulated phospholipase C, but not phospholipase A2, pathway, the former pathway may be more significant in 2C-I drug discrimination. Similarly, Parrish et al. (2005) observed 2C-I to be a partial agonist with high potency at the phospholipase C pathway. Consistent with the possible importance of the phospholipase C pathway, a conformationally restricted 2C-B analog ((R)-4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)methyl-amine) was equipotent to LSD in rats trained to discriminate LSD from saline and was 65-fold more potent at stimulating inositol phosphate turnover than AA release, although the authors suggested that preferential stimulation of this pathway may indicate lower psychoactivity (McLean et al. 2006). Using a phospholipase C inhibitor, Schindler et al. (2013) found that phospholipase C activation is necessary for DOI-elicited head bobs in mice while LSD-elicited head bobs are independent of this pathway, suggesting distinct pathways for these structurally dissimilar hallucinogens. A caveat to the possible relative contribution of 5-HT2A pathways to hallucinogenic activity is the abundant evidence that the cell types used to express serotonin receptors, as well as the second messenger systems characterized, have impact on whether a drug is an agonist, partial agonist, or antagonist (Acuna-Castillo et al. 2002;Kurrasch-Orbaugh et al. 2003;Moya et al. 2007;Rabin et al. 2002;Villalobos et al. 2004).
To summarize, the relationship between drug substitution, mechanism of action, and potential for abuse for these drugs is not uniform. 2C-C, 2C-D, 2C-E and DOC fully substituted for the discriminative stimuli of the hallucinogenic phenethylamine DOM and fully to partially substituted for LSD, and have agonist profiles similar to DOM and LSD at 5-HT2A/2C receptors. In contrast, 2C-I substituted fully for hallucinogenic compounds, but its pharmacology is more complex, as it fully stimulated the 5-HT2A/2C receptors phospholipase C pathways but inhibited the 5-HT2A receptor phospholipase A2 pathway, as discussed above. Finally, 2C-T-2 was a full, high potency agonist at 5-HT2A/2C receptors but produced appreciable drug-appropriate responding for only one training drug (DMT). Overall, there was not a good correlation between potencies and efficacies in drug discrimination assays and binding/functional assays, suggesting that complex mechanisms or additional receptor systems may modulate behavior.
These behavioral and biochemical findings, together with ongoing reports of use on the internet (Erowid.com), suggest that the compounds have high abuse liability. In addition, the adverse effects we observed in rat and mouse behavioral assays included tremor, muscle spasms, hind limb paralysis and lethality and indicate toxicity at higher doses. Recent reports of seizures and serotonin syndrome following 2C-I ingestion (Bosak et al. 2013), fatal toxic leukoencephalopathy and acute kidney failure following 2C-E ingestions (Sacks et al. 2012;Van Vrancken et al. 2013), and seizures and rhabdomyloysis following DOC and MDMA co-ingestion (Ovaska et al. 2008) suggest consumption of these phenethylamines carries significant health risk.
Acknowledgments
Support: Funding for this study was provided by National Institutes of Health National Institute on Drug Abuse/Veterans Affairs Interagency agreements [Y1 DA 5007-05, Y1-DA-0101-01], The Methamphetamine Abuse Research Center [P50 DA018165], the Veterans Affairs Research Career and Merit Review programs, and by National Institute on Drug Abuse contracts [N01DA2-8822 and N01DA-7-8872]. NIDA project officers contributed to study design and had no further role in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Conflict of interest.
All authors declare that they have no conflicts of interest.
Reference List
- Acuna-Castillo C, Villalobos C, Moya PR, Saez P, Cassels BK, Huidobro-Toro JP. Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT(2A) and 5-HT(2C) receptors. Br J Pharmacol. 2002;136:510–519. doi: 10.1038/sj.bjp.0704747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom JR, Chang MS, Chu H, Niswender CM, Sanders-Bush E. Agonist-directed signaling of serotonin 5-HT2C receptors: differences between serotonin and lysergic acid diethylamide (LSD) Neuropsychopharmacology. 1999;21:77S–81S. doi: 10.1016/S0893-133X(99)00005-6. [DOI] [PubMed] [Google Scholar]
- Baker LE, Broadbent J, Michael EK, Matthews PK, Metosh CA, Saunders RB, West WB, Appel JB. Assessment of the discriminative stimulus effects of the optical isomers of ecstasy (3,4-methylenedioxymethamphetamine; MDMA) Behav Pharmacol. 1995;6:263–275. [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- Bosak A, Lovecchio F, Levine M. Recurrent Seizures and Serotonin Syndrome Following “2C-I” Ingestion. J Med Toxicol. 2013 doi: 10.1007/s13181-013-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cohen S. Psychotomimetic agents. Annu Rev Pharmacol. 1967;7:301–318. doi: 10.1146/annurev.pa.07.040167.001505. [DOI] [PubMed] [Google Scholar]
- de Boer D, Bosman I. A new trend in drugs-of-abuse; the 2C-series of phenethylamine designer drugs. Pharm World Sci. 2004;26:110–113. doi: 10.1023/b:phar.0000018600.03664.36. [DOI] [PubMed] [Google Scholar]
- DEA. DEA Title 21 Code of federal regulations, Section 1308.11 Schedule 1(d) hallucinogenic substances. 2013 http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm.
- Dean BV, Stellpflug SJ, Burnett AM, Engebretsen KM. 2C or Not 2C: Phenethylamine Designer Drug Review. J Med Toxicol. 2013;9:172–178. doi: 10.1007/s13181-013-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees JC, Stone JA, Wu AH. Morbidity involving the hallucinogenic designer amines MDA and 2C-I. J Forensic Sci. 2009;54:1485–1487. doi: 10.1111/j.1556-4029.2009.01199.x. [DOI] [PubMed] [Google Scholar]
- Egan C, Grinde E, DuPre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors. Synapse. 2000;35:144–150. doi: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Carmolli M, Cumbay M, Martens CR, Neve KA, Janowsky A. Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J Pharmacol Exp Ther. 1999;289:877–885. [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology (Berl) 2005;181:496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ. The behavioral pharmacology of hallucinogens. Biochem Pharmacol. 2008;75:17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology (Berl) 1995;121:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ. Abuse liability profile of three substituted tryptamines. J Pharmacol Exp Ther. 2011;338:280–289. doi: 10.1124/jpet.111.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, Carbonaro T, Forster MJ. Comparison of the discriminative stimulus effects of dimethyltryptamine with different classes of psychoactive compounds in rats. Psychopharmacology (Berl) 2009;204:715–724. doi: 10.1007/s00213-009-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Mathis CA, Shulgin AT, Hoffman AJ, Nichols DE. [125I]-2-(2,5-dimethoxy-4-iodophenyl)aminoethane ([125I]-2C-I) as a label for the 5-HT2 receptor in rat frontal cortex. Pharmacol Biochem Behav. 1990;35:211–217. doi: 10.1016/0091-3057(90)90228-a. [DOI] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:114–123. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- Krebs KM, Geyer MA. Cross-tolerance studies of serotonin receptors involved in behavioral effects of LSD in rats. Psychopharmacology (Berl) 1994;113:429–437. doi: 10.1007/BF02245219. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther. 2003;304:229–237. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- Li JX, Koek W, Rice KC, France CP. Differential effects of serotonin 5-HT1A receptor agonists on the discriminative stimulus effects of the 5-HT2A receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rats and rhesus monkeys. J Pharmacol Exp Ther. 2010;333:244–252. doi: 10.1124/jpet.109.163451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE. 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J Med Chem. 2006;49:5794–5803. doi: 10.1021/jm060656o. [DOI] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutierrez-Hernandez MA, Saez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther. 2007;321:1054–1061. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi KK. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Gavaudan S, Conte C, Chaput C, Touzard M, Verriele L, Audinot V, Millan MJ. Agonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: a [35S]GTPgammaS binding study. Eur J Pharmacol. 1998;355:245–256. doi: 10.1016/s0014-2999(98)00483-x. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Differences between the mechanism of action of MDMA, MBDB, and the classic hallucinogens. Identification of a new therapeutic class: entactogens. J Psychoactive Drugs. 1986a;18:305–313. doi: 10.1080/02791072.1986.10472362. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Studies of the relationship between molecular structure and hallucinogenic activity. Pharmacol Biochem Behav. 1986b;24:335–340. doi: 10.1016/0091-3057(86)90362-x. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nonaka R, Nagai F, Ogata A, Satoh K. In vitro screening of psychoactive drugs by [(35)S]GTPgammaS binding in rat brain membranes. Biol Pharm Bull. 2007;30:2328–2333. doi: 10.1248/bpb.30.2328. [DOI] [PubMed] [Google Scholar]
- Oberlender R, Nichols DE. Drug discrimination studies with MDMA and amphetamine. Psychopharmacology (Berl) 1988;95:71–76. doi: 10.1007/BF00212770. [DOI] [PubMed] [Google Scholar]
- Ovaska H, Viljoen A, Puchnarewicz M, Button J, Ramsey J, Holt DW, Dargan PI, Wood DM. First case report of recreational use of 2,5-dimethoxy-4-chloroamphetamine confirmed by toxicological screening. Eur J Emerg Med. 2008;15:354–356. doi: 10.1097/MEJ.0b013e3282fc765b. [DOI] [PubMed] [Google Scholar]
- Parrish JC, Braden MR, Gundy E, Nichols DE. Differential phospholipase C activation by phenylalkylamine serotonin 5-HT 2A receptor agonists. J Neurochem. 2005;95:1575–1584. doi: 10.1111/j.1471-4159.2005.03477.x. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Regina M, Doat M, Winter JC. 5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol Biochem Behav. 2002;72:29–37. doi: 10.1016/s0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Sacks J, Ray MJ, Williams S, Opatowsky MJ. Fatal toxic leukoencephalopathy secondary to overdose of a new psychoactive designer drug 2C-E (“Europa”) Proc (Bayl Univ Med Cent) 2012;25:374–376. doi: 10.1080/08998280.2012.11928883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders-Bush E, Burris KD, Knoth K. Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J Pharmacol Exp Ther. 1988;246:924–928. [PubMed] [Google Scholar]
- Schechter MD. MDMA-like stimulus effects of hallucinogens in male Fawn-Hooded rats. Pharmacol Biochem Behav. 1998;59:265–270. doi: 10.1016/s0091-3057(97)00415-2. [DOI] [PubMed] [Google Scholar]
- Schindler EA, Harvey JA, Aloyo VJ. Phospholipase C mediates (+/−)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-, but not lysergic acid diethylamide (LSD)-elicited head bobs in rabbit medial prefrontal cortex. Brain Res. 2013;1491:98–108. doi: 10.1016/j.brainres.2012.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin AT, Shulgin A. Pihkal: A Chemical Love Story. Transform Press; Berkeley, CA: 1991. [Google Scholar]
- Titeler M, Lyon RA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology (Berl) 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O’Brien A, White A, Kennedy JM, Craymer K, Farrington L, Auh JS. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr. 1998;178:440–466. [PubMed] [Google Scholar]
- Van Vrancken MJ, Benavides R, Wians FH., Jr Identification of designer drug 2C-E (4-ethyl-2, 5-dimethoxy-phenethylamine) in urine following a drug overdose. Proc (Bayl Univ Med Cent) 2013;26:58–61. doi: 10.1080/08998280.2013.11928922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos CA, Bull P, Saez P, Cassels BK, Huidobro-Toro JP. 4-Bromo-2,5-dimethoxyphenethylamine (2C-B) and structurally related phenylethylamines are potent 5-HT2A receptor antagonists in Xenopus laevis oocytes. Br J Pharmacol. 2004;141:1167–1174. doi: 10.1038/sj.bjp.0705722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JC. Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology (Berl) 2009;203:251–263. doi: 10.1007/s00213-008-1356-8. [DOI] [PubMed] [Google Scholar]


