Abstract
Objective
To evaluate the efficacy of real relative to sham peripheral prism glasses for patients with complete homonymous hemianopia and without visual neglect.
Methods
Patients recruited at 13 clinics were allocated by minimization into a double-masked, crossover trial with two groups. One group received real (57Δ) oblique and sham (≤ 5Δ) horizontal prisms; the other received real horizontal and sham oblique, in counterbalanced order. A masked data collector at each clinic administered questionnaires after each 4-week crossover period.
Main outcome measure
The primary outcome was the overall difference, across the two periods of the crossover, between the proportion of participants who wanted to continue with (said “yes” to) real prisms and the proportion who said yes to sham prisms. The secondary outcome was the difference in perceived mobility improvement between real and sham prisms.
Results
Of 73 patients randomized, 61 completed the crossover. A significantly higher proportion said yes to real than sham prisms (64% vs. 36%; odds ratio 5.3, 95% CI 1.8 to 21.0). Participants who continued wear after 6 months reported greater improvement in mobility with real than sham prisms at crossover end (p=0.002); participants who discontinued wear reported no difference.
Conclusion
Real peripheral prism glasses were more helpful for obstacle avoidance when walking than sham glasses, with no differences between the horizontal and oblique designs.
Applications to clinical practice
Peripheral prism glasses provide a simple and inexpensive mobility rehabilitation intervention for hemianopia.
INTRODUCTION
Although prismatic corrections have been used in the rehabilitation of homonymous hemianopia (HH) for at least the last 80 years,1 evidence for their effectiveness is almost exclusively based on anecdotal case reports2-4 and open-label evaluations without a control condition.5-10 Recent reviews of a range of interventions for patients with homonymous visual field loss have underscored the need for randomized controlled trials in this area.11-14 To the best of our knowledge, there have only been three controlled studies15-17 of prismatic devices for HH, and each had substantial limitations (eTable 1).
In 2000, Peli7 described a new approach - peripheral prism glasses - to fitting prisms for HH. High-power prism segments fitted unilaterally on the upper and lower peripheral parts of the spectacle lens provide up to 30° lateral visual field expansion with 57Δ prisms (Figures 1 and 2). As the prism images fall on peripheral retina, central diplopia, common with other designs, is avoided. An evidence base for the efficacy of peripheral prism glasses has gradually been built through a series of open-label studies, including a laboratory-based study,8 a multi-center clinical trial9 and most recently an independent (not initiated by Peli) single-center clinical study.10 Clinical success rates were good in each study with 47%9 to 83%10 of participants continuing to use the prism glasses in the long term, reporting that they were helpful for obstacle avoidance when walking. While these findings are promising, none of the studies included a control group or a control treatment.
Figure 1. Permanent peripheral prism glasses as fitted for the study.
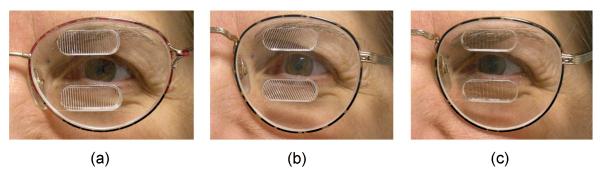
Shown here with prisms on the left spectacle lens for a patient with left hemianiopia, with 12mm inter-prism separation: (a) Horizontal design, 57Δ (base-apex axis horizontal); (b) Oblique design, 57Δ (base-apex axis at 25°); and (c) Sham horizontal, 5Δ. The oblique design provided visual field expansion in more central areas of the visual field than the horizontal design (Figure 2). Each patient wore real (57Δ) prisms of one design and sham (5Δ) prisms of the other design (e.g., real oblique (b) and sham horizontal (c))
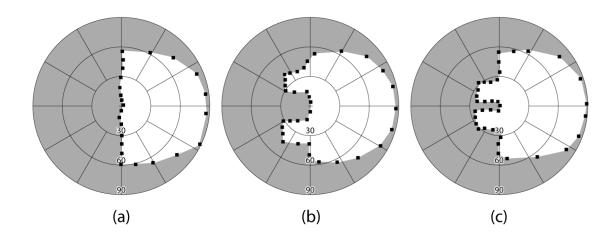
Figure 2. Binocular visual field (Goldmann V4e) of a patient with left HH.
(a) Without peripheral prisms; (b) with 57Δ horizontal peripheral prisms; and (c) with 57Δ oblique peripheral prisms, as fitted for the study with a 12mm inter-prism separation. Both designs provide close to 30° of lateral expansion into the blind hemifield (slightly more for the horizontal than the oblique design). The expansion is in more central areas of the field with the oblique design. Small black squares are the individual points mapped during the perimetry.
Here we report a controlled multi-center trial of the peripheral prism glasses employing a crossover design in which each patient wore a pair of real (57Δ) and a pair of sham prism glasses (<5Δ). Our primary hypothesis was that participants would be more likely to want to continue to use the real than the sham prism glasses, as they would find them more helpful for detecting hazards when walking. Our secondary study goal was to establish preliminary comparative data on two peripheral prism configurations: the original “horizontal” design,7 and a more recent “oblique” design18 (Figures 1a and b). We hypothesized that there would be no difference in continuation rates for the two designs as both provide visual field expansion in areas likely to be helpful when walking (Figures 2b and c). However, the oblique design may be advantageous for driving.19
METHODS
Schepens was the coordinating and data management center for the study. Data were collected at 13 study sites, including Dr. Peli’s lab at Schepens, 11 vision rehabilitation clinics in the US, and one in the UK. The clinics included university, hospital and private-practice clinics. Each site recruited a median of 7 participants (range 3 to 12). The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) at Schepens and by local IRBs for study sites with an IRB. Data were collected in the period October 2007 to January 2010. Study visits are summarized in Table 1. Procedures are detailed in supplementary online materials (eProcedures).
Table 1.
Summary of study visits and assessments
| Visit* | Timing | Assessments and procedures | Personnel |
|---|---|---|---|
| 1 | Week 0 | Screening tests Spectacle and prism measurements |
Practitioner |
| Mobility questionnaire (base line) | Masked data collector | ||
| 2 | Week 4 (approx) |
Dispense 1st pair prism glasses and train in use | Practitioner |
| 3 | Week 8 | Mobility questionnaire 1st pair Would you want to continue with 1st pair (yes/no) |
Masked data collector |
| Dispense 2nd pair prism glasses | Practitioner | ||
| 4 | Week 12 | Mobility questionnaire 2nd pair Would you want to continue with 2nd pair (yes/no) Comparison questionnaire (1st and 2nd pair) |
Masked data collector |
| Debriefing Clinical decision whether to continue wear |
Practitioner | ||
| 5 | 6 months after Visit 4 |
Telephone Interview to assess longer-term experience of wearing prism glasses (only subjects who decided at week 12 to continue) |
Practitioner |
Visits 1 to 4 were in-office; Visit 5 was a telephone interview
Participants
Participants were recruited by practitioners at each study site. The primary inclusion criteria were: complete HH8 of greater than 3 months duration, no visual neglect (Bells test20 and Schenkenberg Line Bisection test21) and no history of having worn peripheral prism glasses. In addition, participants had corrected monocular visual acuity of at least 20/50 in each eye, refractive error within the −5D to +5D range, no strabismus, no significant cognitive decline (Short Portable Mental Status Questionnaire22), and no balance problems or other deficits that could impair ability to walk or use the prism glasses. Visual field mapping extended to at least 50° from fixation in all directions and was performed using Goldmann perimetry (V4e target), Humphrey Field Analyzer 120 point full field screening test or similar tests, depending on the equipment available at each clinic. Before screening, the nature of the study was explained and informed consent was obtained from all participants. To ensure that study inclusion criteria were uniformly applied, screening data were sent to the Principal Investigator (ARB) who determined eligibility.
Study design
The study was a double-masked, multi-center crossover trial of real and sham peripheral prism glasses with a counterbalanced AB/BA design. Each crossover period was four weeks. A washout period was not included as no carry-over effects were anticipated. To address our secondary goal of providing preliminary comparative data on the oblique and horizontal designs, participants were allocated to receive either real oblique and sham horizontal prism glasses, or real horizontal and sham oblique.
At the end of the crossover a clinical decision whether or not to continue wearing the real prism glasses was made. For participants who continued, a follow-up telephone interview was conducted approximately 6 months after their final in-office visit (Table 1 and eProcedures).
Treatment allocation
The clinical coordinator at Schepens assigned participants to one of four possible treatment allocations (real oblique AB/BA, and real horizontal AB/BA) using minimization23 (Minim software; Evans S., Day S., and Royston P.; available at http://www-users.york.ac.uk/~mb55/index.html). The first participant was assigned randomly with each subsequent participant assigned in such a way as to minimize imbalances among the four treatment allocations. We could realistically balance for only two factors. Study site was the primary factor (as continuation rates varied significantly across sites in our first multicenter study9) and side of HH (right or left) was the second factor (as the side of the lesion could potentially affect performance with the prism glasses). We did not balance for age as it was not a significant factor affecting continuation rates in our previous study.9
Letter codes, randomly assigned to each of the treatment allocations by a researcher external to the study, were used by the Minim software and in all data records and spreadsheets. There were two copies of the code breaker: the first was kept in a sealed envelope in a location known only to the external researcher and the second was sent to Chadwick Optical so that the correct combinations of prism glasses could be manufactured for each participant. The code was not broken at Schepens until data analyses were completed.
Real and sham prism glasses
The real and sham prism glasses (manufactured by Chadwick Optical, Inc.) both comprised an upper and lower rigid Fresnel prism segment with a 12mm separation9 embedded in a regular distance-vision spectacle lens in front of the eye on the side of the HH. They differed only in the design (horizontal vs. oblique) and in the prism power: 57Δ for the real prisms (Figures 1 a and b) and 5Δ for the sham prisms (Figure 1 c). An extra optical element included with the sham prisms provided visual acuity reduction and chromatic dispersion similar to those experienced with the 57Δ prisms and also reduced the prism power by about 2Δ from the original 5Δ. Hence the sham prisms provided no useful field expansion (<3°). For the horizontal design, the prisms were base-out. For the oblique design, the upper prism was placed base-out and -down and the lower prism was placed base-out and -up, with the base-apex line at an angle of tilt of 25° to the horizontal (Figure 1 c).
Masking
Double-masking was employed with participants and data collectors being masked as far as possible. In addition, the principal investigator (ARB) who conducted data analyses was masked. However it was impossible to mask all study personnel; there was an unmasked practitioner at each site who fitted the prism glasses and dealt with clinical aspects of patient care.
Participants were informed that they were evaluating two different designs of prism glasses; they were not told that one pair was a sham. If they asked about the difference, the practitioner commented on the physical difference of the vertical versus tilted grooves on the Fresnel prism inserts for the horizontal and oblique designs, respectively. To prevent investigator bias, the data collector at each site was unaware of the treatment allocation and the study glasses were retained by the (unmasked) practitioner while the questionnaires were administered. Patients never had possession of both pairs together.
Primary outcome measure
At the end of each crossover period, participants were asked a yes/no question: “If the study were to end today, would you want to continue with these prism glasses (i.e. the prism glasses worn in that period)?” Our primary outcome was the overall difference, across the two periods of the crossover, between the proportion of participants saying “yes” to real glasses and the proportion saying “yes” to sham glasses.
Secondary outcome measure
Perceived difficulties with mobility were quantified using a 5-point rating scale (no difficulty to extreme difficulty) for 7 situations (items) relevant to HH, including at home, in stores, outdoors, in unfamiliar areas, in familiar areas, in crowded areas, and noticing objects off to the side when walking.8, 24 The questionnaire was administered at baseline (without prisms) and after each period of the crossover. Interval scale measures25 of perceived difficulty with overall mobility for each participant were estimated using Rasch analysis of the responses to all seven items (Winsteps software, version 3.70.0.226). Rasch measures were expressed as logits (log odds ratios). Mobility improvement scores for real and sham prisms were defined as the difference in perceived difficulty relative to baseline (in logits). Psychometric properties of the questionnaire were good (eTable 2).
Comparison questionnaire
At the end of the crossover participants completed a comparison questionnaire about the two pairs of glasses. They did not have access to the glasses while answering the questions and the questionnaire was administered before they were told that one pair was a sham (debriefing came later; see eProcedures). Questions included: “Which pair would you select (1st pair, 2nd pair, neither)?”; “Which pair was better for obstacle avoidance when walking?”; and “Which pair gave more comfortable vision?”. These last two questions were scored on a 5-point scale from 1st pair much better to 2nd pair much better.
Statistical analyses
The sample size calculation for the primary outcome measure was based on a McNemar test for a 2-by-2 contingency table of the yes/no responses to real and sham prism glasses for data combined across both periods of the crossover (StudySize software, version 2.0.4; CreoStat HB, Sweden). In our previous open-label multicenter study,9 74% of participants continued with (real) peripheral prism glasses after an initial 4-week trial. We therefore estimated that 70% of participants would say yes to the real prism glasses in this study and that half that number (35%) would say yes to the sham prism glasses. For a two-tailed test, the minimum sample size to detect a 35% difference in yes responses to real and sham prism glasses was 57 participants, assuming 30% overlap (i.e., 30% said yes to both pairs of glasses), power of 90% and significance (α) level of 1%. Assuming9 an attrition rate of 20%, we planned to enroll at least 68 participants.
As planned, the primary outcome measure was analyzed using a McNemar test for data combined across both periods of the crossover. In addition, the proportions of participants saying yes to real and yes to sham prism glasses at the end of each period were compared using a two-proportion z-test. As a secondary measure, the proportion expressing a preference for the real prism glasses at the end of the crossover was analyzed using a binomial confidence interval test.
Mobility improvement scores, the secondary outcome measure, were normally distributed. Our primary analysis was a within-subjects comparison of the crossover differences in mobility scores between real and sham prism glasses, analyzed using a paired t-test. In addition, differences in mobility scores between patients wearing real and sham prisms were analyzed for each period of the crossover using an independent samples t-test. In our prior open-label multicenter trial,9 participants who continued wearing peripheral prism glasses gave significantly higher mobility-helpfulness ratings for the glasses than participants who discontinued wear. We therefore conducted subgroup analyses of mobility improvement scores based on final status (continuing wear or discontinued wear) at the 6 month interview.
When questionnaires were administered, patients did not know that one pair of glasses was real and one pair was sham; however, for clarity in reporting of results, participant responses have been converted to real or sham glasses rather than first or second pair. All analyses were 2-sided. An α-value ≤ 0.01 was considered to indicate statistical significance for the primary analysis and ≤ 0.05 for the secondary analyses.
RESULTS
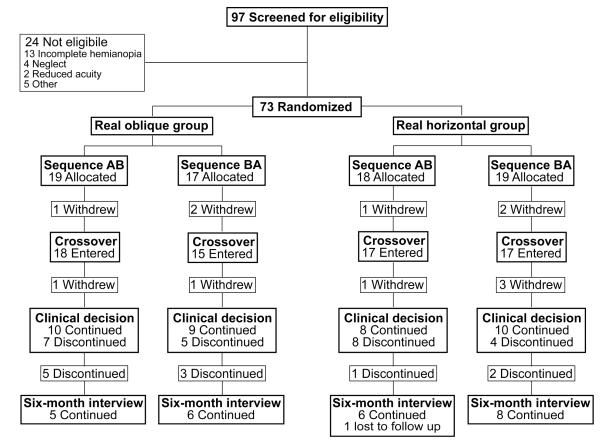
Seventy-three patients were enrolled with 36 allocated to the real oblique group and 37 to the real horizontal group (Figure 3). Twelve participants subsequently withdrew: six before the start of the crossover (3 due to transportation problems and 3 no reason) and six more during the crossover (3 for health reasons, 1 visual field recovery, 1 transportation problems, 1 no reason). Thus 61 participants (66% male) with a median age of 58 years (range 18 to 89) completed the crossover; 64% had left hemianopia. The median time since onset was 18 months (range 3 to 396) with stroke the predominant cause (77%).
Figure 3. Participant flow through the study.
Minimization was used to allocate participants to treatment group and sequence: real oblique AB/BA and real horizontal AB/BA (AB = Real first; BA = Sham first).
At the end of the crossover, 61% (19/31) continued prism wear in the oblique group and 60% (18/30) in the horizontal group (p = 0.918). At the long-term interview, 36% (11/31) and 47% (14/30) were still wearing the prism glasses in each group, respectively (p = 0.3). Thus the overall continuation rate at 6 months was 41% (25/61).
In agreement with our prediction, there were no statistically significant differences between the oblique and horizontal groups for any of the outcome measures (eTable 3); therefore data were pooled across the two groups for the main analyses reported below. Additional analyses are summarized in supplementary materials (see eResults), including: a summary of reported difficulties with real and sham prism glasses; reasons for discontinuing wear; predictors of long-term wear; and debriefing data.
Primary outcome measure
In response to the question “would you want to continue with these prism glasses”, the difference between the proportions of participants who said yes to real and yes to sham at the end of the first crossover period was not significant (p = 0.39), but was highly significant at the end of the second period (p = 0.001; Table 2). For data combined across the two periods of the crossover, the overall proportion of participants who said yes to the real prism glasses (64%, 39/61) was higher than the overall proportion saying yes to the sham prism glasses (36%, 22/61; Tables 2 and 3). The 28% difference in these proportions, the primary outcome, was significant (95% CI 12% to 42%, McNemar test p = 0.001; Tables 2 and 3).
Table 2.
Number of participants responding “yes” to real and sham prisms in each crossover period, and across both periods
| Yes to real | Yes to sham | Odds ratio | |
|---|---|---|---|
| Period 1 a | 19 / 33 (58%) | 13 / 28 (46%) | 1.6 (95% CI 0.6 to 4.3); z = 0.87, p = 0.39 |
| Period 2 a | 20 / 28 (71%) | 9 / 33 (27%) | 6.7 (95% CI 2.2 to 20.4); z = 3.44, p = 0.001 |
| Periods 1 and 2 b | 39 / 61 (64%) | 22 / 61 (36%) | 5.3c (95% CI 1.8 to 21.0); z = 3.40, p = 0.001 |
Analyzed as if for a parallel arm trial (two-proportion z-test)
Matched pairs analysis for the crossover (McNemar test); See Table 3 for the two-by-two contingency table
Marginal odds ratio based on discordant pairs
Table 3.
Two-by-two (matched pairs) classification of the number of participants responding “yes” or “no” to real and sham prism glasses for data combined across the two periods of the crossover.
| Sham | ||||
|---|---|---|---|---|
| Yes | No | Total | ||
| Real | Yes | 18 | 21 | 39 |
| No | 4 | 18 | 22 | |
| Total | 22 | 39 | 61 | |
Overall mobility improvement score
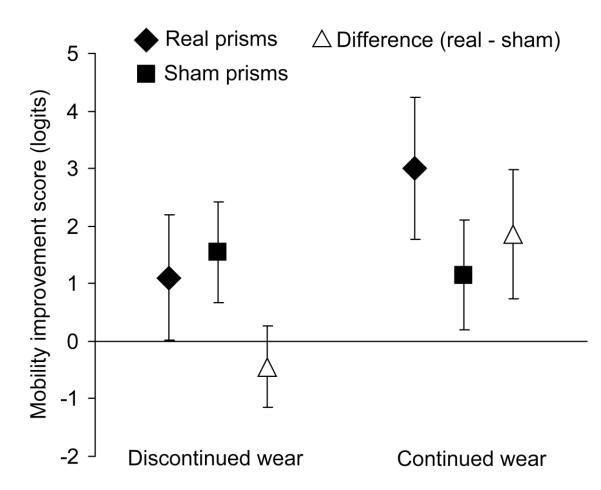
Relative to baseline, there was a significant improvement in the overall mobility score for both real and sham prism glasses in both crossover periods (p < 0.01; Table 4). However, the difference in the amount of improvement between participants wearing real and sham prism glasses was not significant in either period (p = 0.38 and 0.50, respectively; Table 4). In contrast, analysis of the within-subjects crossover differences revealed a trend toward greater improvement with the real than the sham prisms (p = 0.09; Table 4). Subgroup analyses further revealed that participants who continued with prism glasses at the 6-month follow up reported markedly more improvement for real than sham prisms at the end of the crossover (p = 0.002); whereas, participants who discontinued wear reported little difference in the amount of perceived improvement for the two pairs of glasses (Table 4 and Figure 4).
Table 4.
Mobility improvement scores (in logits) for each crossover period, and across both periods
| Real Mean (SD) |
Sham Mean (SD) |
Difference Mean (SD) |
T-test (2 tailed) | |
|---|---|---|---|---|
| Period 1 a | 1.9b (3.3) n = 33 |
1.2b (2.2) n = 28 |
0.7 (2.9) 95% CI −0.8 to 2.1 |
t(59) = 0.88, p = 0.38 |
| Period 2 a | 2.0b (3.4) n = 28 |
1.4b (2.8) n = 33 |
0.6 (3.1) 95% CI −2.1 to 1.1 |
t(59) = 0.69, p = 0.50 |
|
Periods 1 and 2
c
(all subjects) |
1.9 (3.3) n = 61 |
1.3 (2.5) n = 61 |
0.6 (2.6) 95% CI −0.1 to 1.3 |
t(60) = 1.73, p = 0.09 |
|
Periods 1 and 2
c
(continued wear) |
3.0 (3.2) n = 25 |
1.1 (2.4) n = 25 |
1.9 (2.7) 95% CI 0.7 to 3.0 |
t(24) = 3.45, p = 0.002 |
|
Periods 1 and 2
c
(discontinued wear) |
(3.3) n = 35 |
1.6 (2.7) n = 35 |
−0.5 (2.1) 95% CI −1.2 to 0.3 |
t(34) = 1.29, p = 0.21 |
Analyzed as if for a parallel arm trial (independent samples t-test)
Mobility improvement scores all significantly different from 0.0 (one-sample t-tests, all p < 0.01)
Matched pairs analysis for the crossover (paired t-test)
Figure 4. Mean mobility improvement scores for real and sham prism glasses.
Participants who continued prism wear reported significantly more improvement with real than sham glasses. Mobility scores are in logit units: more positive values represent greater improvement. For real and sham prisms, error bars are 95% confidence intervals of the mean scores. For the difference between real and sham, errors bars are 95% confidence intervals of the mean paired differences.
Comparison questionnaire
When asked which pair of glasses they would select at the end of the crossover, 37/61 (61%) chose the real prism glasses, 16/61 (26%) the sham glasses and 8/61 (13%) neither pair. The number of participants selecting real prism glasses approached significance when expressed as a proportion of the total number completing the crossover (61%, 95% CI 48% to 72%; p = 0.07) and was significant when expressed as a proportion of those who actually stated a preference (37/53, 70%, 95% CI 56% to 80%; p = 0.01). These results support the findings of the primary outcome measure.
Participants who selected real prism glasses rated them as much better for obstacle avoidance and vision comfort than sham prism glasses (median ratings; Figure 5). By comparison, participants who selected sham prism glasses rated them as only slightly better than real prism glasses for obstacle avoidance and vision comfort (median ratings; Figure 5). Participants who selected neither pair of glasses gave a median rating of “no difference” for both these aspects. In a similar vein, the main reason given for selecting real prism glasses was that they were the pair of glasses that was more helpful when walking (92%; 34/37); whereas, the main reasons for selecting sham prism glasses were that they were the pair with which vision was more comfortable (81%; 13/16) and with which fewer difficulties had been encountered.
Figure 5. Median relative ratings of real and sham prism glasses from the comparison questionnaire.
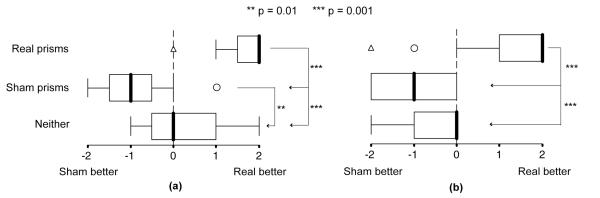
(a) Ratings for obstacle avoidance and (b) ratings for vision comfort, grouped by whether participants selected real prism glasses (n = 37), sham prism glasses (n = 16) or neither pair of prism glasses (n = 8). Responses of participants who selected real prism glasses were significantly different from those who selected sham or neither. Participants who selected real prism glasses rated them as much better than the sham; whereas, those who selected sham glasses rated them as only slightly better than the real glasses. (Participants, still masked when this questionnaire was administered, gave rankings in terms of first pair or second pair, which were subsequently converted to real or sham. Scale: −2 = Sham much better; −1 = Sham slightly better; 0 = No difference; 1 = Real slightly better; 2 = Real much better). Thick vertical line within each box is the median; box length is the interquartile range (IQR); whiskers represent the range of the data within 1.5x IQR; open circle: outlier within 1.5x – 3x IQR; open triangle: far outlier beyond 3x IQR.
DISCUSSION
Participants demonstrated a preference for real peripheral prism glasses over sham peripheral prism glasses. They were about five-times more likely to say yes only to real prism glasses than yes only to sham prism glasses during the crossover (Table 2; marginal odds ratio 5.3), and 64% selected real prisms over sham prisms at the end of the crossover. Moreover, real prism glasses were rated as much more helpful than the sham for obstacle avoidance when walking. The proportion of participants that continued with real prism glasses was similar for the horizontal and oblique designs, suggesting that both designs were helpful for everyday pedestrian mobility. However, a preference for the oblique design might be expected for driving.19
The participants in this study were patients with complete homonymous hemianopia without spatial neglect and without significant cognitive decline attending a range of hospital, university and private practice clinics. As such, we believe the results to be highly generalizable to clinical rehabilitation of patients with similar characteristics. Furthermore, all procedures and data collection methods were based on current clinical practice.
Our results demonstrate the importance of including a control condition when evaluating a rehabilitation intervention. Specifically, 26% of participants selected the sham prism glasses at the end of the crossover. The reasons for their choice were related to vision comfort and lack of difficulties in using the glasses rather than improved functional performance. These are patients that in an open-label trial might artificially inflate success rates when only a short-term follow up is included (e.g., 1 month) as they would like to continue with the study intervention but for the wrong reasons, and would likely discontinue use of the device before a longer-term follow up (e.g., 6 months). Indeed, the short-term success rate (continuation rate at the end of the crossover) was lower in this controlled trial than in our prior open-label trial9 of the peripheral prism glasses (61% vs. 74%), while long-term success rates were more similar (41% vs. 47%). Furthermore, placebo effects were evident in the self-ratings of mobility difficulties; participants reported an improvement in overall mobility for both sham and real prism glasses. However, for participants who continued to wear prism glasses in the long term, the improvement was greater for the real than the sham glasses. Thus, for this subgroup, we were able to measure both treatment and placebo effects.
Although not a goal of this study, we evaluated the ability of a range of factors to predict long-term success (continuation rates) (eTable 6). The strongest predictors were participants’ responses to the prism glasses at the end of the crossover. Unsurprisingly, those who said “yes” to real prism glasses, those who rated them as better than the sham for obstacle avoidance, and those who did not report any difficulties with them were more likely to continue wearing prism glasses in the long term. By comparison, age was only a weak predictor, and side and duration of hemianopia were not predictive (consistent with our prior open-label trial9). Difficulty interpreting the prism image was a major reason for discontinuing wear (eFigure 1 and eTable 5). Limited training in how to use the prism glasses was provided, similar to that implemented in our prior study; however, it is possible that some participants might have benefited from more extensive training. We are currently evaluating the effects of intensive computer-based training for use of the peripheral prism glasses.27
In planning this study, our aim was to achieve a robust, but practical design that would fit within a busy clinic schedule; however, some limitations need to be considered. Differing numbers of participants were recruited at each clinic and we were unable to ensure total masking of data collectors. Furthermore, our outcome measures were based on patient preference and self-report questionnaires. For practical reasons, evaluations of functional mobility performance, such as those employed in lab-based studies of devices for visual field loss,28-30 could not be used.
Our primary outcome measure was limited by period effects. Specifically, after the first crossover period, the difference in the proportion of participants saying yes to real and sham prism glasses was only 12%, compared with 44% after the second period. While responses at the end of the first period might have been affected by the knowledge that another pair of glasses was to be worn in the second period, responses at the end of the second period were clearly influenced by having already worn either real or sham glasses in the first period. Interestingly, period effects were less evident in the mobility improvement scores as the magnitude of the difference in perceived improvement between those wearing real and sham prism glasses was similar at the end of each period (Table 4).
In order to evaluate the evidence base for a given treatment or intervention, systematic reviews synthesize data across trials. Combining results from crossover and parallel-arm trials is not easy; various methods have been proposed.31-33 One straightforward approach is to use data from the first period only, as if from a parallel arm trial; however, this means that valuable information from the second period may be lost and ignores the fact that the study was designed as a crossover. We suggest that the period effects present in our original primary outcome measure provide an example of a situation in which it would have been potentially misleading to include data from only the first crossover period.
In conclusion, this study addresses the lack of controlled trials identified in recent systematic reviews of interventions for homonymous visual field loss11-14 and strengthens the evidence base for the efficacy of peripheral prism glasses as a mobility aid for patients with homonymous hemianopia. The next step should be a clinical trial with outcome measures evaluating functional performance on real-world or simulated mobility tasks.
Supplementary Material
ACKNOWLEDGEMENTS AND DISCLOSURES
EP has financial interest in a patent related to the peripheral prism glasses (assigned to Schepens Eye Research Institute). KK has licensed that patent for Chadwick Optical, Inc. Chadwick Optical, Inc. funded the study in part from NIH grant EY014723 through a subcontract with Schepens Eye Research Institute. EP was a paid consultant to Chadwick Optical, Inc. on the design of the permanent prisms.
Funding/Support and Role of Sponsor Supported in part by NIH grants EY014723 to Karen Keeney (Chadwick Optical, Inc.) and EY12890 to Eli Peli (Schepens Eye Research Institute). The NIH had no role in: the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication.
Doris Apfelbaum, BA, maintained the study database. Practitioners at study sites included: Henry A Greene, OD, Academy Eye Associates, Durham, NC; Susan A. Primo, OD, MPH, Emory Eye Center, Atlanta, GA; David Lewerenz, OD, NSU Oklahoma College of Optometry, Tahlequah, OK; Tracy Matchinski, OD, Illinois Eye Institute, Chicago, IL; Elli Kollbaum, OD, Community Eye Care Center, Indiana University School of Optometry, Bloomington, IN; Barry Kavanaugh, OD, Seven Lakes Eye Care, West End, NC; Dawn K. DeCarlo, OD, MS, MSPH and Marsha Swanson Snow, OD, UAB Center for Low Vision Rehabilitation, Birmingham, AL; Rosalind Creer, PhD, Manchester Royal Eye Hospital Optometry Department, Manchester, UK; Paul Levine, OD, Vision Care Specialists, P.C., Southborough, MA; Selma Chin, OD, Vista Center for the Blind & Visually Impaired, Palo Alto, CA; Richard (Scott) Hearing, OD, Visual Health@Jupiter Eye Center, Jupiter, FL; Thomas Whittaker, MS, JD, MD, Departments of Ophthalmology and Neurology, University of Kansas Medical Center, Prairie Village, KS.
Footnotes
Financial/Proprietary Interest ARB has no financial interests.
Data Access and Responsibility The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Clinical trial registration number: NCT00494676 (ClinicalTrials.gov)
REFERENCES
- 1.Young CA. Homonymous hemianopsia during pregnancy aided by reflecting prism. Arch Ophthalmol. 1929;2:560–565. [Google Scholar]
- 2.Smith JL, Weiner IG, Lucero AJ. Hemianopic Fresnel prisms. J Clin Neuroophthalmol. 1982;2:19–22. [PubMed] [Google Scholar]
- 3.Woo GC, Mandelman T. Fresnel prism therapy for right hemianopia. Am J Optom Physiol Opt. 1983;60:739–743. doi: 10.1097/00006324-198308000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Perez AM, Jose RT. The use of Fresnel and ophthalmic prisms with persons with hemianopic visual field loss. J Vis Impair Blind. 2003;97:173–176. [Google Scholar]
- 5.Hedges TR, Jr., Stunkard J, Twer A. Fresnel prisms--their value in the rehabilitation of homonymous hemianopsias. Klin Monbl Augenheilkd. 1988;192:568–571. doi: 10.1055/s-2008-1050180. [DOI] [PubMed] [Google Scholar]
- 6.Lee AG, Perez AM. Improving awareness of peripheral visual field using sectorial prism. J Am Optom Assoc. 1999;70:624–628. [PubMed] [Google Scholar]
- 7.Peli E. Field expansion for homonymous hemianopia by optically induced peripheral exotropia. Optom Vis Sci. 2000;77:453–464. doi: 10.1097/00006324-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Giorgi RG, Woods RL, Peli E. Clinical and laboratory evaluation of peripheral prism glasses for hemianopia. Optom Vis Sci. 2009;86:492–502. doi: 10.1097/OPX.0b013e31819f9e4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowers AR, Keeney K, Peli E. Community-based trial of peripheral prism visual field expansion device for hemianopia. Arch Ophthalmol. 2008;126:657–664. doi: 10.1001/archopht.126.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neill EC, Connell PP, O’Connor JC, Brady J, Reid I, Logan P. Prism therapy and visual rehabilitation in homonymous visual field loss. Optom Vis Sci. 2011;88:263–268. doi: 10.1097/OPX.0b013e318205a3b8. [DOI] [PubMed] [Google Scholar]
- 11.Pollock A, Hazelton C, Henderson CA, et al. Interventions for visual field defects in patients with stroke. Cochrane Database of Systematic Reviews. 2011 doi: 10.1002/14651858.CD008388.pub2. Art. No: CD008388 DOI: 008310.001002/14651858.CD14008388.pub14651852. [DOI] [PubMed] [Google Scholar]
- 12.Lane AR, Smith DT, Schenk T. Clinical treatment options for patients with homonymous visual field defects. Clin Ophthalmol. 2008;2:93–102. doi: 10.2147/opth.s2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pambakian A, Currie J, Kennard C. Rehabilitation strategies for patients with homonymous visual field defects. J Neuroophthalmol. 2005;25:136–142. [PubMed] [Google Scholar]
- 14.Plow EB, Maguire S, Obretenova S, Pascual-Leone A, Merabet LB. Approaches to rehabilitation for visual field defects following brain lesions. Expert Rev Med Devices. 2009;6:291–305. doi: 10.1586/erd.09.8. [DOI] [PubMed] [Google Scholar]
- 15.Rossi PW, Kheyfets S, Reding MJ. Fresnel prisms improve visual perception in stroke patients with homonymous hemianopia or unilateral visual neglect. Neurology. 1990;40:1597–1599. doi: 10.1212/wnl.40.10.1597. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb DD, Freeman P, Williams M. Clinical research and statistical analysis of a visual field awareness system. J Am Optom Assoc. 1992;63:581–588. [PubMed] [Google Scholar]
- 17.Szlyk JP, Seiple W, Stelmack J, McMahon T. Use of prisms for navigation and driving in hemianopic patients. Ophthalmic Physiol Opt. 2005;25:128–135. doi: 10.1111/j.1475-1313.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- 18.Peli E. Peripheral field expansion device. US 7,374,284 B2 United States patent. 2008
- 19.Bowers AR, Tant M, Peli E. A pilot evaluation of on-road detection performance by drivers with hemianopia using oblique peripheral prisms. Stroke Res Treat. 2012 doi: 10.1155/2012/176806. doi:10.1155/2012/176806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanier M, Gauthier L, Lambert J, et al. Evaluation of left visuospatial neglect: Norms and discrimination power of two tests. Neuropsychology. 1990;4:87–96. [Google Scholar]
- 21.Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30:509–517. doi: 10.1212/wnl.30.5.509. [DOI] [PubMed] [Google Scholar]
- 22.Pfeiffer E. Short Portable Mental Status Questionnaire for Assessment of Organic Brain Deficit in Elderly Patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 23.Altman DG. Practical Statistics for Medical Research. Chapman and Hall; London: 1991. [Google Scholar]
- 24.Turano KA, Geruschat DR, Stahl JW, Massof RW. Perceived visual ability for independent mobility in persons with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1999;40:865–877. [PubMed] [Google Scholar]
- 25.Massof RW, Rubin GS. Visual function assessment questionnaires. Surv Ophthalmol. 2001;45:531–548. doi: 10.1016/s0039-6257(01)00194-1. [DOI] [PubMed] [Google Scholar]
- 26.Linacre JM. WINSTEPS® (Version 30.70.02) Rasch measurement computer program. Winsteps.com; Beaverton, Oregon: 2010. [Google Scholar]
- 27.Houston K, Churchill J, Wiegand J, et al. Perceptual-motor adaptation in hemianopes wearing peripheral prisms is possible: Preliminary results. Invest Ophthalmol Vis Sci. 2013;54 ARVO E-abstract 2759. [Google Scholar]
- 28.Bowers AR, Mandel AJ, Goldstein B, Peli E. Driving with hemianopia: 1. Detection performance in a simulator. Invest Ophthalmol Vis Sci. 2009;50:5137–5147. doi: 10.1167/iovs.09-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowers AR, Luo G, Rensing NM, Peli E. Evaluation of a prototype Minified Augmented-View device for patients with impaired night vision. Ophthalmic Physiol Opt. 2004;24:296–312. doi: 10.1111/j.1475-1313.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 30.Luo G, Woods RL, Peli E. Collision judgment when using an augmented-vision head-mounted display device. Invest Ophthalmol Vis Sci. 2009;50:4509–4515. doi: 10.1167/iovs.08-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; [updated March 2011]. 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- 32.Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31:140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- 33.Stedman MR, Curtin F, Elbourne DR, Kesselheim AS, Brookhart MA. Meta-analyses involving crossover trials: methodological issues. Int J Epidemiol. 2011;40:1732–1734. doi: 10.1093/ije/dyp345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.