Abstract
The CD3 zeta chain (CD247), a gene involved in T cell signaling, has been shown to associate with blood pressure in human genetic studies. To test the functional role of CD247 in hypertension and renal disease, zinc-finger nucleases targeting CD247 were injected into Dahl SS/JrHsdMcwi embryos. The resulting 11-bp frameshift deletion in exon 1 of CD247 led to a predicted premature stop codon. Western blotting confirmed the absence of CD247 protein in the thymus, and flow cytometry (n=5-9/group) demonstrated that the mutant rats (CD247−/−) have a greater than 99% reduction in circulating CD3+ T-cells compared to littermate controls (CD247+/+). Studies were performed on age-matched, littermate male, CD247+/+ and −/− rats fed a 4.0% NaCl diet for three weeks. The infiltration of CD3+ T-cells into the kidney following high salt was significantly blunted in CD247−/− (1.4±0.4 × 105 cells/kidney) compared to the CD247+/+ (8.7±2.0 × 105 cells/kidney). Accompanying the reduced infiltration of T-cells, mean arterial blood pressure was significantly lower in CD247−/− than in CD247+/+ (134±1 vs 151±2 mmHg). As an index of kidney disease, urinary albumin and protein excretion rates were significantly reduced in CD247−/− (17±1 and 62±2 mg/day, respectively) compared to CD247+/+ (49±3 and 121±5 mg/day, respectively). Glomerular and renal tubular damage were also attenuated in the CD247−/−. These studies demonstrate that functional T cells are required for the full development of Dahl SS hypertension and indicate that the association between CD247 and hypertension in humans may be related to altered immune cell function.
Keywords: kidney, hypertension, lymphocytes, immune system, rats
INTRODUCTION
Studies in humans have demonstrated associations between genetic variants and hypertension in GWAS1, and other association studies.2 Many associated genes do not have an obvious link to blood pressure regulation; it is therefore important to explore the mechanisms whereby these genes alter blood pressure to elucidate pathways of disease and develop new approaches for drug therapy. One such gene is CD247 in which a SNP variant in intron 1 is associated with diastolic and systolic blood pressure in hypertensive African-American and European-American subjects.3 CD247, which encodes the CD3 zeta chain, is involved in the assembly and expression of the T cell receptor complex and in signal transduction upon antigen triggering.4,5,6 Mice with a disruption of the CD3 zeta chain demonstrate a marked reduction in thymocytes and peripheral T cells.6,7 Furthermore, a patient with somatic mutations in the CD3 zeta chain was shown to have a reduction in circulating T cells with no change in B cells.8 In addition, GWAS have demonstrated an association of variants in CD247 with systemic sclerosis, rheumatoid arthritis, and other autoimmune-related disorders.9,10 Although the role of CD247 in immune-related disease appears obvious, the role of this gene in hypertension is not known.
A potential link between CD247, a gene involved in T cell signaling, and hypertension is provided by many studies that have implicated the immune system in experimental hypertension and renal disease in rats and mice. Moreover, human data are consistent with the observations in animals and indicate that the immune system plays a role in hypertension and renal disease in patients.11,12,13 Recent work in our laboratory has focused upon the role of infiltrating immune cells in the kidney in the development of salt-sensitive hypertension and renal disease in Dahl SS rats. Dahl SS rats develop salt-sensitive hypertension, albuminuria, and renal histological damage which is accompanied by a significant increase in infiltrating T-lymphocytes and macrophages in the kidney.14,15,16 Interestingly, immunosuppression blocked the infiltration of T-cells and macrophages into the kidney and attenuated hypertension and renal damage in Dahl SS rats fed high salt.14,15,16,17,18 Since CD247 is involved in T cell signaling, the present experiments addressed the role of CD247 in the infiltration of immune cells into the kidney in Dahl SS rats fed high salt and the resulting hypertension and renal disease.
Initial experiments examined the infiltration and activation of T lymphocytes in the kidney of Dahl SS rats. Zinc-finger nuclease (ZFN) technology was then utilized to delete CD247 in the Dahl SS genetic background in order to examine the importance of T cell activation in the disease response. Experiments were then performed in the CD247 mutant rats to investigate the infiltration of immune cells into the kidney and to assess the hypertensive and renal disease phenotypes.
METHODS
All animal procedures were performed at the Medical College of Wisconsin under protocols approved by the Institutional Animal Care and Use Committee. The experimental methods detailing generation of the mutant animals, phenotyping, and statistics are described in the online-only Data Supplement.
RESULTS
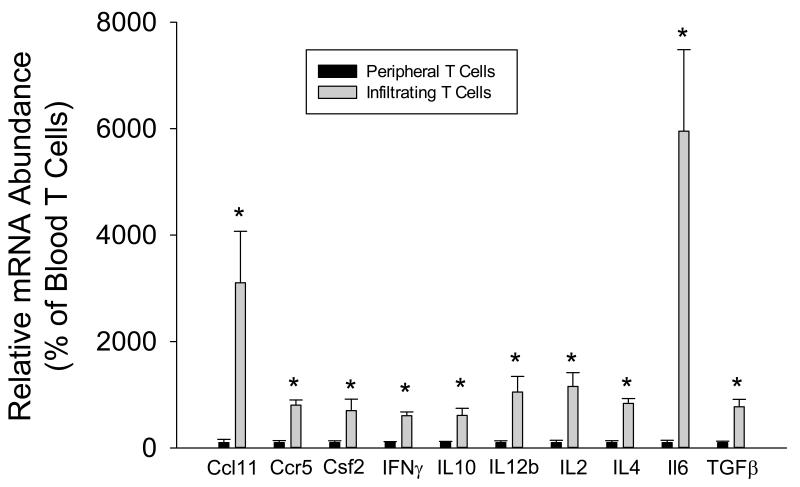
A gene expression analysis with real-time PCR was initially performed on RNA isolated from circulating T cells and T cells isolated from the kidneys of 12 Dahl SS rats (4 pooled samples of 3 individual rats each) fed high salt for three weeks (Figure 1). The infiltrating T cells in the kidney expressed significantly greater (by 5-54 fold) mRNA of inflammatory molecules associated with T cell signaling. These included proliferation factors such as Il-2 and factors associated with TH1, TH2, Treg, and TH17 subsets such as IFN-γ, Il-4, Il-10, and Il-6, respectively. The increased expression of these factors along with other molecules associated with T cell activity indicate that the infiltrating T cells in the kidney have proliferated and are activated relative to circulating T-lymphocytes.
FIGURE 1.
Cytokine expression in T cells isolated from the blood and kidney of Dahl SS rats fed a high salt (4.0% NaCl) diet for three weeks. * indicates P<0.05 vs. peripheral cells.
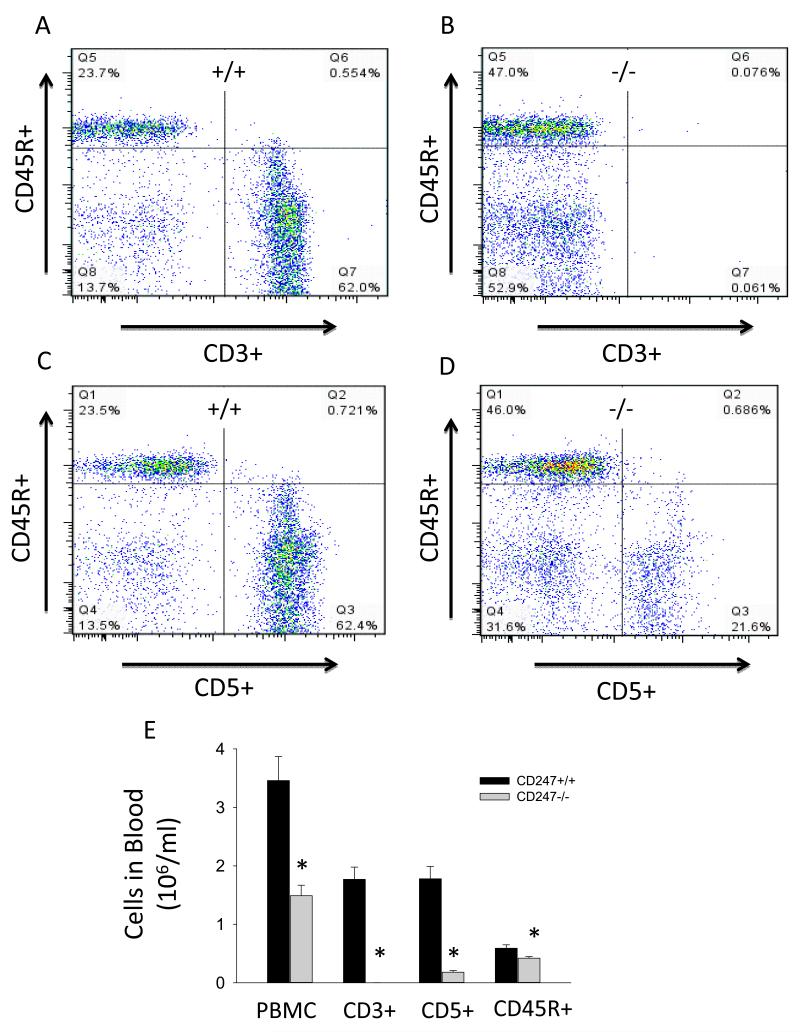
To examine the importance of activation of the infiltrating cells in the Dahl SS disease response, rats with a null mutation in CD247 were generated with ZFN technology. As described in the Methods, DNA sequencing revealed an 11 base pair frameshift deletion of bases 154-164 in the SS-Cd247em1Mcwi mutant (CD247−/−) rats. Western blotting experiments demonstrated a deficit of CD247 protein in homogenates of the thymus of CD247−/− rats compared to CD247+/+ (Figure S1A). Flow cytometry experiments demonstrated that, in the rat, CD5 is a T cell marker expressed on the same cells as CD3 (Figure S1B); in contrast to the CD247+/+, CD3 is absent on CD5+ cells in the CD247−/− (Figure S1C). Furthermore, CD5+ cells in the CD247−/− are negative for the TCRαβ (Figure S1D-E). Together, these data demonstrate the absence of the T cell receptor complex in the mutant animals. Figure 2 illustrates a flow cytometric analysis of peripheral blood mononuclear cells (PBMC) for CD3+, CD5+ and CD45R+ cells (n=5-6/group). Representative two dimensional profiles from CD247+/+ (Figures 2A and C) and CD247−/− rats (Figures 2B and D) demonstrate the percentage distribution of each cell type in the PBMC population. Those data, along with the PBMC count, were used to assess the total circulating cells (Figure 2E). Total PBMC were reduced by greater than 55% in CD247−/− compared to the CD247+/+. Circulating CD3+ cells were virtually undetectable in the CD247−/− rats, and CD5+ cells were reduced by 90%. B-lymphocytes, as assessed by CD45R, were also significantly reduced in the CD247−/− by 28%. At 12 weeks of age (n=6-8/group), thymus weight tended to be reduced in the CD247−/− (0.30±0.08 grams) compared with wild type littermates (0.56±0.03 grams); body weights (367±10 and 347±6 grams) were not different between CD247+/+ and −/− rats, respectively.
FIGURE 2.
Representative flow cytometric analysis of CD5+, CD3+ and CD45R+ cells in CD247+/+ (A,C) and CD247−/− rats (B,D). The graph illustrates mean numbers of PBMC and CD3+, CD5+ and CD45R+ lymphocytes in CD247+/+ and CD247−/− rats (E). * indicates P<0.05 vs. CD247+/+.
As indicated above, CD5, a T cell-specific marker, was still present on circulating and splenic cells in the mutant rats. To confirm the influence of CD247 deletion on T cell function, the proliferative response was examined in enriched CD5+ cells isolated from CD247+/+ and −/− rats. Results of stimulation of cultured CD5+ cells with Conconavalin A for 4-6 days are illustrated in Figure S2. The fluorescent peaks illustrated in Figure S2A-D, moving from right to left, represent successive generations of live CD5+ cells. Compared to control cells (Figure S2A), Concanavalin A stimulation of CD5+ cells from WT rats led to a marked proliferative response (Figure S2B) with over 80% of the cells dividing at least once. In contrast, cell proliferation of CD5+ splenocytes obtained from CD247−/− rats was virtually absent, averaging approximately 1% of the total cells in either control or Concanavalin A-stimulated conditions (Figures S2C and D, respectively). These experiments illustrate the importance of CD247 in T-cell proliferation. Lacking a functional CD247 gene inhibits the activation and proliferation of CD5+ cells (T cells) via the TCR, indicating an inability to participate in the inflammatory responses to antigens.
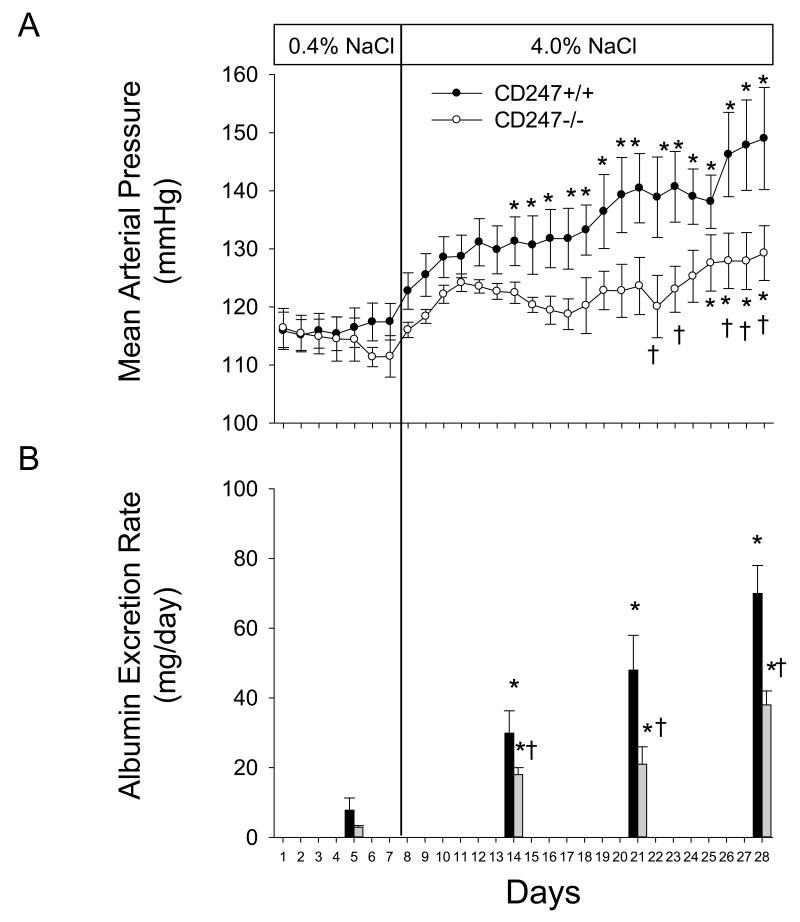
The changes in arterial blood pressure and albumin excretion rate in wild type and SS-CD247em1Mcwi littermates during high salt intake are illustrated in Figure 3. The 24 hour average daily MAP values measured by telemetry were not different between the CD247+/+ and −/− rats during 7 days of low salt intake (0.4% NaCl, n=6-8/group). Blood pressure increased in both groups following the high salt intake, though the increase occurred more rapidly and reached a greater magnitude in the CD247+/+ rats. The average 24 hour MAP value was significantly increased after 7 days of 4.0% NaCl chow in the WT rats. In contrast, a significant increase in MAP was only observed in CD247−/− rats after 18 days of high salt. Moreover, a comparison of blood pressure values between groups indicated significant differences in arterial pressure on 5 of the final 6 days of blood pressure measurement. The albumin excretion rate was elevated in CD247+/+ rats fed low NaCl compared to the CD247−/−, though the differences did not reach statistical significance. In parallel to the increase in blood pressure, albumin excretion rate was increased on Days 7, 14, and 21 of high salt in comparison to the low salt value in the CD247+/+ rats. The albumin excretion rate also increased in the CD247−/− rats when NaCl intake was increased, but the albumin excretion rate in the null mutant rats was significantly less than observed in the CD247+/+. No differences in steady state sodium excretion rate were observed between CD247+/+ and −/− rats on the low salt (1.0±0.1 vs 0.9±0.1 mEq/day) or high salt diet (14.4±0.6 vs 15.0±0.6 mEq/day).
FIGURE 3.
Changes in mean arterial pressure (A) and albumin excretion rate (B) in CD247+/+ and CD247−/− rats fed AIN-76A diet containing 0.4% NaCl followed by 21 days of 4.0% NaCl. * indicates P<0.05 vs.values obtained on the final day of 0.4% NaCl chow, † indicates P<0.05 vs. CD247+/+ on the same day.
Blood pressure, albumin excretion rate, and conscious creatinine clearance were measured in a separate set of age-matched CD247+/+ and −/− littermates fed the 4.0% NaCl diet and implanted with chronic indwelling femoral arterial catheters (data not shown). Consistent with the studies described above, MAP was significantly lower in the CD247−/− than in the CD247+/+ (134±1 vs 151±2 mmHg; n=8-10/group) following three weeks of 4.0% NaCl diet. As an index of kidney disease, urinary albumin excretion rate was significantly reduced in CD247−/− (17±1 mg/day) compared to WT rats (17±1 vs 49±3 mg/day). Conscious creatinine clearance, as an index of GFR, tended to be greater in the CD247−/− rats than in the WT (612±50 vs 466±73 ml/min/gram kidney weight) following three weeks of high salt, though the difference did not reach statistical significance.
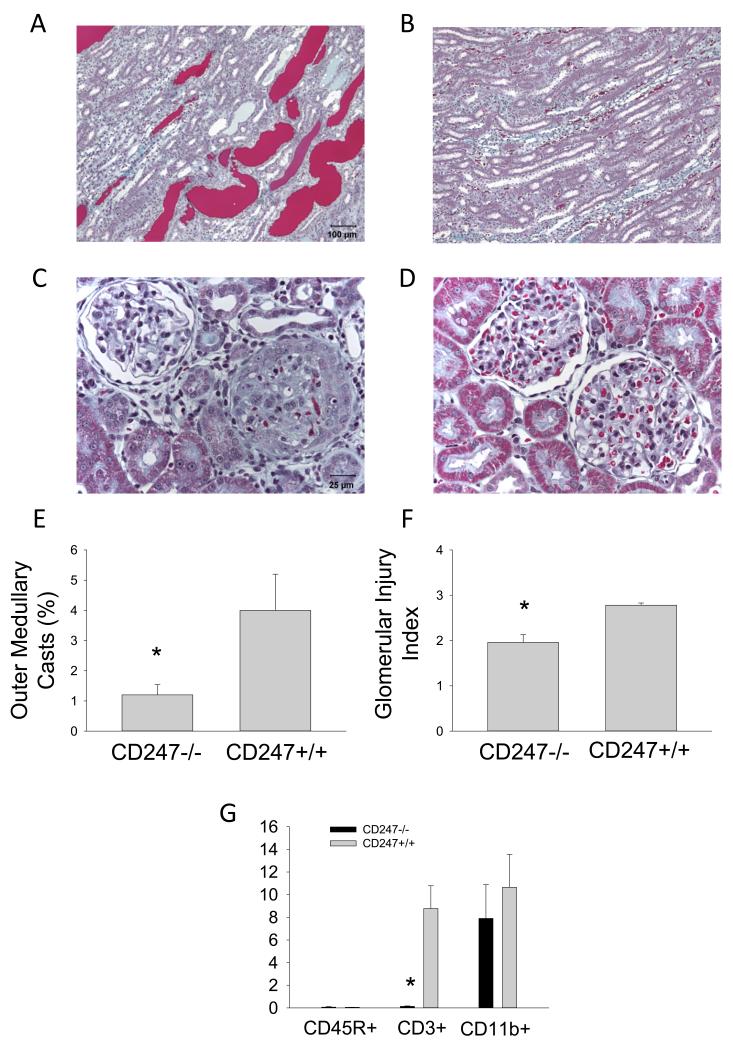
Renal histological changes and infiltration of immune cells into the kidneys are illustrated in Figure 4. The renal histological damage typically observed in Dahl SS fed high salt, including blocked and dilated tubules in the outer medulla, was reduced in the CD247−/− rats (n=4-5/group, Figure 4A-F). Histological scoring demonstrated a significant reduction in the glomerular damage index as well as a reduction in damaged tubules in the outer medulla. Finally, isolation and counting of infiltrating T-cells in the kidney showed significantly fewer infiltrating CD3+ T-cells in the kidneys of the CD247−/− compared to the CD247+/+ kidneys after three weeks of high salt; the absolute number of CD11b+ cells (monocytes and macrophages) in the kidney were not different between groups (Figure 4G).
FIGURE 4.
Light microscopy of trichome stained sections of the renal outer medulla (A and B, 10× original magnification) and renal cortex (C and D, 40× original magnification) of CD247+/+ (A and C) or CD247−/− (B and D) fed 4.0% NaCl chow for three weeks. The percentage of the renal outer medulla consisting of protein casts (E), the calculated glomerular injury score (F), and the number of infiltrating B lymphocytes (CD45R+), T lymphocytes (CD3+) and macrophages/monocytes (CD11b+) in the kidney of CD247+/+ and −/− rats following three weeks of 4.0% NaCl diet are also plotted (G). * indicates P<0.05 vs. CD247−/−.
DISCUSSION
Previous studies from our lab have indicated an infiltration of T lymphocytes into the kidney of Dahl SS rats fed a high salt diet. During an immune response, antigen presenting cells endocytose foreign material which is presented as antigen to the T helper cells; T cells expressing the specific T cell receptor against the antigen are activated and proliferate in a process dependent upon the release of a variety of cytokines.19 The present data indicate increased expression of proliferation factors and other cytokines in the infiltrating T cells in the kidney when compared to circulating T-lymphocytes. The present study describes the development of a rat in which a null mutation in CD247 was induced in the Dahl SS genetic background using ZFN technology. The genetic deletion of CD247 resulted in the depletion of the cell surface expression of CD3 and the TCRαβ chain and inhibited the ability of the T cells to proliferate. This unique model permitted us to examine the role of T cell activation in Dahl salt-sensitive hypertension and renal damage.
Previous studies from our lab have indicated that immunosuppressive agents mitigate the development of salt-sensitive hypertension and renal damage in the Dahl SS rat.14,15,16,17 These and similar observations in other animal models of hypertension and/or renal disease20,21,22,23 indicate an important role of the immune system in cardiovascular disease. Potential non-specific effects of these pharmacological agents may cloud the interpretation of these studies. Moreover, it is difficult to ascertain the importance of a single immune cell type or to determine the time course of disease development with a pharmacological approach. To begin to address specific immune cell types in Dahl SS hypertension, we recently deleted the Rag1 gene in the SS rat; this resulted in a depletion of mature T- and B-cells and attenuated Dahl SS hypertension and renal damage.18 The present study permitted us to examine the influence of deletion of a gene associated with human hypertension and to determine the influence of selective T-cell depletion in the Dahl SS since previous studies demonstrated a central role of T-cells in AngII-induced hypertension in mice.21 These experiments demonstrate that selective depletion of CD3+ cells blunts Dahl SS hypertension and renal damage, indicating a central role of T-cells in the disease response. Considering the significant increase in inflammatory gene expression in infiltrating T cells in the kidneys of Dahl SS, the absence of functionality of CD247 mutant T cells suggests an important role for T cell signaling in the development of Dahl SS hypertension and renal disease.
Despite the attenuation of salt-sensitive hypertension, the CD247 mutant rats still developed a significant elevation of arterial blood pressure, albuminuria, and renal damage when dietary NaCl intake was increased. Moreover, despite the large differences in blood pressure and renal damage in the rats fed high NaCl, arterial blood pressure and albumin excretion values were not significantly different between the groups on the low NaCl diet. These data indicate that the infiltration of T cells into the kidney amplifies salt-sensitive hypertension in Dahl SS rats; the significant elevation in blood pressure and renal damage that develops in the CD247 mutants is apparently due to effects independent of T-cells.
The development of hypertension in the Dahl SS rat follows a pattern in which there is an initial increase in MAP during days 1-5 of high salt and then a secondary phase which commences after approximately 10 days of high salt.14,18 The present data indicate that the magnitude of this secondary phase of hypertension in the Dahl SS is significantly attenuated and the initial increase in MAP tended to be lower in the CD247−/− rats. We previously reported a significant blunting of the hypertension throughout the high salt period in Dahl SS rats lacking both T- and B-cells.18 The immune system therefore appears capable of altering the salt-sensitive hypertensive response throughout the period of elevated NaCl intake. The exact mechanisms mediating the hypertensive response are unknown. The present study focused upon cells infiltrating the kidney; we interpret the present data to indicate that the renal effects of T-cells are mediating the disease phenotype. It is important to note that other potential effects of T-cells to alter vascular reactivity, sympathetic outflow, storage of sodium in the skin, or effects in other organs could be participating in the observed phenotypic responses. The vascular sensitivity to AngII or other vasoactive molecules in these rats is unknown.
The present experimental approach utilized a zinc-finger nuclease strategy to mutate CD247. This approach, which has been documented for a number of other genes,18,24,25 led to an 11 base pair frameshift deletion in exon 1 of CD247 and resulted in a predicted stop codon downstream of the mutation. Phenotyping experiments demonstrated a loss of immunoreactive CD247 protein in the thymus of the mutant rats and a marked reduction in CD3+ cells in the circulation. The reduction of total lymphocytes and CD3+ cells is consistent with observations in a patient with somatic mutations in CD2478 and also in a knockout mouse model in which the CD3 zeta chain was deleted by homologous recombination.7,26 Though the CD247 mutants have almost no CD3+ cells, the CD247 mutant animals retained a reduced number of circulating CD3-/CD5+ cells compared to CD3+/CD5+ cells in the WT. Proliferation studies of CD5+ splenocytes from both strains demonstrated that CD5+ cells in CD247 mutant animals did not respond to nonspecific activation, indicating the importance of CD247 in T-cell proliferation and activation. Since the infiltrating cells in the kidney are activated relative to circulating T cells, these results indicate that interference with T-cell activation is responsible for the attenuation of the salt-sensitive disease in the Dahl SS.
PERSPECTIVES
Based upon these data, we speculate that the initial increase in arterial blood pressure upon ingestion of high salt in Dahl SS rats leads to a cellular immune response and the infiltration of activated T lymphocytes in the renal interstitium. The infiltrating T cells, which are localized around blood vessels, glomeruli, and renal tubules,14,15,17 can participate in the formation of cytokines, free radicals,15 and AngII.14 The release of these or other factors can presumably alter renal tubular and vascular function leading to the further retention of sodium and amplification of hypertension and renal damage. Since renal infiltration of immune cells has also been observed in hypertensive patients,12,27 we speculate that CD247 participates in human disease by mechanisms similar to those described in the Dahl SS rat.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is New?
Deletion of CD247 in the Dahl SS genetic background resulted in an almost complete loss of circulating CD3+ T-lymphocytes, a reduction in infiltration of T–lymphocytes into the kidney, and an attenuation of salt-sensitive hypertension and renal damage.
What is Relevant?
A genetic variant in CD247 is associated with blood pressure in hypertensive patients. A potential link between CD247, a gene involved in T cell signaling, and hypertension, is provided by a large number of studies that have implicated the immune system in experimental hypertension and renal disease in rats and mice. The present studies demonstrate the important role of CD247 in salt-sensitive hypertension in rats.
Summary
The CD3 zeta chain (CD247), a gene involved in T cell signaling, has been demonstrated to associate with hypertension in human genetic studies. To test the functional role of the CD3 zeta chain in hypertension and renal disease, CD247 was deleted in the Dahl SS rat using a zinc finger nuclease-mediated approach. Deletion of CD247 in the Dahl SS genetic background resulted in an almost complete loss of CD3+ T-lymphocytes in affected rats. Compared to control animals, Dahl SS rats lacking CD247 exhibited reduced renal infiltration of T–lymphocytes and an attenuation of salt-sensitive hypertension and renal damage. Since renal infiltration of immune cells has also been observed in hypertensive patients, we speculate that CD247 participates in human disease by mechanisms similar to those described in the Dahl SS rat.
SOURCES OF FUNDING
This work was partially supported by National Institutes of Health Grants DK-96859, HL116264, and RC2-HL101681.
Footnotes
DISCLOSURES/CONFLICT OF INTEREST
Sigma and MCW have a license agreement that could send royalties based on rat sales to MCW.
REFERENCES
- 1.National Human Genome Research Institute [accessed 4-25-2013];A Catalog of Published Genome-Wide Association Studies. http://www.genome.gov/gwastudies/
- 2.Cowley AW., Jr. The genetic dissection of essential hypertension. Nature Reviews Genetics. 2006;7:829–840. doi: 10.1038/nrg1967. [DOI] [PubMed] [Google Scholar]
- 3.Ehret GB, O’Connor AA, Weder A, Cooper RS, Chakravarti A. Follow-up of a major linkage peak on chromosome 1 reveals suggestive QTLs associated with essential hypertension: GenNet study. Eur J Hum Genet. 2009;17:1650–1657. doi: 10.1038/ejhg.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irving BA, Chan AC, Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor zeta chain. J Exp Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh Y, Matsuura A, Kinebuchi M, Honda R, Takayama S, Ichimiya S, Kon S, Kikuchi K. Structural analysis of the CD3 zeta/eta locus of the rat. Expression of zeta but not eta transcripts by rat T cells. Journal of Immunology. 1993;151:4705–4717. [PubMed] [Google Scholar]
- 6.Sussman JJ, Bonifacino JS, Lippincott-Schwartz J, Weissman AM, Saito T, Klausner RD, Ashwell JD. Failure to synthesize the T Cell CD3-ζ chain: Structure and function of a partial T cell receptor complex. Cell. 1988;52:85–96. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- 7.Malissen N, Gillet A, Rocha B, Trucy J, Vivier E, Boyer C, Köntgen F, Brun N, Mazza G, Spanopoulou E, Guy-Grand D, Malissen B. T cell development in mice lacking the CD3-zeta/eta gene. EMBO J. 1993;12:4347–4355. doi: 10.1002/j.1460-2075.1993.tb06119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rieux-Laucat F, Hivroz C, Lim A, Mateo V, Pellier I, Selz F, Fischer A, Le Deist F. Inherited and Somatic CD3ζ Mutations in a Patient with T-Cell Deficiency. N Engl J Med. 2006;354:1913–1921. doi: 10.1056/NEJMoa053750. [DOI] [PubMed] [Google Scholar]
- 9.Radstake TR, Gorlova O, Rueda B, Martin JE, Alizadeh BZ, Palomino-Morales R, Coenen MJ, Vonk MC, Voskuyl AE, Schuerwegh AJ, Broen JC, van Riel PL, van ‘t Slot R, Italiaander A, Ophoff RA, Riemekasten G, Hunzelmann N, Simeon CP, Ortego-Centeno N, González-Gay MA, González-Escribano MF, Spanish Scleroderma Group. Airo P, van Laar J, Herrick A, Worthington J, Hesselstrand R, Smith V, de Keyser F, Houssiau F, Chee MM, Madhok R, Shiels P, Westhovens R, Kreuter A, Kiener H, de Baere E, Witte T, Padykov L, Klareskog L, Beretta L, Scorza R, Lie BA, Hoffmann-Vold AM, Carreira P, Varga J, Hinchcliff M, Gregersen PK, Lee AT, Ying J, Han Y, Weng SF, Amos CI, Wigley FM, Hummers L, Nelson JL, Agarwal SK, Assassi S, Gourh P, Tan FK, Koeleman BP, Arnett FC, Martin J, Mayes MD. Genome-wide association study of systemic sclerosis identifies CD247 as a new susceptibility locus. Nat Genet. 2010;42:426–429. doi: 10.1038/ng.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhernakova A, Stahl EA, Trynka G, Raychaudhuri S, Festen EA, Franke L, Westra H-J, Fehrmann RSN, Kurreeman FAS, Thomson B, Gupta N, Romanos J, McManus R, Ryan AW, Turner G, Brouwer E, Posthumus MD, Remmers EF, Tucci F, Toes R, Grandone E, Mazzilli MC, Rybak A, Cukrowska B, Coenen MJH, Radstake TRDJ, van Riel PLCM, Li Y, de Bakker PIW, Gregersen PK, Worthington J, Siminovitch KA, Klareskog L, Huizinga TWJ, Wijmenga C, Plenge RM. Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci. PLoS Genetics. 2011;7:e1002004. doi: 10.1371/journal.pgen.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, Immunity, and Hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughson MD, Gobe GC, Hoy WE, Manning RD, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and Whites. Am J Kid Dis. 2008;52:18–28. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2011;10:1440–1681. doi: 10.1111/j.1440-1681.2011.05482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Miguel C, Das S, Lund H, Mattson DL. T-lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol. 2010;298:R1136–R1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Miguel C, Lund H, Di F, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and lead to hypertension and renal disease. Am J Physiol. 2011;300:F734–742. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 17.De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl salt-sensitive (SS) rats by increasing infiltrating immune cells. Hypertension. 2011;57:269–274. doi: 10.1161/HYPERTENSIONAHA.110.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt sensitive rats attenuates hypertension and renal damage. Am J Physiol. 2013;304:R407–R414. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 6th ed. Saunders Elsevier; 2007. [Google Scholar]
- 20.Franco M, Martinez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodriguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol. 2007;293:R251–R256. doi: 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 21.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrera J, Ferrebuz A, García MacGregor E, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:218–225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 23.Muller DN, Shagdarsuren E, Park J-K, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against Angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geurts AM, Cost GJ, Remy S, Cui X, Tesson L, Usal C, Menoret S, Jacob HJ, Anegon I, Buelow R. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol Biol. 2010;597:211–225. doi: 10.1007/978-1-60327-389-3_15. [DOI] [PubMed] [Google Scholar]
- 25.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Ménoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;24:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohno H, Aoe T, Taki S, Kitamura D, Ishida Y, Rajewsky K, Saito T. Developmental and functional impairment of T cells in mice lacking CD3 zeta chains. EMBO J. 1993;12:4357–4366. doi: 10.1002/j.1460-2075.1993.tb06120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol. 1958;34:685–715. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.