Abstract
Development of angiotensin II (Ang II)-dependent hypertension involves microglial activation and proinflammatory cytokine actions in the hypothalamic paraventricular nucleus (PVN). Cytokines activate receptor signaling pathways that can both acutely grade neuronal discharge and trigger long-term adaptive changes that modulate neuronal excitability through gene transcription. Here, we investigated contributions of PVN cytokines to maintenance of hypertension induced by infusion of Ang II (150 ng/kg/min, SC) for 14 days in rats consuming a 2% NaCl diet. Results indicate that bilateral PVN inhibition with the GABA-A receptor agonist muscimol (100 pmol/50 nL) caused significantly greater reductions of renal and splanchnic sympathetic nerve activity (SNA) and mean arterial pressure (MAP) in hypertensive than normotensive rats (P<0.01). Thus ongoing PVN neuronal activity appears required for support of hypertension. Next, the role of the prototypical cytokine tumor necrosis factor alpha (TNF-α) was investigated. Whereas PVN injection of TNF-α (0.3 pmol/50 nL) acutely increased lumbar and splanchnic SNA and MAP, interfering with endogenous TNF-α by injection of etanercept (10 µg/50 nL) was without effect in hypertensive and normotensive rats. We next determined that although microglial activation in PVN was increased in hypertensive rats, bilateral injections of minocycline (0.5 µg/50 nL), an inhibitor of microglial activation, failed to reduce lumbar or splanchnic SNA or MAP in hypertensive or normotensive rats. Collectively, these findings indicate that established Ang II-salt hypertension is supported by PVN neuronal activity, but short term maintenance of SNA and ABP does not depend on ongoing local actions of TNF-α.
Keywords: inflammation, cytokines, sympathetic nerve activity, blood pressure
Introduction
Low dose systemic infusion of angiotensin II (Ang II) combined with a high salt (2% NaCl) diet leads to development of neurogenic hypertension maintained by sympathetic nerve activity (SNA)1–3. Circulating Ang II and elevated plasma sodium are known to act at forebrain circumventricular organs to recruit sympathoexcitatory neurons of the hypothalamic paraventricular nucleus (PVN)4–6 and their downstream targets in autonomic control areas in the brainstem and spinal cord7, 8.
Cellular mechanisms through which systemic Ang II and high salt intake elevate SNA and arterial blood pressure (ABP) have not been fully elucidated. Available evidence indicates that centrally acting Ang II promotes development of hypertension and other cardiovascular diseases4, 9, 10 through induction of pro-inflammatory cytokines (PICs) in the PVN11–13. Of particular importance for the present study is that PICs have two well established modes of action14–18. Activation of PIC receptors and their downstream signaling cascades can acutely and chronically enhance neuronal activity by modulation of ion channel gating and by transcriptional regulation of gene expression, respectively14–18.
Tumor necrosis factor alpha (TNF-α), a PIC that is elevated in the PVN of rats with Ang II hypertension12, 19, can acutely increase neuronal activity through mechanisms that include stimulation of L-glutamate release20 and positive modulation of voltage-gated sodium channels16. In addition, TNF-α driven nuclear factor kappa B (Nf-κB) signaling, can transcriptionally modify expression of numerous genes that lead, in the longer term, to enhanced neuronal activity/excitability14, 17, 21.
To date, studies have directly interfered with PVN expression of PICs19 and have indirectly interfered with PIC induction by activated microglia via minocycline12. Both approaches were shown to abrogate the development of Ang II dependent hypertension12, 19. However, it remains unclear if anti-hypertensive effects are attributable to interruption of acute PIC actions that continuously drive neuronal discharge or to interruption of a critical triggering phase of PIC-induced transcriptional adaptation that is required in order for hypertension to develop. The present study sought to differentiate between these possibilities. We focused on TNF-α as a prototypical PIC and tested the hypothesis that acute blockade of TNF-α in PVN and acute PVN delivery of minocycline would each reduce SNA and ABP more in rats with established Ang II-salt hypertension than normotensive controls.
Materials and Methods
Animals
Male Sprague-Dawley rats (225–250 g, Charles River Laboratory, Wilmington, MA) were housed in a temperature controlled room (22–23°C) with a 14:10 hour light-dark cycle. Tap water and laboratory chow were available ad libitum except where otherwise noted. All surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Induction of Ang II-Salt Hypertension
Rats in the normotensive (NT) group consumed a normal salt diet (0.4% NaCl), and rats in the hypertensive (HT) group were placed on a high salt diet (2% NaCl). Diets were otherwise identical in calories from fat, protein, and carbohydrates (Research Diets, New Brunswick, NJ). Radio telemetry was used to record ABP and monitor the development of Ang II-salt hypertension in conscious rats as described previously9, 22. Ang II or saline was infused via osmotic mini-pump for 14 days in the HT and NT groups, respectively, prior to performing microinjection studies.
Experimental Preparation
On the day of experiments, rats were anesthetized with an intraperitoneal injection of a mixture of urethane (750 mg/kg) and α-chloralose (75 mg/kg). Catheters (PE-50 tubing) were implanted in a femoral artery and vein for recording ABP and administration of drugs, respectively. Because the role of PVN cytokines on various regional sympathetic outflows in Ang II-salt hypertension has not been previously investigated, rats in the present study rats were prepared for recording of renal (RSNA), splanchnic (SSNA), or lumbar (LSNA) SNA as previously described by our laboratory23–25. Animals were artificially ventilated with oxygen-enriched room air, paralyzed with gallamine triethiodide (20 mg/mL, 0.25 mL/h, IV) and end tidal CO2 was monitored and maintained between 4–5%. An adequate depth of anesthesia was determined by lack of a limb withdrawal reflex to noxious pinching of the foot prior to paralysis. Thereafter, adequacy of anesthesia was determined by lack of a pressor or sympathoexcitatory response to noxious foot pinch. Supplemental anesthesia (10% of initial dose) was given as needed. Body temperature was maintained at 37±1°C. Recorded variables were allowed to stabilize for ~1 h after surgery before an experiment began.
Contribution of PVN Neuronal Activity to Maintenance of Ang II-Salt Hypertension
PVN microinjections were performed as previously described24, 25. Unless otherwise noted, only the specified compound was microinjected into the PVN of each animal. To determine the contribution of PVN neuronal activity to maintenance of established Ang II-salt hypertension, NT (n=7) and HT (n=7–14) rats were prepared as described above. Following a 10 min baseline period, the GABA-A receptor agonist muscimol (100 pmol/50 nL) or vehicle aCSF (50 nL) was bilaterally microinjected into PVN. Variables were recorded for an additional 30 minutes.
Involvement of TNF-α in PVN Maintenance of Ang II-Salt Hypertension
Experiments were performed to determine doses of the TNF-α antibody etanercept and the microglial activation inhibitor minocycline that blocked responses to TNF-α. Following a 10 minute baseline recording of LSNA, SSNA, ABP and HR, TNF-α (0.3 pmol/50nL) or aCSF (50 nL) was bilaterally microinjected into PVN to elicit a sympathoexcitatory response. In separate groups of rats (n=5/group), either etanercept (10 µg/50 µL) or minocycline (0.5 µg/50 nL) was microinjected into PVN and responses to TNF-α were tested again 5 minutes later.
To determine effects of etanercept and minocycline in PVN on maintenance of SNA and elevated ABP in Ang II-salt HT rats, separate groups of HT and NT rats were prepared as described above. Following a 10 minute baseline period, the TNF-α antibody etanercept or the inhibitor of microglial activation minocycline was bilaterally microinjected into PVN.
Histological Verification of Microglial Activation in PVN
A separate group of rats was deeply anesthetized (5% isoflurane) 14 days after the start of Ang II (HT, n=4) or saline (NT, n=4) infusion and perfused transcardially with 200 mL of 0.1 M phosphate-buffered saline (PBS) followed by 200 mL of 4% paraformaldehyde (PFA) in 0.1 M PBS. As a positive control for microglial activation, naïve rats (n=6) received unilateral PVN microinjections of lipopolysaccharide (LPS, 10 mg/1 mL, 200 nL). 2 h prior to undergoing transcardiac perfusion as described above fixed brain tissue that included the PVN was sectioned at 30 µM and three serial sets of sections were placed in vials of cryoprotectant and stored at −20°C.
For microglial immunostaining, sections were incubated with a monoclonal mouse anti-rat OX-42 primary antibody (1:100) and a biotinylated goat anti-mouse secondary antibody (1:200) (Vector Laboratories) (see data supplement online at http://hyper.ahajournals.org for detailed methods).
PVN sections were examined using an Olympus IX50 microscope. Images were captured with a Spot digital camera (Diagnostic Instruments, Inc.). ImageJ software (http://rsbweb.nih.gov/ij) was used to quantify staining density.
Data Analysis
Summary data are expressed as mean ± SEM. Responses of integrated SNA are expressed either as a percent of baseline or percent change from baseline as indicated in specific figures. In all cased responses were quantified after subtraction of background noise, which was determined as the signal remaining 5–10 minutes after treatment with the ganglionic blocker hexamethonium (30 mg/kg, IV). For all variables, the average value of a 2 minute data segment was obtained at baseline and compared to a similar average obtained 10, 20 and 30 minutes after each PVN microinjection. Data were analyzed by 1- or 2-way ANOVA, with repeated measures as appropriate. Post hoc tests were performed with independent or paired t tests, with a layered Bonferroni correction. P<0.05 was deemed statistically significant.
Results
Ang II-Salt Hypertension
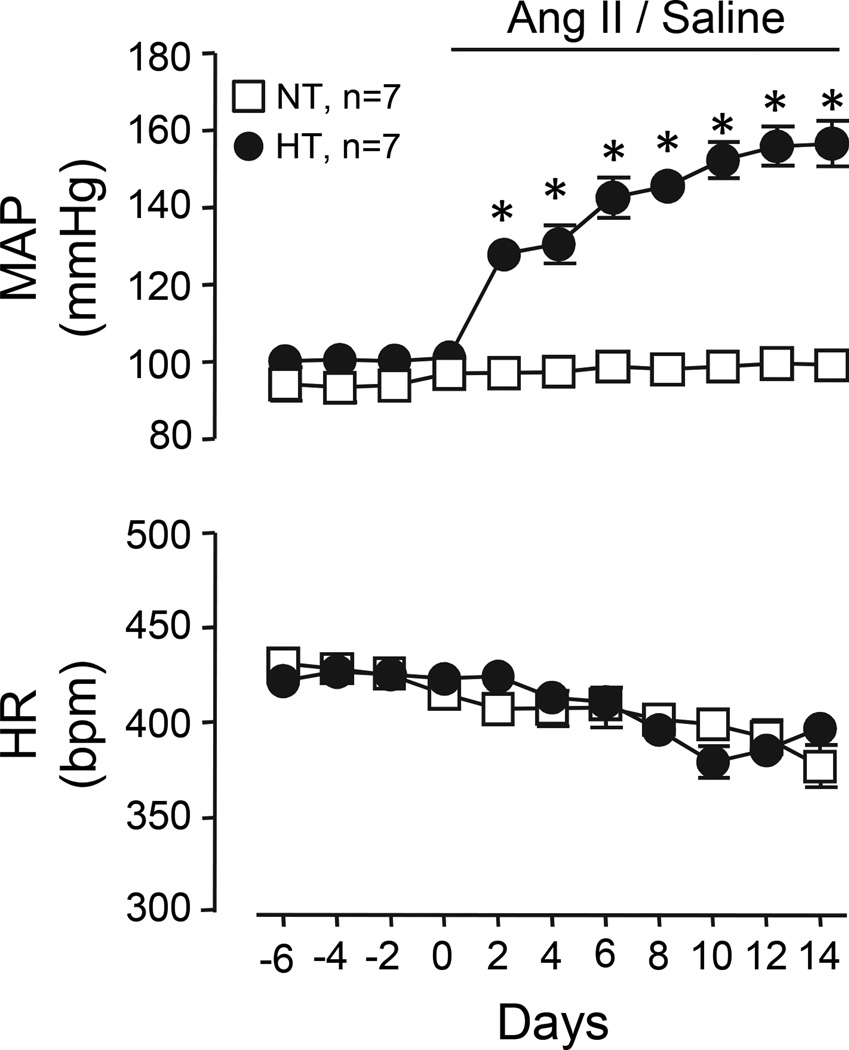
Telemetric recordings from conscious Ang II-salt HT and NT rats (n=7/group) revealed that mean arterial pressure (MAP) was increased by day 2 of Ang II infusion and remained significantly elevated throughout the remainder of the 14 day infusion period compared to NT rats (P<0.05). Throughout the infusion, HR did not differ between groups. Summary data are shown in Figure 1. During acute experiments under anesthesia, MAP was reduced in HT rats (113±3 mmHg), but remained significantly elevated compared to NT (97±2 mmHg) controls (total ANOVA n=19, P<0.0001).
Figure 1.
Summary data of MAP and HR in NT and Ang II-salt HT rats at baseline and during 14 days of Ang II or saline infusion. Baseline MAP and HR were similar across groups despite HT rats consuming a high salt (2% NaCl) diet. Within 2 days of Ang II infusion, MAP increased significantly and remained elevated throughout the infusion period. HR did not change in either group throughout the infusion. *P<0.05 vs. NT.
PVN Neuronal Activity Maintains Ang II-Salt Hypertension
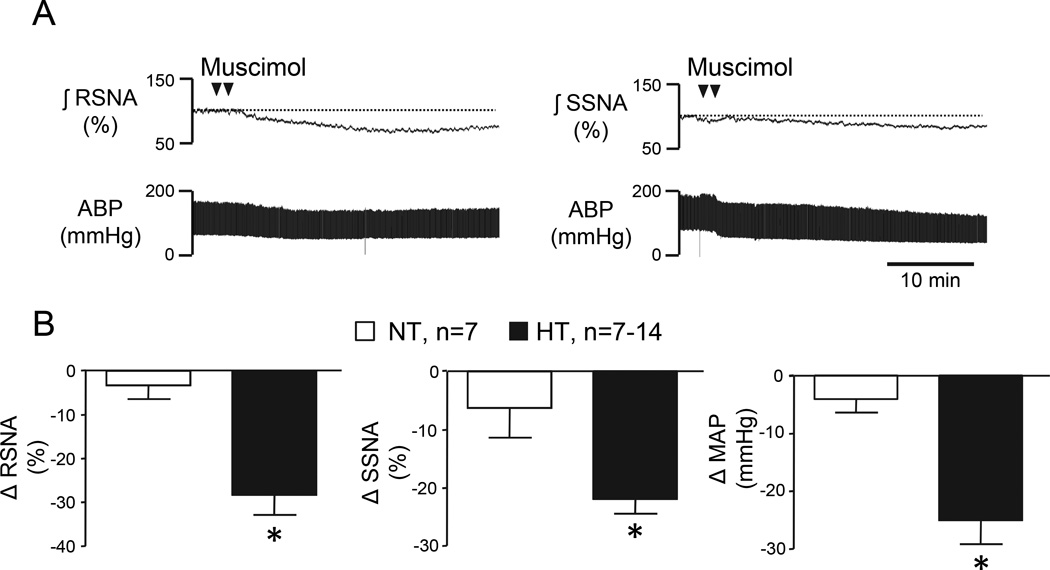
A major goal of this study was to determine the contribution of PVN neuronal activity to maintenance of SNA and elevated MAP in Ang II-salt HT rats. Figure 2A shows representative responses to bilateral PVN microinjections of the GABA-A receptor agonist muscimol in separate Ang II-salt HT rats with RNSA (left) or SSNA (right) recording. Figure 2B shows summary data of peak reductions of RSNA (left, n=7), SSNA (center, n=7), and MAP (right, n=14) among HT and NT rats. Note that muscimol significantly reduced RSNA, SSNA, and MAP in HT rats (P<0.05), but was without affect in NT controls.
Figure 2.
(A) Representative examples of RSNA (left), SSNA (right), and ABP responses during PVN microinjection of muscimol in separate HT rats. (B) Peak changes in RSNA, SSNA, and MAP after bilateral microinjection of muscimol into the PVN of NT and HT. Note that PVN muscimol caused significantly greater reductions of RSNA, SSNA, and MAP in HT than NT rats. *P<0.05 vs. NT.
TNF-α in PVN Increases SNA but TNF-α Inhibition Does Not Change SNA or MAP in Ang II-salt HT Rats
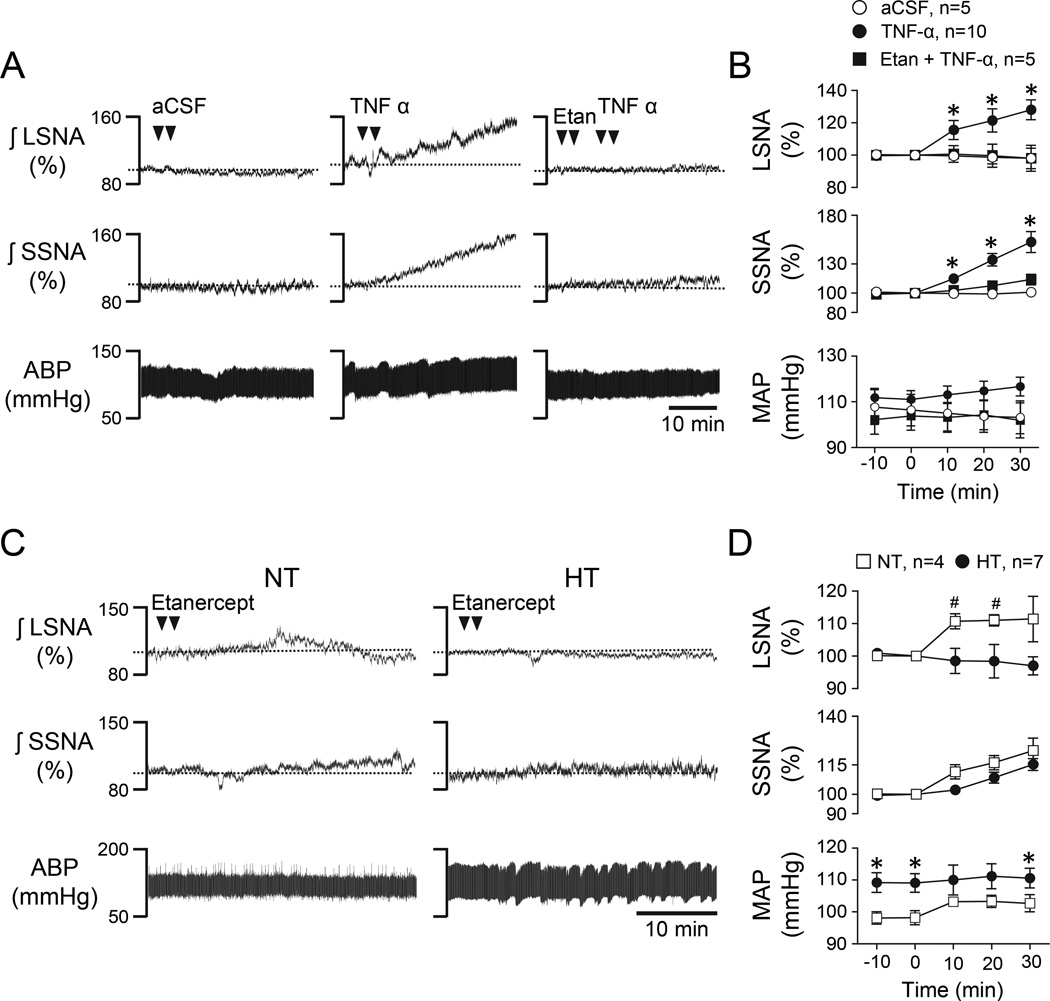
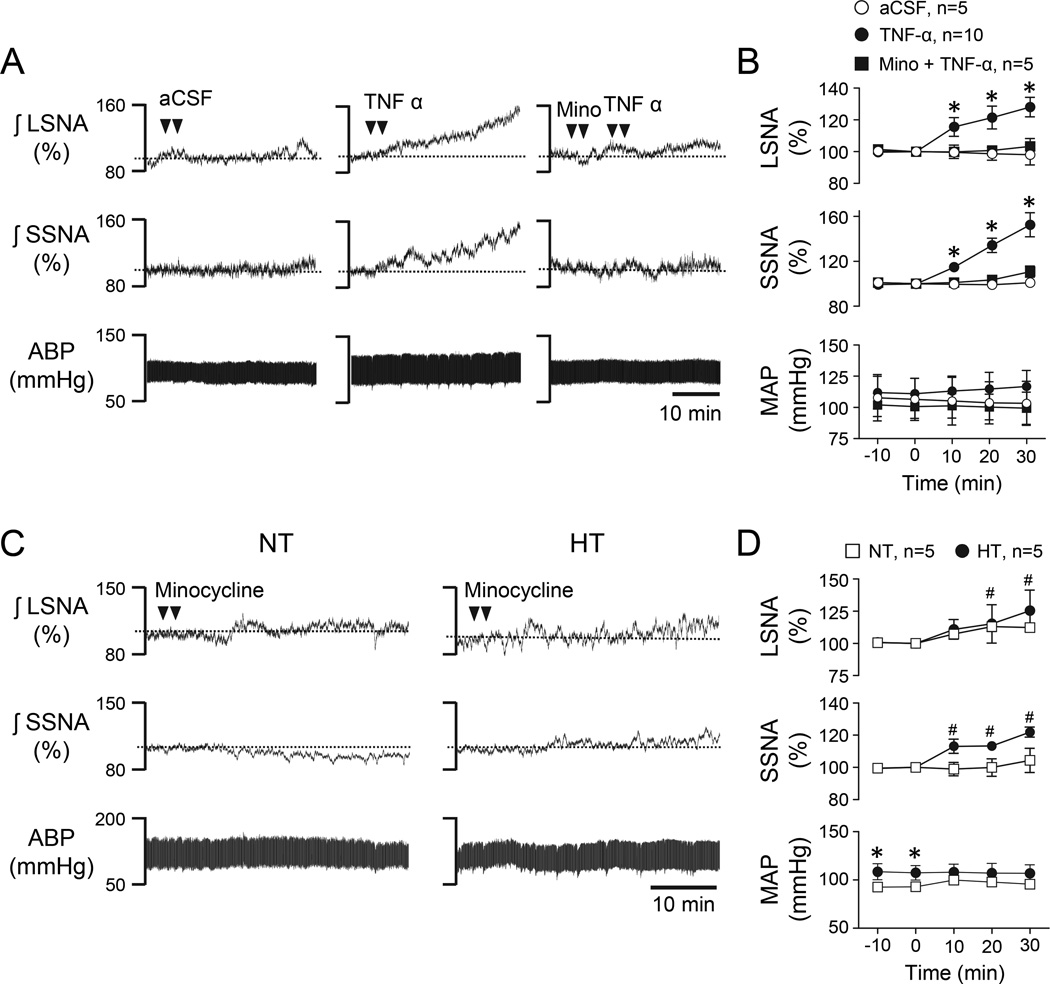
Initial experiments were performed to assess the SNA response to PVN-microinjected TNF-α and to determine a dose of the TNF-α antibody etanercept that was able to prevent the response. Figure 3A shows representative responses to PVN microinjection of aCSF vehicle (left), TNF-α alone (center), and etanercept followed by TNF-α (right) in an NT rat. Group data are summarized in Figure 3B and indicate that TNF-α significantly increased LSNA and SSNA compared to aCSF over 30 minutes (P<0.05) and this response was prevented by prior treatment with etanercept.
Figure 3.
(A) Representative LSNA, SSNA, and ABP responses to PVN microinjection of aCSF (left), TNF-α alone (center), and etanercept followed by TNF-α (right). (B) Corresponding summary data. Note that prior injection of etanercept significantly blunted sympathoexcitatory responses to TNF-α. *P<0.05 vs aCSF. (C) Representative LSNA, SSNA, and ABP responses to PVN microinjection of etanercept alone in an NT (left) and an HT (right) rat. (D) Corresponding summary data. *P<0.05 vs. NT, #P<0.05 vs baseline.
Another goal of this study was to determine the role of endogenous TNF-α in PVN in maintaining Ang II-salt hypertension. Figure 3C shows representative responses of an NT (left) and an HT (right) rat to PVN microinjection of the same dose of etanercept that blocked SNA responses to PVN TNF-α (see Figure 3A). Summary data in Figure 3D reveal that PVN etanercept did not significantly alter LSNA, SSNA, or MAP among HT rats but slightly increased LSNA in NT rats.
Microglial Activation in Ang II-Salt Hypertensive Rats
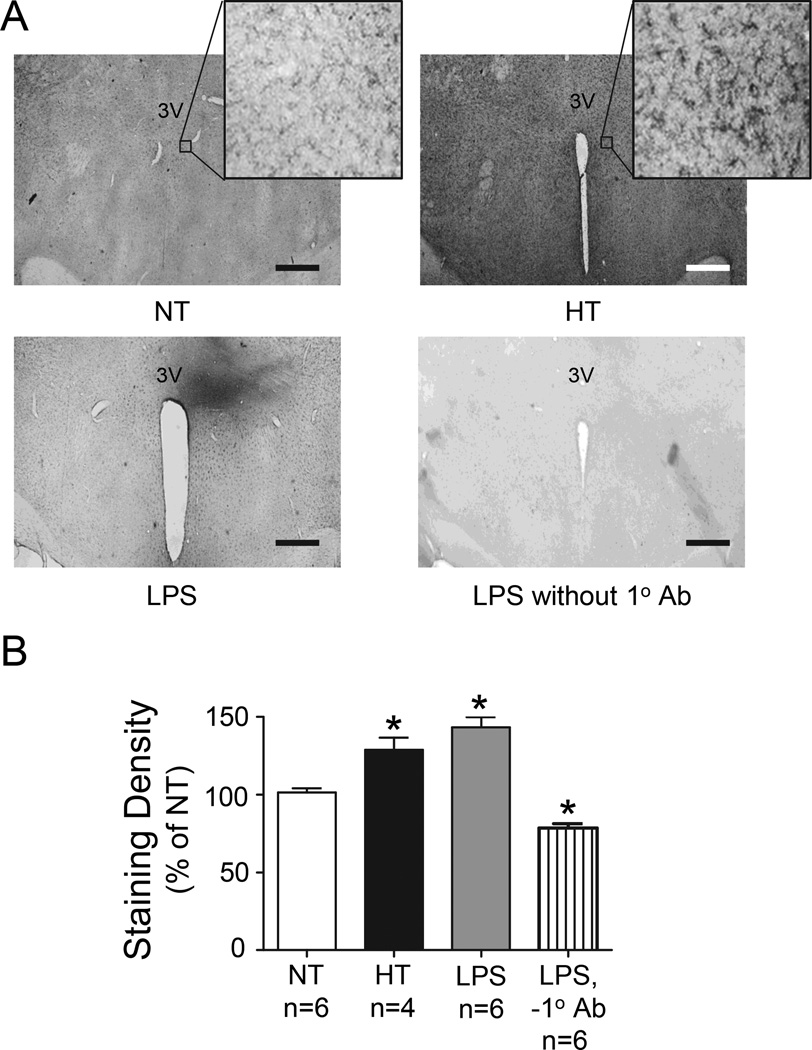
Given that selective inhibition of endogenous TNF-α had no effect on SNA or MAP in Ang II-salt HT rats and previous reports that Ang II-dependent hypertension elevates PVN microglial activation12, we sought to confirm that PVN microglia were in fact activated in our HT rats. Figure 4A shows representative PVN photomicrographs of OX-42 staining from an NT and HT rat as well as from control rats injected with LPS in PVN with (bottom left) and without (bottom right) inclusion of 1° antibody. A comparison of staining density across groups is shown in Figure 4B. Both Ang II + salt treatment and LPS microinjection significantly increased OX-42 staining above that of NT controls. Of note, staining was not localized to the area of PVN but was elevated in HT rats throughout the hypothalamus, including the PVN. Unilateral microinjection of LPS caused localized staining. There was no significant difference in density between HT and LPS positive controls. As expected, PVN sections processed without 1° antibody showed only minimal background staining that was significantly below that of NT controls.
Figure 4.
(A) Digital photomicrographs of OX-42 staining of PVN sections from an NT (left, top) and an HT rat (right, top). Also shown is a section from a positive control rat in which LPS was injected unilaterally into PVN (left, bottom) and a control for non-specific staining (no 1° Ab, right, bottom). Scale bar = 25 µM. Note: Higher magnification (400x) images in NT and HT panels show characteristic morphology of activated microglia. (B) Quantification of relative OX-42 staining across NT, HT, PVN LPS and PVN LPS without 1° Ab. *P<0.05 vs. NT. 3V, third ventricle.
Minocycline in PVN Does Not Effect SNA or MAP in Ang II-Salt HT Rats
To investigate the role of microglial in PVN support of SNA and MAP in HT rats, experiments first determined a dose of PVN minocycline that abrogated the sympathoexcitatory response to TNF-α. Figure 5A shows representative responses to PVN microinjected aCSF (left), TNF-α alone (center), and minocycline followed by TNF-α (right). Note that similar to studies with etanercept (see Figure 3A), TNF-α alone increased LSNA and SSNA compared to aCSF and this response was blocked by prior treatment with minocycline. Summary data are shown in Figure 5B. Next, minocycline alone was microinjected bilaterally into PVN of NT and HT rats. Figure 5C shows representative responses to minocycline microinjected into PVN of an NT (left) and an HT (right) rat. Group data are summarized in Figure 5D. Minocycline did not significantly change LSNA, SSNA, or MAP over 30 min in HT compared to NT rats. It did increase LSNA in NT rats and SSNA in HT rats from baseline.
Figure 5.
(A) Representative LSNA, SSNA, and ABP responses to PVN microinjection of aCSF (left), TNF-α alone (center), and minocycline followed by TNF-α (right). (B) Corresponding summary data. Note that minocycline significantly blunted the sympathoexcitatory responses to TNF-α. *P<0.05 vs. aCSF. (C) Representative LSNA, SSNA, and ABP responses to PVN microinjection of minocycline alone in an NT (left) and an HT (right) rat. (D) Corresponding summary data. *P<0.05 vs. NT, #P<0.05 vs baseline.
Histology
Injection sites marked with rhodamine microspheres were confined to an area encompassing the PVN as previously described by our laboratory24 (Figure 1S).
Discussion
Chronic infusion of Ang II in rats consuming a high salt diet elevates whole body norepinephrine spillover2 and results in neurogenic hypertension1, 4, 9. Brain regions contributing to the development of Ang II-salt hypertension include the subfornical organ (SFO)26 and median preoptic area27 of the forebrain. Here we show that ongoing PVN neuronal activity is also necessary to maintain SNA and elevated ABP in anesthetized rats with established Ang II-salt hypertension. Furthermore, although cytokines in PVN have been reported to contribute to specific cardiovascular disease models28, 29, we demonstrate here that although TNF-α delivered into PVN promptly increases SNA, acute blockade of local TNF-α actions did not acutely reduce SNA or elevated MAP. And although microglial activation was significantly increased in the PVN of Ang II-salt hypertensive rats, PVN injection of minocycline, an inhibitor of microglial activation that blocked acute sympathoexcitatory responses to TNF-α, also did not change ongoing levels of SNA or MAP. It should be emphasized that although PVN minocycline blocked acute sympathoexcitatory responses to PVN injection of TNF-α, it may not have effectively reversed microglial activation within the time course of these experiments. Taken together with literature evidence12, 19, our findings suggest that involvement of PVN cytokines in neurogenic Ang II-salt hypertension is likely attributable to their capacity to induce stable transcriptionally driven adaptive responses that enhance neuronal excitability and/or efficacy of excitatory synaptic transmission rather than their acute signaling mechanisms that directly regulate neuronal activity. An important caveat to this interpretation is that experiments were performed under anesthesia, which reduced the magnitude of the hypertension. Consequently, it is possible that failure to observe an acute effect of etanercept or minocycline in PVN could reflect anesthesia-induced blunting of neuronal responsiveness to cytokines.
A number of diseases and physiological challenges drive sympathetic outflow by mechanisms that involve PVN neuronal activation. These include heart failure30, essential hypertension31, chronic intermittent hypoxia25, and water deprivation24. Sympathetic regulatory PVN neurons receive mono- and poly-synaptic input from forebrain regions that include the organum vasculosum of the lamina terminalis (OVLT) and SFO, which detect changes in body fluid osmolality, as well as circulating Ang II5, 6, 32, 33. Here we provide the first evidence that acute inhibition of PVN by microinjection of muscimol reduces SNA and MAP in rats with established Ang II-salt hypertension. One caveat is the possibility that drug microinjections spread outside of PVN. However, this is unlikely because of the small volume (50 nL) used and evidence from dye diffusion experiments which show that a volume as large as 100 nL does not spread appreciably beyond the lateral most boundaries of PVN34.
When taken together with recent reports5, 26, 27, the present findings begin to elucidate key components of a possible pathway supporting the neurogenic component of Ang II-salt hypertension. Elevated Ang II and salt activate neurons in the SFO and OVLT which lack a complete blood brain barrier. Activating a projection from MnPO to PVN35 in turn activates sympathetic PVN neurons projecting to the RVLM and intermediolateral cell column thereby elevating SNA and MAP36, 37.
Although crosstalk exists between Ang II and cytokines, little is known about the specific role of TNF-α in linking Ang II treatment with neuronal activation. A multifunctional cytokine, TNF-α plays key roles in inflammation, cell growth, differentiation, and apoptosis38. Interestingly, TNF-α knockout mice do not develop Ang II-dependent hypertension39. Moreover, treatments such as intracerebroventricular minocycline or etanercept that preclude the increase of central TNF-α also prevent the hypertensive response to chronic Ang II infusion12, 19. While studies provide evidence to support the concept that central TNF-α plays a critical role in the development of hypertension, experiments have yet to establish a role for TNF-α in the sustained drive of PVN neurons and sympathetic outflow. Here we demonstrated that TNF-α microinjected directly into PVN elevates LSNA, SSNA, and MAP. This extends a recent study by Shi et al.40, which reported that TNF-α in PVN acutely increases RSNA and MAP.
Mechanisms through which TNF-α in the PVN elicits sympathoexcitation are not known, but TNF-α has been reported to stimulate release of L-glutamate in hippocampus20 and to increase NMDA receptor insertion into the plasma membrane41. Furthermore, TNF-α enhances current through voltage-gated sodium channels in dorsal root ganglion neurons16 and disinhibits GABAergic neurons42 while increasing spontaneous EPSC frequency and decreasing spontaneous IPSC frequency in spinal neurons43. In the present study, sympathoexcitation by PVN TNF-α was blocked by local delivery of either a TNF-α antibody (etanercept) or an inhibitor of microglial activation (minocycline). Minocycline directly inhibits activation of p38 mitogen activated protein kinase (MAPK)44, 45 and NFκB46, both major downstream signaling molecules activated by TNF-α47, 48. Additional studies are needed to investigate the mechanism(s) of action of TNF-α in PVN in order to determine whether excitatory or disinhibitory actions predominate and whether TNF-α alone is capable of increasing microglial activation.
In light of our findings that TNF-α in PVN increased LSNA and SSNA and our demonstration that microglial activation was increased in PVN of Ang II-salt rats, we anticipated that TNF-α blockade or minocycline would significantly decrease SNA and MAP in hypertensive rats. However, these effects were not observed. As noted above, a possible explanation is the effect of anesthesia. Although MAP of anesthetized HT rats was significantly elevated above that of NT controls, it was significantly decreased relative to the pre-anesthesia level. We consider this explanation unlikely given that muscimol significantly lowered SNA and ABP even under anesthesia. In specific regard to the lack of effect of minocycline, it is possible that our study did not allow sufficient time for reversal of microglial activation to occur. Additional studies are needed to determine the time course and mechanisms of minocycline-induced inhibition/reversal of microglial activation.
Upon initial inspection, our findings appear to conflict with those of Shi et al.12 and Sriramula et al.19, who reported that the developmental phase of Ang II-dependent hypertension was significantly attenuated by pre-treatment of rats with intracerebroventricular minocycline and etanercept, respectively. There are several possible explanations for these disparate results. First, PICs have been reported to initiate the onset of rheumatoid disease, but their role in maintenance is less clearly established49, 50. It is possible that their etiology in hypertension could be similar such that cytokine signaling is necessary for initiation/development of hypertension but perhaps not for its maintenance. It should be stressed that because we recorded SNA and ABP for only one hour after PVN microinjection of minocycline or etanercept we might have failed to detect slow onset effects to lower SNA and/or ABP51. It is also important to bear in mind that there are key differences in the design of the present and previous studies. Here, we investigated acute effects of etanercept and minocycline on SNA and ABP in hypertension. Whereas, previous studies used chronic ICV infusions of minocycline or etanercept throughout the 14 day Ang II infusion period to ascertain the role of TNF-α and microglial activation on hypertension development. Another possible confound is that in previous studies, all rats consumed a normal salt diet (0.3–0.4% NaCl), whereas in our study they consumed a high salt diet (2% NaCl) for 14 days before and throughout the 14 day Ang II infusion period. Possible pro-inflammatory interactions of Ang II and salt need further study to determine if a high salt diet precludes or abrogates the ability of TNF-α/microglial activation to drive increased PVN neuronal discharge.
Perspectives
Predicting which individuals will become clinically hypertensive is not currently possible. As a result, it is important to improve treatments for already established hypertension. Doing so, while minimizing unwanted side effects, requires identification and selective targeting of specific mechanisms that actively sustain the hypertension. The present study is an effort toward this goal. Previous studies have revealed a strong correlation between circulating Ang II, inflammatory signaling in the brain and elevated ABP11, 12, 19. However, the neural circuitry impacted by inflammation that actively maintains neurogenic hypertension has not been fully elucidated. Here, we provide evidence that neuronal activity in the hypothalamic PVN is required for support of SNA and ABP in Ang II-salt hypertensive rats. Although exogenous TNF-α in PVN acutely increased SNA, blockade of endogenous TNF-α in PVN did not acutely lower ongoing SNA or ABP in hypertensive rats. Likewise, although microglial activation in PVN is elevated in Ang II-salt hypertensive rats, ongoing SNA and ABP were unaffected by PVN injection of minocycline, a prototypical inhibitor of microglial activation. So while the present results highlight the importance of PVN in the sympathoexcitatory circuit that supports established Ang II-salt hypertension, additional studies are needed to fully elucidate neural mechanisms that underlie cytokine and microglia involvement in neurogenic hypertension.
Supplementary Material
Novelty and Significance.
1. What is new?
-
➢
PVN neuronal activity maintains ongoing SNA and elevated MAP in Ang II-salt hypertensive rats.
-
➢
TNF-α microinjected into PVN increases lumbar SNA, splanchnic SNA, and MAP.
-
➢
Maintenance of SNA and elevated MAP in Ang II-salt hypertensive rats does not depend on actions of TNF-α in the PVN.
2. What is relevant?
-
➢
Consumption of a high salt (2% NaCl) diet together with systemic infusion of Ang II results in sustained hypertension that is largely reversed by inhibition of PVN neuronal activity.
-
➢
Although Ang II dependent hypertension is associated with systemic and central inflammation, acute inhibition of TNF-α in PVN does not affect ongoing SNA or maintenance of MAP.
3. Summary
We conclude that PVN neuronal activity maintains SNA and MAP in Ang II-salt hypertension, and although central cytokines are hypothesized to play a crucial role in the development of Ang II dependent hypertension, maintenance of elevated SNA and MAP in rats with Ang II-salt hypertension does not acutely depend on actions of TNF-α in the PVN.
Acknowledgements
The authors thank Alfredo S. Calderon for technical assistance, Mary Ann Andrade for help with analysis of injection site histology, and Walter W. Holbein for helpful discussions of this work.
Sources of Funding
This work was supported by NIH NHLBI grants P01 HL088052 and R01 HL102310 (GMT). MEB was supported by NIH NHLBI grant T32 HL07446.
Footnotes
Disclosures
None
References
- 1.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin ii salt hypertension in rats. Hypertension. 2007;50:547–556. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- 2.King AJ, Novotny M, Swain GM, Fink GD. Whole body norepinephrine kinetics in ang ii-salt hypertension in the rat. American journal of physiology. Regulatory, integrative and comparative physiology. 2008;294:R1262–R1267. doi: 10.1152/ajpregu.00819.2007. [DOI] [PubMed] [Google Scholar]
- 3.Kuroki MT, Guzman PA, Fink GD, Osborn JW. Time-dependent changes in autonomic control of splanchnic vascular resistance and heart rate in ang ii-salt hypertension. American journal of physiology. Heart and circulatory physiology. 2012;302:H763–H769. doi: 10.1152/ajpheart.00930.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin ii and dietary salt: Converging signals for neurogenic hypertension. Current hypertension reports. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 5.Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;293:R2279–R2289. doi: 10.1152/ajpregu.00160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toney GM, Stocker SD. Hyperosmotic activation of cns sympathetic drive: Implications for cardiovascular disease. The Journal of physiology. 2010;588:3375–3384. doi: 10.1113/jphysiol.2010.191940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience. 2003;118:797–807. doi: 10.1016/s0306-4522(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 8.Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. Journal of neurophysiology. 2010;103:4–15. doi: 10.1152/jn.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen QH, Andrade MA, Calderon AS, Toney GM. Hypertension induced by angiotensin ii and a high salt diet involves reduced sk current and increased excitability of rvlm projecting pvn neurons. Journal of neurophysiology. 2010;104:2329–2337. doi: 10.1152/jn.01013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleiber AC, Zheng H, Sharma NM, Patel KP. Chronic at1 receptor blockade normalizes nmda-mediated changes in renal sympathetic nerve activity and nr1 expression within the pvn in rats with heart failure. American journal of physiology. Heart and circulatory physiology. 2010;298:H1546–H1555. doi: 10.1152/ajpheart.01006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. The American journal of cardiology. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 12.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei SG, Zhang ZH, Yu Y, Weiss RM, Felder RB. Central actions of the chemokine stromal cell-derived factor 1 contribute to neurohumoral excitation in heart failure rats. Hypertension. 2012;59:991–998. doi: 10.1161/HYPERTENSIONAHA.111.188086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cellular signalling. 2010;22:977–983. doi: 10.1016/j.cellsig.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nygard M, Lundkvist GB, Hill RH, Kristensson K. Rapid nitric oxide-dependent effects of tumor necrosis factor-alpha on suprachiasmatic nuclei neuronal activity. Neuroreport. 2009;20:213–217. doi: 10.1097/WNR.0b013e32831f1ca2. [DOI] [PubMed] [Google Scholar]
- 16.Jin X, Gereau RWt. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razani B, Reichardt AD, Cheng G. Non-canonical nf-kappab signaling activation and regulation: Principles and perspectives. Immunological reviews. 2011;244:44–54. doi: 10.1111/j.1600-065X.2011.01059.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor JJ. Targeting tumour necrosis factor-alpha in hypoxia and synaptic signalling. Irish journal of medical science. 2013;182:157–162. doi: 10.1007/s11845-013-0911-4. [DOI] [PubMed] [Google Scholar]
- 19.Sriramula S, Cardinale JP, Francis J. Inhibition of tnf in the brain reverses alterations in ras components and attenuates angiotensin ii-induced hypertension. PloS one. 2013;8:e63847. doi: 10.1371/journal.pone.0063847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santello M, Bezzi P, Volterra A. Tnfalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69:988–1001. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Napetschnig J, Wu H. Molecular basis of nf-kappab signaling. Annual review of biophysics. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedrino GR, Calderon AS, Andrade MA, Cravo SL, Toney GM. Discharge of rvlm vasomotor neurons is not increased in anesthetized angiotensin ii - salt hypertensive rats. American journal of physiology. Heart and circulatory physiology. 2013 doi: 10.1152/ajpheart.00657.2013. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holbein WW, Toney GM. Sympathetic network drive during water deprivation does not increase respiratory or cardiac rhythmic sympathetic nerve activity. Journal of applied physiology. 2013;114:1689–1696. doi: 10.1152/japplphysiol.00078.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. The Journal of physiology. 2005;563:249–263. doi: 10.1113/jphysiol.2004.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharpe AL, Andrade MA, Herrera-Rosales M, Britton SL, Koch LG, Toney GM. Rats selectively bred for differences in aerobic capacity have similar hypertensive responses to chronic intermittent hypoxia. American journal of physiology. Heart and circulatory physiology. 2013;305:H403–H409. doi: 10.1152/ajpheart.00317.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osborn JW, Hendel MD, Collister JP, Ariza-Guzman PA, Fink GD. The role of the subfornical organ in angiotensin ii-salt hypertension in the rat. Experimental physiology. 2012;97:80–88. doi: 10.1113/expphysiol.2011.060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ployngam T, Collister JP. Role of the median preoptic nucleus in chronic angiotensin ii-induced hypertension. Brain research. 2008;1238:75–84. doi: 10.1016/j.brainres.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. American journal of physiology. Heart and circulatory physiology. 2008;295:H227–H236. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang YM, Wang Y, Yang LM, Elks C, Cardinale J, Yu XJ, Zhao XF, Zhang J, Zhang LH, Yang ZM, Francis J. Tnf-alpha in hypothalamic paraventricular nucleus contributes to sympathoexcitation in heart failure by modulating at1 receptor and neurotransmitters. The Tohoku journal of experimental medicine. 2010;222:251–263. doi: 10.1620/tjem.222.251. [DOI] [PubMed] [Google Scholar]
- 30.Li YF, Cornish KG, Patel KP. Alteration of nmda nr1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circulation research. 2003;93:990–997. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- 31.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- 32.Simpson JB. The circumventricular organs and the central actions of angiotensin. Neuroendocrinology. 1981;32:248–256. doi: 10.1159/000123167. [DOI] [PubMed] [Google Scholar]
- 33.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta physiologica Scandinavica. 2003;177:43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- 34.Cardoso LM, Colombari E, Toney GM. Endogenous hydrogen peroxide in the hypothalamic paraventricular nucleus regulates sympathetic nerve activity responses to l-glutamate. Journal of applied physiology. 2012;113:1423–1431. doi: 10.1152/japplphysiol.00912.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stocker SD, Toney GM. Vagal afferent input alters the discharge of osmotic and ang ii-responsive median preoptic neurons projecting to the hypothalamic paraventricular nucleus. Brain research. 2007;1131:118–128. doi: 10.1016/j.brainres.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. The Journal of comparative neurology. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guyenet PG. The sympathetic control of blood pressure. Nature reviews. Neuroscience. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 38.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. The New England journal of medicine. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 39.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin ii-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, De W, Zhu GQ. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 2011;203:289–297. doi: 10.1111/j.1748-1716.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- 41.Han P, Whelan PJ. Tumor necrosis factor alpha enhances glutamatergic transmission onto spinal motoneurons. Journal of neurotrauma. 2010;27:287–292. doi: 10.1089/neu.2009.1016. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Dougherty PM. Acute inhibition of signalling phenotype of spinal gabaergic neurons by tumour necrosis factor-alpha. The Journal of physiology. 2011;589:4511–4526. doi: 10.1113/jphysiol.2011.215301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Nei H, Dougherty PM. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:12844–12855. doi: 10.1523/JNEUROSCI.2437-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pi R, Li W, Lee NT, Chan HH, Pu Y, Chan LN, Sucher NJ, Chang DC, Li M, Han Y. Minocycline prevents glutamate-induced apoptosis of cerebellar granule neurons by differential regulation of p38 and akt pathways. Journal of neurochemistry. 2004;91:1219–1230. doi: 10.1111/j.1471-4159.2004.02796.x. [DOI] [PubMed] [Google Scholar]
- 45.Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against n-methyl-d-aspartate neurotoxicity by inhibiting microglia. J Immunol. 2001;166:7527–7533. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- 46.Nikodemova M, Duncan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and ikappabalpha degradation in a stimulus-specific manner in microglia. Journal of neurochemistry. 2006;96:314–323. doi: 10.1111/j.1471-4159.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- 47.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 mapk in primary sensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and fas signaling mechanisms. Annual review of immunology. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 49.Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Nightingale P, Koutedakis Y, Kitas GD. Anti-tumour necrosis factor alpha therapy improves insulin sensitivity in normal-weight but not in obese patients with rheumatoid arthritis. Arthritis research & therapy. 2012;14:R160. doi: 10.1186/ar3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas HE, Graham KL, Chee J, Thomas R, Kay TW, Krishnamurthy B. Proinflammatory cytokines contribute to development and function of regulatory t cells in type 1 diabetes. Annals of the New York Academy of Sciences. 2013;1283:81–86. doi: 10.1111/j.1749-6632.2012.06797.x. [DOI] [PubMed] [Google Scholar]
- 51.Jobe LJ, Melendez GC, Levick SP, Du Y, Brower GL, Janicki JS. Tnf-alpha inhibition attenuates adverse myocardial remodeling in a rat model of volume overload. American journal of physiology. Heart and circulatory physiology. 2009;297:H1462–H1468. doi: 10.1152/ajpheart.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.