Abstract
The speed and severity of clinical progression after Alzheimer’s Disease (AD) diagnosis varies and depends on multiple factors, most not well elucidated. We assessed whether body mass index (BMI) and one-year weight change (WC) are associated with clinical progression in amnestic mild cognitive impairment (aMCI) and early-stage AD. Longitudinal data comprising 2,268 aMCI and 1,506 AD participants in the National Alzheimer’s Coordinating Center’s Uniform Data Set were used to examine nuances of clinical progression by BMI and WC, as well as potential variations in associations by age, sex, BMI (WC model), or apolipoprotein E (APOE) genotype. In aMCI, high BMI (versus moderate BMI) was associated with slower progression; weight loss (versus no WC) was associated with faster progression. In AD, no significant differences were observed in clinical progression by BMI or WC. The association between BMI and clinical progression varied significantly by APOE genotype in AD, and the association between WC and clinical progression varied significantly by sex and BMI in aMCI. Baseline BMI and one-year WC in late-life may serve as early prognostic indicators in aMCI and early-stage AD. If replicated, these results may help in counseling patients on anticipated clinical progression and suggest windows of opportunity for intervention.
Keywords: Body Mass Index, Body Weight Changes, Weight Loss, Alzheimer Disease, Mild Cognitive Impairment, Disease Progression
INTRODUCTION
Age of diagnosis1 and presence of comorbidities2, 3 are prognostic indicators of cognitive and functional decline and survival time in Alzheimer’s Disease (AD). This study aimed to determine if body mass index (BMI) and body weight change (WC) in late life are prognostic indicators of clinical progression in AD and in amnestic mild cognitive impairment (aMCI), a condition that often precedes AD.
Approximately 30-40% of patients with mild to moderate AD lose weight.4, 5 Weight loss may begin ten years before diagnosis, be more rapid one to two years preceding diagnosis, and be greater than expected for similarly aged individuals without AD.6, 7 Low BMI and weight loss in later life have been associated with increased risk of AD.8, 9
Reasons for weight loss in AD remain unclear and may differ depending on AD stage and severity. Proposed biological mechanisms for weight loss in AD have included atrophy of the mesial temporal cortex, a region associated with eating behavior, or disruption of energy-regulating mechanisms.10, 11 In addition, for a variety of social, environmental, medical, and health care reasons, healthy eating behaviors may be abandoned during progression and later stages of AD, resulting in weight loss and lower BMI. Thus, weight loss and low BMI could be useful predictors of clinical progression in AD and aMCI.
Few studies have examined if BMI and WC are associated with clinical progression after aMCI or AD diagnosis. One study found that AD patients with ≥4% weight loss over one year experienced a large drop in MMSE score (≥3 points) over six months.12 Another study found that there were faster declines in cognition over one year among MCI patients with lower baseline BMI and slower declines among those with higher BMI.13 Focusing on individuals with incident AD, another study showed an 8% faster rate of cognitive decline for every 1-unit (kg/m2) lower baseline BMI and a 40% faster rate of cognitive decline for every 1-unit decrease in BMI per year.8
This study examined, in both aMCI and AD, if: (1) clinical progression, defined as annual change in Clinical Dementia Rating sum of boxes (CDR-SB), is associated with BMI; (2) clinical progression is associated with one-year weight change (WC); (3) these associations vary by age, sex, or apolipoprotein E (APOE) ε4 status; and (4) the association between WC and clinical progression varies by BMI. Both BMI and WC were examined because BMI allows comparison to previous studies, whereas WC is a simple clinical marker of change in nutritional status. No studies to our knowledge have investigated these aims in both aMCI and AD. Additionally, this study improves upon previous studies by defining the outcome measure using CDR-SB, a more sensitive measure of clinical progression.14
METHODS
Participants
Longitudinal data from the National Alzheimer’s Coordinating Center’s (NACC) Uniform Data Set (UDS) were used to study participants with aMCI and early-stage AD at 32 U.S. Alzheimer’s Disease Centers (ADC). ADCs have collected demographic, clinical, diagnostic, and neuropsychological data on UDS participants with normal cognition, MCI, and dementia annually since 2005. Participants come from population-based samples, clinic samples, public recruitment efforts, participant referrals, and other ongoing studies. Because recruitment methods vary by ADC, UDS participants are best described as a clinical case series of patients from each ADC. Additional details about the UDS population are found elsewhere.15, 16 Data collected between September 2005 and February 2012 were included in this study.
Amnestic MCI sample
UDS participants diagnosed with aMCI had a cognitive complaint, abnormal cognition for their age (but no dementia), memory impairment, and essentially normal functional activities.17 The participant’s first UDS visit with an aMCI diagnosis (termed the index visit) was the starting point for including his/her data in the analysis (Figure, Supplemental Digital Content 1, http://links.lww.com/WAD/A74). To ensure that the sample best represented aMCI, the MCI type most likely to progress to AD,18 additional sample restrictions included: (1) global Clinical Dementia Rating (CDR) score of 0.5; (2) CDR memory box score ≥ 0.5; (3) Mini Mental State Exam (MMSE) score ≥ 24; and (4) age at index visit ≥ 55 years (See Figure, Supplemental Digital Content 2, http://links.lww.com/WAD/A74). A younger age cut-off was used for aMCI than AD because it often precedes AD. Our restrictions based on CDR global score, CDR memory box score, and MMSE parallel those made for Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants.19
Early Stage AD sample
Participants included in the AD sample were diagnosed with primary probable AD according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria (NINCDS-ADRDA).20 The participant’s first UDS visit with an AD diagnosis was the index visit (Figure, Supplemental Digital Content 1, http://links.lww.com/WAD/A74). To ensure our sample best represented early-stage AD, additional sample restrictions included: (1) global CDR score of 0.5 or 1.0; (2) MMSE between 20 and 26, inclusive; and (3) age at index visit ≥ 65 years (Figure, Supplemental Digital Content 2, http://links.lww.com/WAD/A74). Restrictions based on CDR global score and MMSE parallel those made in ADNI.19 For our study, aMCI participants later diagnosed with AD were never included in the AD sample.
Outcome
The CDR, a well-accepted global measure of dementia severity based primarily on neurological exam and informant report,21 was assigned to participants at each visit by a clinician. CDR-SB,22 the outcome variable in all analyses, is a summary measure of the participant’s scores for each of the six categories assessed: memory, orientation, judgment, community affairs, home and hobbies, and personal care (range: 0 to 18; higher score indicates greater impairment). The change in CDR-SB over time is best described as clinical progression as it assesses both cognitive and functional impairment.
Predictors
Participant height (inches) and body weight (pounds) were recorded at each visit. BMI (kg/m2) was calculated from the height and weight at the index visit (iBMI) and categorized as low (<20.0 kg/m2), moderate (20.0 to <27.5 kg/m2), or high (≥27.5 kg/m2). The upper boundary chosen for moderate iBMI (27.5 kg/m2) was based on mortality studies suggesting a higher cut point might be more appropriate when defining normal BMI in older adults.23, 24 One-year WC was calculated by dividing the difference between a participant’s weight at the index visit and one year later by the index visit weight. The one-year WC variable was then categorized as >4% weight loss, >4% weight gain, or no change (≤4% weight gain or loss); the 4% WC cut point was based on published studies.4, 25
BMI and WC were analyzed as categorical versus continuous variables because of past studies that have indicated a U-shaped relationship between BMI/WC and AD risk and cognitive decline,8, 26 and because these categories provide clinically relevant comparisons and have been associated with a variety of health outcomes previously. Results remained similar when iBMI and WC were included as continuous variables in post-hoc analyses (data not shown).
Covariates
Potential confounders included age at index visit (years), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, non-Hispanic other race, Hispanic of any race), years of education (≤12 years, 13-16 years, ≥17 years), ≥1 comorbidity (reported history of diabetes, heart attack, heart failure, or cerebrovascular disease [TIA or stroke]), history of hypercholesterolemia, history of hypertension, depression (Geriatric Depression Scale [GDS]27 score ≥6 [range: 0-15]), and years since cognitive decline began (<5 years, 5-9 years, or ≥10 years between age of onset of cognitive decline and index visit). Age of onset of cognitive decline was assigned by clinicians after considering patient and informant report and other available clinical evidence. Presence versus absence of the APOE ε4 allele, a recognized predictor of AD risk, was examined as a potential effect modifier. APOE genotyping is provided to NACC on a voluntary basis and was available for approximately 72% of our study sample.
Analyses
Descriptive statistics (mean and standard deviation, or percentage) were calculated for the aMCI and AD samples. Linear mixed effects models were used to examine whether there were significant differences in the clinical progression rates by iBMI and one-year WC.
All models were adjusted for clustering at the ADC- and participant-level and were run separately for aMCI and AD participants using SAS Proc MIXED. Time was represented in the longitudinal models as years from index visit. Each independent variable was considered time-invariant. The participant’s CDR-SB score was allowed to vary by visit, permitting an estimation of the average change in CDR-SB over time. All models allowed for a random slope and intercept, based on indication of random effects from spaghetti plots. An exchangeable correlation structure was assumed for the random effects.
The unadjusted iBMI model contained iBMI, time, and interaction terms between iBMI and time. CDR-SB was included as the outcome variable in the iBMI analysis starting at the index visit. The unadjusted one-year WC model contained one-year WC, time, and interaction terms between one-year WC and time. CDR-SB was included as the outcome variable in the analysis starting with the index+1 visit, which ensured that the one-year WC temporally preceded the measure of the annual clinical progression rate. The model coefficients for the interaction terms (i.e., iBMI×time and WC×time) provide the difference in clinical progression rates by iBMI and WC. A positive coefficient indicates a faster rate of clinical progression and a negative coefficient indicates a slower rate of clinical progression, always in comparison to the moderate iBMI/no WC participants.
Multivariable models adjusted for iBMI (one-year WC model only), age at index visit, sex, race/ethnicity, education, comorbidities, years since cognitive decline began, and depression. These models estimated the average difference in clinical progression rates by iBMI or one-year WC category, separately for aMCI and AD participants. Education, years since cognitive decline began, and depression were included as categorical measures in the model because the categories are more easily interpretable and including continuous measures did not change the estimates in a meaningful way. A single variable for presence of ≥1 comorbidity was used in the final model because so few participants had comorbidities and thus the model would not converge when comorbidities were included separately. Multivariable models were restricted to participants with non-missing data. Unadjusted and adjusted models provided very similar results; therefore, the results section focuses on the multivariable analyses.
In each multivariable model, three-way interactions between the main predictor (iBMI or one-year WC), time, and the following covariates were tested (separately): >75 years old at index visit, female sex, and APOE ε4 status. Interactions between these variables were of interest because iBMI or WC may be stronger predictors of clinical progression for those who are older, male or female, or have ≥1 APOE ε4 allele. In addition, a three-way interaction term between time, iBMI, and one-year WC was evaluated. The estimated average clinical progression rate (CDR-SB change over time) was plotted only if the three-way interaction term(s) significantly contributed to the multivariable model. An alpha level of 0.05 was used for all analyses.
Standard protocol approvals, registrations, and patient consents
The Institutional Review Board at the University of Washington approved this study. Informed consent was obtained from all participants and informants at the individual ADCs.
RESULTS
Demographics
Our final sample consisted of 2,268 aMCI participants and 1,506 early-stage AD participants (Table 1). Mean follow-up time was 2.3 years (SD: 1.3 years). The mean age at index visit was 76.0 and 78.2 years for aMCI and AD participants, respectively. The majority of aMCI and AD participants were non-Hispanic white and a greater percentage of the AD group had lower levels of education compared with the aMCI group. AD participants more frequently experienced cognitive decline ≥5 years prior to the index visit compared with aMCI participants. The mean CDR-SB at index visit was 1.3 and 4.6 for the aMCI and AD groups, respectively. In both diagnostic groups, approximately 5% had a low iBMI (<20.0 kg/m2) and 35% had high iBMI (≥27.5 kg/m2). About 18% of aMCI participants and 21% of AD participants lost >4% of their index visit weight over one year, whereas 13% of aMCI participants and 18% of AD participants gained >4% of their index visit weight over one year. Among those with APOE data, the frequency of having ≥1 APOE ε4 allele was 45% and 62% for the aMCI and AD groups, respectively.
Table 1.
Characteristics of UDS Participants With aMCI and AD at Index Visit, 2005-2012
| Characteristic at Index Visita | aMCI (n=2,268) | AD (n=1,506) |
|---|---|---|
| Age in years, mean (SD) | 76.0 (8.4) | 78.2 (6.7) |
| Primary reported race/ethnicity (%) | ||
| Non-Hispanic White | 81.8 | 82.8 |
| Non-Hispanic Black | 10.4 | 9.3 |
| Other Non-Hispanic | 2.3 | 2.6 |
| Hispanic | 5.6 | 5.3 |
| Male sex (%) | 51.1 | 48.9 |
| Education (%) | ||
| ≤12 years | 23.8 | 36.9 |
| 13-16 years | 43.9 | 38.1 |
| ≥17+ years | 32.3 | 25.0 |
| ≥1 Comorbidity (%)b | 16.0 | 12.9 |
| Hypercholesterolemia (%) | 52.3 | 53.5 |
| Hypertension (%) | 51.6 | 50.3 |
| Depression (GDS score ≥6) (%) | 10.8 | 9.5 |
| Years since cognitive decline began (%) | 58.4 | |
| <5 years | 69.0 | 34.7 |
| 5 to 9 years | 24.5 | 6.9 |
| ≥ 10 years | 6.5 | |
| CDR-SB, mean (SD) | 1.3 (0.9) | 4.6 (1.7) |
| Index visit BMI (iBMI) (%) | ||
| Low iBMI (<20.0 kg/m2) | 3.8 | 5.8 |
| Middle iBMI (20 to <27.5 kg/m2) | 59.7 | 56.6 |
| High iBMI (≥27.5 kg/m2)) | 36.6 | 34.5 |
| One-year WC (%) | ||
| Weight loss of >4% | 18.1 | 20.9 |
| Weight gain of >4% | 12.9 | 18.2 |
| No change (≤ 4% weight loss or gain) | 69.1 | 61.0 |
| ≥1 APOE ε4 allele (%) | 44.6 | 61.8 |
Abbreviations: UDS, Uniform Data Set; aMCI; amnestic MCI; AD; Alzheimer’s Disease; SD; standard deviation; iBMI, index visit BMI; GDS; Geriatric Depression Scale; CDR-SB; Clinical Dementia Rating Sum of Boxes; WC, weight change; APOE, Apolipoprotein E
Missing data (aMCI/AD): age (0.0%/0.0%), race/ethnicity (0.1%/0.3%), sex (0.0%/0.0%), education (0.2%/0.2%), comorbidities (0.0%/0.0%), hypercholesterolemia (0.0%/0.0%), hypertension (0.0%/0.0%), depression (1.5%/2.7%), years since cognitive decline began (7.5%/0.9%), CDR-SB (0.0%/0.0%), iBMI (0.0%/0.0%), one-year WC (0.0%/0.0%), APOE (33.3%/21.2%).
Participants have one or more of the following comorbidities: Diabetes, heart failure, cardiac arrest, or cerebrovascular disease (TIA and/or stroke).
Adjusted iBMI model, aMCI
Among aMCI participants, high iBMI (versus moderate iBMI) was associated with an average 0.13 point higher baseline CDR-SB score and slower annual clinical progression rate (0.20 points slower per year) (Table 2). No significant differences in baseline CDR-SB score or clinical progression rate were observed when comparing individuals in the low versus moderate iBMI groups. The association between iBMI and clinical progression did not vary by sex, age, or APOE ε4 status (results not shown).
Table 2.
Baseline CDR-SB Differences and Differences in Mean CDR-SB Annual Change by BMI at Index Visit, UDS Participants, 2005-2012
| aMCI |
AD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted iBMI Model |
Adjusted iBMI Modelb |
Unadjusted iBMI Model |
Adjusted iBMI Modelb |
|||||
| Model Term | Estimate | 95% CI (P value) |
Estimate | 95% CI (P Value) |
Estimate | 95% CI (P Value) |
Estimate | 95% CI (P Value) |
| Baseline differences in mean CDR-SB | ||||||||
| Low iBMIa | 0.17 | −0.05, 0.40 (0.13) |
0.17 | −0.05, 0.39 (0.12) |
−0.01 | −0.41, 0.38 (0.94) |
−0.14 | −0.57, 0.29 (0.52) |
| Moderate iBMIa | Ref | Ref | Ref | Ref | ||||
| High iBMIa | 0.11 | 0.02, 0.19 (0.02) |
0.13 | 0.04, 0.22 (0.003) |
0.18 | 0.01, 0.34 (0.03) |
0.17 | 0.02, 0.32 (0.03) |
| Differences in mean CDR-SB change per year | ||||||||
| Low iBMIa | 0.22 | −0.04, 0.49 (0.09) |
0.22 | −0.04, 0.49 (0.09) |
0.25 | −0.14, 0.64 (0.21) |
0.26 | −0.13, 0.66 (0.19) |
| Moderate iBMIa | Ref | Ref | Ref | Ref | ||||
| High iBMIa | −0.18 | −0.30, −0.07 (0.001) |
−0.20 | −0.32, −0.08 (0.001) |
−0.17 | −0.36, 0.02 (0.08) |
−0.16 | −0.34, 0.02 (0.09) |
Abbreviations: CDR-SB, Clinical Dementia Rating Sum of Boxes; UDS, Uniform Data Set; iBMI, body mass index at index visit; aMCI, amnestic MCI; AD, Alzheimer's Disease; Ref, reference group
Low iBMI is <20 kg/m2, Moderate iBMI is 20 to ≥27.5 kg/m2, High iBMI is ≥27.5 kg/m2
Adjusting for age at index visit, years of education, sex, race/ethnicity, comorbidities, hypertension, hypercholesterolemia, years since cognitive decline began, and depression
Adjusted iBMI model, AD
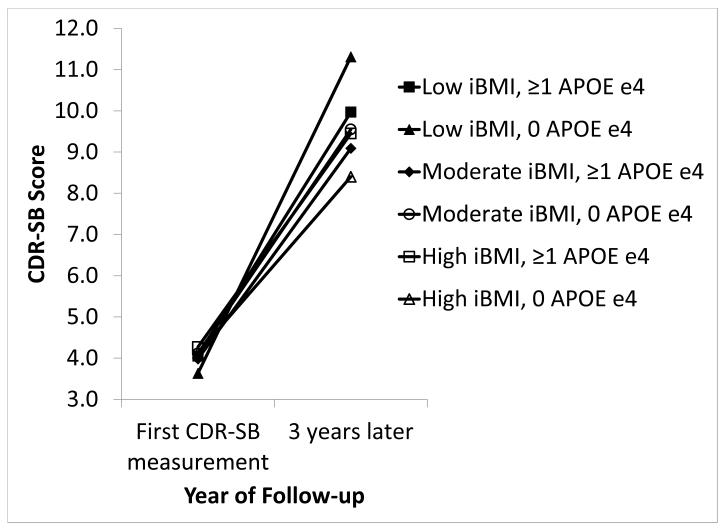
Among AD participants, high iBMI (versus moderate iBMI) was associated with an average 0.17 point higher baseline CDR-SB score but was not associated with clinical progression rate (Table 2). Low iBMI was not associated with baseline CDR-SB score or clinical progression rate. The association between iBMI and clinical progression did not differ by sex or age (results not shown); however, a slower clinical progression rate was observed for high iBMI participants without APOE ε4 versus moderate iBMI participants without APOE ε4 (p=0.014) (Figure 1).
Figure 1. Clinical Progression by iBMI and APOE ε4 Status in UDS Participants With AD, 2005-2012.
Significantly slower clinical progression among AD participants with no APOE ε4 alleles and high iBMI versus participants with no APOE ε4 alleles and moderate iBMI (p=0.014). No other significant differences by APOE ε4 status.
Adjusted one-year WC model, aMCI
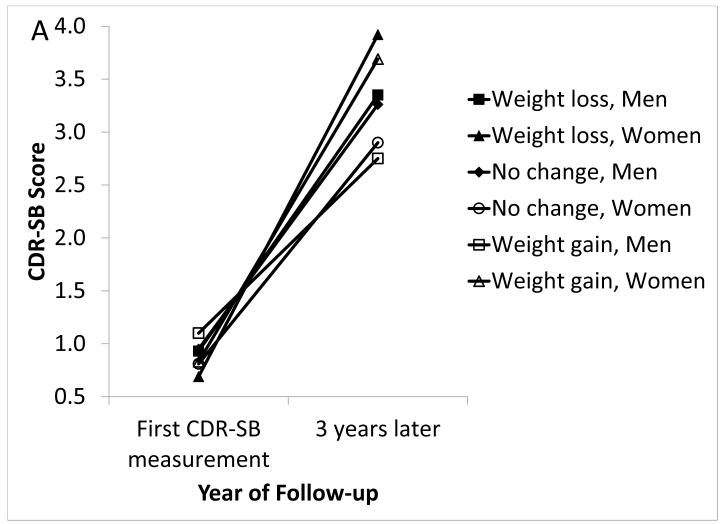
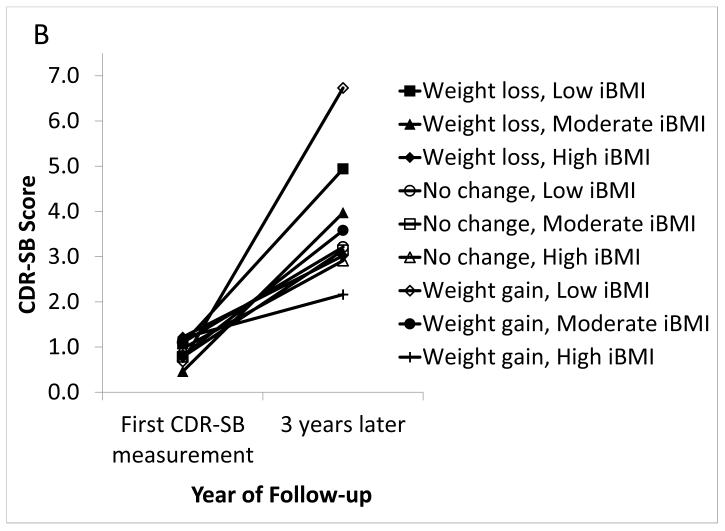
Among aMCI participants, weight loss and weight gain of >4% were not associated with baseline CDR-SB score, and >4% weight gain was not associated with clinical progression rate (Table 3). Weight loss of >4% (versus no WC) was associated with a faster clinical progression rate (0.20 points faster per year) (p=0.014). Among women, the clinical progression rate was faster for those experiencing >4% weight loss compared to those with no WC (p<0.0001) (Figure 2a). In moderate iBMI participants, clinical progression was faster among those experiencing >4% weight loss compared to those with no WC (p=0.0001) (Figure 2b). In low iBMI participants, clinical progression was faster for those experiencing >4% weight gain compared to those with no WC (p=0.020). In high iBMI participants, clinical progression was slower for those experiencing >4% weight gain compared to those with no WC (p=0.049). Clinical progression rates did not differ by one-year WC and APOE ε4 status (data not shown).
Table 3.
Baseline CDR-SB Differences and Differences in Mean CDR-SB Annual Change by One-year Weight Change, UDS Participants, 2005-2012
| aMCI |
AD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted WC Model |
Adjusted WC Modela |
Unadjusted WC Model |
Adjusted WC Modela |
|||||
| Model Term | Estimate | 95% CI (P value) |
Estimate | 95% CI (P value) |
Estimate | 95% CI (P value) |
Estimate | 95% CI (P value) |
| Baseline Differences in mean CDR-SB | ||||||||
| Weight loss of >4% | 0.03 | −0.32, 0.27 (0.87) |
−0.06 | −0.34, 0.22 (0.67) |
0.71 | 0.34, 1.08 (<0.001) |
0.72 | 0.33, 1.10 (<0.001) |
| No WC (≤4%) | Ref | Ref | Ref | Ref | ||||
| Weight gain of >4% | 0.04 | −0.21, 0.30 (0.74) |
0.09 | −0.16, 0.34 (0.48) |
0.72 | 0.09, 1.35 (0.03) |
0.52 | 0.05, 0.99 (0.03) |
| Differences in mean CDR-SB change per year | ||||||||
| Weight loss of >4% | 0.17 | 0.00, 0.34 (0.04) |
0.20 | 0.04, 0.35 (0.014) |
−0.02 | −0.25, 0.21 (0.86) |
−0.02 | −0.25, 0.21 (0.85) |
| No WC (≤4%) | Ref | Ref | Ref | Ref | ||||
| Weight gain of >4% | 0.01 | −0.16, 0.18 (0.87) |
0.02 | −0.17, 0.20 (0.86) |
0.10 | −0.29, 0.49 (0.62) |
0.21 | −0.07, 0.50 (0.14) |
Abbreviations: CDR-SB, Clinical Dementia Rating Sum of Boxes; UDS, Uniform Data Set; WC, weight change; aMCI, amnestic MCI; AD, Alzheimer’s Disease; Ref, reference group
Adjusting for iBMI, age at index visit, years of education, sex, race/ethnicity, comorbidities, hypertension, hypercholesterolemia, years since cognitive decline began, and depression
Figure 2. Clinical Progression by Weight Change and Sex (Figure 2a) or iBMI (Figure 2b) in UDS Participants With Amnestic MCI, 2005-2012.
In aMCI: (A) Significantly faster clinical progression among women with >4% weight loss versus women with no WC (p<0.0001). There were no other significant differences by sex. (B) Significantly faster clinical progression among moderate iBMI participants with >4% weight loss versus moderate iBMI participants with no WC (p=0.0001), significantly faster clinical progression in low iBMI participants with >4% weight gain versus low iBMI participants with no WC (p=0.020), and significantly slower clinical progression in high iBMI participants with >4% weight gain versus high iBMI participants with no WC (p=0.049). There were no other significant differences by iBMI.
Adjusted one-year WC model, AD
Compared with AD participants experiencing no WC, baseline CDR-SB scores were 0.72 points higher for those with >4% weight loss and 0.52 points higher for those with >4% weight gain. No association was observed between WC and clinical progression rate. The association between WC and clinical progression did not vary by age, sex, APOE ε4 status, or iBMI (data not shown).
DISCUSSION
In our study, high iBMI was associated with higher baseline impairment in aMCI and AD and slower clinical progression in aMCI. The magnitude and the direction of the estimated associations between high iBMI and clinical progression were similar in aMCI and AD, suggesting that high iBMI may also be associated with slower clinical progression in AD. Additionally, the magnitude and direction of the estimated association between low iBMI and clinical progression were similar in aMCI and AD, suggesting a potential relationship between low iBMI and faster clinical progression.
It is interesting that high iBMI was associated with slower clinical progression for aMCI participants and for AD participants with no APOE ε4 alleles. The UDS may capture a critical inflection point in the BMI-AD relationship, whereby higher average iBMI reflects the midlife BMI exposure and perhaps higher vascular risk for cognitive impairment, yet as aMCI or AD progresses, higher iBMI protects against progression. This inflection point dividing a risk state from a protective state has not been well described in the literature. The natural history of BMI in relation to aging and dementia has been illustrated in the Swedish birth cohort studies.28, 29 It may also be that UDS participants with high BMI are a subset of individuals not experiencing other life-threatening comorbidities associated with higher BMI, and thus represent more robust individuals who are less susceptible to cognitive decline.
Results for one-year WC were inconsistent when comparing the aMCI and AD groups. A >4% one-year WC was associated with higher baseline CDR-SB scores among AD participants but not among aMCI participants. While weight loss was associated with faster clinical progression in aMCI participants, this was not observed in AD participants. Weight gain was not significantly associated with clinical progression in either aMCI or AD participants; however, the magnitude of the estimate for the AD participants suggests a possible relationship between weight gain and faster clinical progression in AD. Since few studies have examined whether a one-year WC measure is associated with clinical progression in either aMCI or AD, these results are fairly novel and suggest that in aMCI, a >4% weight loss over one year could be predictive of faster clinical progression compared to those who maintain their weight.
Associations observed in our study were mostly limited to aMCI. One potential explanation is that weight loss is simply a statistical marker of neurodegeneration and associated cognitive decline during prodromal stages of AD (such as aMCI). Once AD is diagnosed, the difference in cognitive decline by degree of WC may be less discernable because, as a group, all are expected to experience similar levels of decline on average.
Our results complement the three studies previously described.8, 12, 13 The primary findings from the Buchman study were replicated in our study. Those with weight loss experienced faster clinical progression and those with higher baseline BMI experienced slower clinical progression. Differences in results between the three studies and ours are likely due to selection of predictor and outcome measures and the populations within which the associations were evaluated. Cronk et al used a continuous measure of BMI and found that MCI patients with lower BMI experienced slower annual declines using multiple measures of cognitive impairment (i.e., MMSE, ADAS-cog, and a global composite measure), but not using CDR-SB. Soto et al focused on AD patients and found faster cognitive decline over 6 months among those with 4% weight loss. Differences between their findings and ours could be related to their use of MMSE as the outcome, a 6-month period to measure decline (versus 1 year), and inclusion of individuals with later-stage AD (i.e., an MMSE score as low as 10). We purposefully excluded those with later-stage AD from our sample to avoid bias due to possible ‘terminal decline’ of cognition and weight experienced close to death.
The biological mechanisms behind weight loss and AD are not clear; however, several have been proposed. Changes in behaviors such as eating and exercise or genetic susceptibility (e.g., APOE ε4 genotype) may lead to weight loss.30 A decline in BMI and in cognition may result from decreased energy metabolism due to declines in adipose and other tissues. Weight loss observed in AD patients may also result from changes in adipose tissue hormone levels, most of which have not been well-characterized in aging or AD. Leptin, a hormone produced by adipose tissue and highly correlated with BMI,31 may be dysregulated in aging and in AD as suggested by changes in the BMI trajectory and the data presented here. Increased leptin, due to obesity, negatively feeds back and suppresses appetite, in the healthy condition. Experimental data show that adipose-derived hormones, such as leptin and adiponectin, interact directly with hypothalamic nuclei and regulate energy expenditure and appetite.32, 33 Leptin also appears to play important neuroprotective and developmental roles, such as shaping the hypothalamus in the earliest stages of development and in enhancing cognition.34 In contrast, associations between low leptin levels and increased AD risk have led some scientists to suggest that leptin replacement therapy might reduce incident AD.35, 36 Higher leptin levels in late life may indicate an adipose tissue mechanism whereby higher body weight in late life is protective against rapid cognitive decline. Hormonal regulation of energy metabolism needs further investigation but seems to be promising as a mechanism for changing body weight and cognitive decline in aMCI and/or AD.
The significant interaction between iBMI and WC depicts a dynamic state of BMI and WC in aMCI. We were interested in the possible interaction between iBMI and WC because it seemed likely that >4% weight loss in one year may be associated with faster clinical progression among those with low iBMI. Consistent with our expectations, we observed that the association between >4% weight loss and faster clinical progression may be primarily driven by participants with low or moderate iBMI who experienced a >4% weight loss. Similarly, the observed slower clinical progression among individuals with high iBMI may be driven primarily by the subgroup experiencing >4% weight gain. Although the mechanism for this association is unclear, our results suggest that individuals with higher BMI and/or who experience weight gain are protected from cognitive decline compared with those who have lower BMI and/or no weight gain. Future studies are needed to replicate our findings and examine the potential role of energy-regulating mechanisms in changing body weight and clinical progression.
Recent research efforts have focused on sex-based differences in AD;37, 38 therefore, observed differences by sex were not unexpected. A few studies have demonstrated significantly different plasma leptin levels by sex, suggesting that sex-based interactions could be related to differences in energy regulating hormones and metabolic changes associated with neurodegeneration.39, 40
Among AD participants with no APOE ε4 alleles, a slower clinical progression rate was observed among those with high iBMI (versus moderate iBMI) and faster clinical progression was suggested for those with low iBMI (versus moderate iBMI). Associations between risk factors and outcomes such as clinical progression have been observed only amongst those with no APOE ε4 alleles in at least a few previous studies.37 Our findings suggest that in the presence of the APOE ε4 allele, other risk factors may not be as strong predictors of clinical progression.
Our selected BMI and WC categories appear to be clinically meaningful and capable of discriminating differences in clinical progression in aMCI. We would not expect a substantial change in CDR-SB over time for individuals with MCI (the maximum expected CDR-SB score for an individual with MCI would be 4.0) and therefore our estimates are consistent with the expected change in CDR-SB among those with MCI. Regardless of the small expected annual change in CDR-SB in the aMCI group, our study found significant differences in annual CDRSB change by BMI and WC category. Therefore, it appears that BMI, WC, and CDR-SB are clinically relevant and potential early indicators of AD.
Major strengths of this study are: 1) the large number of aMCI and AD participants who have been followed longitudinally allowing for the study of clinical progression; 2) the standardized data collection protocols employed across the ADCs; and 3) the availability of data on a number of potential clinical and demographic confounders. Weaknesses of this study include the: 1) potential lack of generalizability; 2) missing APOE genotype data for 28% of the sample; 3) lack of data on mid-life BMI, which could be a significant confounder if long-term obesity contributes to greater impairment or a faster clinical progression; 4) small number of participants with low iBMI, limiting conclusions we can make about clinical progression among these individuals; and 5) relatively short follow-up time (average of 3 visits, up to 6 years of follow-up).
Our study suggests useful predictors of clinical progression rates following aMCI or AD diagnosis. Differences in iBMI and one-year WC in late-life may serve as early indicators of the underlying disease process in aMCI and early-stage AD, and thus may be useful and practical predictors of future clinical progression. If these findings are replicated, they may be useful in advising patients about expected clinical progression. This study adds to the literature because it uses a more sensitive measure of dementia and decline (CDR-SB) than measures used in past studies, and it is the first known study to assess whether WC is a predictor of clinical progression among individuals diagnosed with aMCI.
Supplementary Material
Acknowledgements
The authors thankfully acknowledge the patients and families enrolled at the ADCs who contributed data to the UDS and the faculty and staff of the ADCs who conducted the evaluations and collected the data used in these analyses. The authors would also like to thank the NIA, which provided support for the ADCs and NACC, as well as NACC staff and faculty who provided useful feedback, including the helpful review by Dr. Stephen Hawes.
Funding Acknowledgement: The National Alzheimer’s Coordinating Center is funded by NIH U01 AG016976. Dr. Gustafson receives support from NIH/NIAID U01-AI-31834, the Swedish Research Council Diarienummer: 523-2005-8460, and the SUNY Research Foundation.
Footnotes
List of Supplemental Digital Content Online SupplementalData_uploadversion.doc
References
- 1.Brookmeyer R, Corrada MM, Curriero FC, et al. Survival following a diagnosis of Alzheimer disease. Archives of Neurology. 2002;59:1764–1767. doi: 10.1001/archneur.59.11.1764. [DOI] [PubMed] [Google Scholar]
- 2.Villarejo A, Benito-Leon J, Trincado R, et al. Dementia-associated mortality at thirteen years in the NEDICES Cohort Study. J Alzheimers Dis. 2011;26:543–551. doi: 10.3233/JAD-2011-110443. [DOI] [PubMed] [Google Scholar]
- 3.Helzner EP, Luchsinger JA, Scarmeas N, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillette-Guyonnet S, Nourhashemi F, Andrieu S, et al. Weight loss in Alzheimer disease. American Journal of Clinical Nutrition. 2000;71:637S–642S. doi: 10.1093/ajcn/71.2.637s. [DOI] [PubMed] [Google Scholar]
- 5.White H. Weight Change in Alzheimer’s Disease. J Am Geriatr Soc. 1996;44:265–272. doi: 10.1111/j.1532-5415.1996.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 6.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Archives of Neurology. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Archives of Neurology. 2006;63:1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 8.Buchman AS, Wilson RS, Bienias JL, et al. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundman M, Corey-Bloom J, Jernigan T, et al. Low body weight in Alzheimer’s disease is associated with mesial temporal cortex atrophy. Neurology. 1996;46:1585–1591. doi: 10.1212/wnl.46.6.1585. [DOI] [PubMed] [Google Scholar]
- 11.Gillette Guyonnet S, Abellan Van, Kan G, Alix E, et al. IANA (International Academy on Nutrition and Aging) Expert Group: weight loss and Alzheimer’s disease. Journal of Nutrition, Health & Aging. 2007;11:38–48. [PubMed] [Google Scholar]
- 12.Soto ME, Secher M, Gillette-Guyonnet S, et al. Weight Loss and Rapid Cognitive Decline in Community-Dwelling Patients with Alzheimer’s Disease. Journal of Alzheimer’s disease. 2011;28:647–654. doi: 10.3233/JAD-2011-110713. [DOI] [PubMed] [Google Scholar]
- 13.Cronk BB, Johnson DK, Burns JM. Body mass index and cognitive decline in mild cognitive impairment. Alzheimer Disease and Associated Disorders. 2010;24:126–130. doi: 10.1097/WAD.0b013e3181a6bf3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coley N, Andrieu S, Jaros M, et al. Suitability of the Clinical Dementia Rating-Sum of Boxes as a single primary endpoint for Alzheimer’s disease trials. Alzheimers Dement. 2011;7:602–610. e602. doi: 10.1016/j.jalz.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 16.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 17.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. discussion 1167. [DOI] [PubMed] [Google Scholar]
- 18.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 22.O’Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s research consortium study. Arch Neurol. 2008;65:1091–1095. doi: 10.1001/archneur.65.8.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deschamps V, Astier X, Ferry M, et al. Nutritional status of healthy elderly persons living in Dordogne, France, and relation with mortality and cognitive or functional decline. European Journal of Clinical Nutrition. 2002;56:305–312. doi: 10.1038/sj.ejcn.1601311. [DOI] [PubMed] [Google Scholar]
- 24.Heiat A. Impact of age on definition of standards for ideal weight. Prev Cardiol. 2003;6:104–107. doi: 10.1111/j.1520-037x.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- 25.Guerin O, Andrieu S, Schneider SM, et al. Different modes of weight loss in Alzheimer disease: a prospective study of 395 patients. American Journal of Clinical Nutrition. 2005;82:435–441. doi: 10.1093/ajcn.82.2.435. [DOI] [PubMed] [Google Scholar]
- 26.Luchsinger JA, Patel B, Tang MX, et al. Measures of adiposity and dementia risk in elderly persons. Archives of Neurology. 2007;64:392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheikh JI, Yesavage JA. Clinical Gerontology: A Guide to Assessment and Intervention. The Haworth Press; NY: 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version; pp. 165–173. [Google Scholar]
- 28.Gustafson DR, Backman K, Waern M, et al. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73:1559–1566. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustafson DR, Bäckman K, Joas E, et al. 37 years of body mass index and dementia: observations from the prospective population study of women in Gothenburg, Sweden. J Alzheimers Dis. 2012;28:163–171. doi: 10.3233/JAD-2011-110917. [DOI] [PubMed] [Google Scholar]
- 30.Vanhanen M, Kivipelto M, Koivisto K, et al. APOE-epsilon4 is associated with weight loss in women with AD: a population-based study. Neurology. 2001;56:655–659. doi: 10.1212/wnl.56.5.655. [DOI] [PubMed] [Google Scholar]
- 31.Gustafson DR. Adiposity hormones and dementia. J Neurol Sci. 2010;299:30–34. doi: 10.1016/j.jns.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Kishi T, Elmquist JK. Body weight is regulated by the brain: a link between feeding and emotion. Mol Psychiatry. 2005;10:132–146. doi: 10.1038/sj.mp.4001638. [DOI] [PubMed] [Google Scholar]
- 33.Qi Y, Takahashi N, Hileman SM, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 34.Harvey J, Shanley LJ, O’Malley D, et al. Leptin: a potential cognitive enhancer? Biochem Soc Trans. 2005;33:1029–1032. doi: 10.1042/BST20051029. [DOI] [PubMed] [Google Scholar]
- 35.Carro EM. Therapeutic approaches of leptin in Alzheimer’s disease. Recent Pat CNS Drug Discov. 2009;4:200–208. doi: 10.2174/157488909789104848. [DOI] [PubMed] [Google Scholar]
- 36.Lieb W, Beiser AS, Vasan RS, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claxton A, Baker LD, Wilkinson CW, et al. Sex and ApoE Genotype Differences in Treatment Response to Two Doses of Intranasal Insulin in Adults with Mild Cognitive Impairment or Alzheimer’s Disease. J Alzheimers Dis. 2013;35:789–797. doi: 10.3233/JAD-122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ancelin ML, Ripoche E, Dupuy AM, et al. Sex differences in the associations between lipid levels and incident dementia. J Alzheimers Dis. 2013;34:519–528. doi: 10.3233/JAD-121228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saad MF, Damani S, Gingerich RL, et al. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82:579–584. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy A, Gettys TW, Watson P, et al. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–1300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.