Abstract
The poor solubility and low dissolution rate in gastro-intestinal fluid, especially for class-II drugs according to Biopharmaceutics Classification System (BCS) the bioavailability enhanced by increasing the solubility and dissolution rate. A novel melt sonocrystallization technique of particle engineering to enhance solubility as well as dissolution of hydrophobic drug and to study its effect on crystal properties of drug. The present study leads to use investigate solubility of melt sonocrystallization technique to modify the undesirable properties of Rosiglitazone is antidiabetic drug in thiozolidione category with (BCS II) to forms agglomerates with number of shallow circular pits on the surface leads to increase solubility. Melt sonocrystallization process was developed for Rosiglitazone in which Rosiglitazone melt was poured in deionized water and simultaneously subjected to ultrasonic energy for 20 min at amplitude 80 %. The product obtained was evaluated using scanning electron microscopy, differential scanning calorimetry, X-ray powder diffractometry (XPRD), Fourier transformed infrared spectroscopy (FTIR), solubility and dissolution rate. The irregular agglomerates with porous surface were obtained having different crystal habit which increases solubility and dissolution rate. FTIR shows thermal behavior of untreated Rosiglitazone and treated Rosiglitazone have no significant difference low intensity peaks in XPRD of treated Rosiglitazone were noticed crystals habit changes and lattice defects during processing have causes favorable changes in the physicochemical properties of Rosiglitazone. The use of melt sonocrystallization technique is promising technique that may affords powder with improved flow as well as improved solubility and dissolution.
Keywords: Melt sonocrystallization, Rosiglitazone, Crystallization, Solubility
Riassunto
La scarsa solubilità e il basso tasso di dissoluzione nei liquidi gastro-intestinali, soprattutto per farmaci di classe II secondo il Biopharmaceutics Classification System (BCS) richiede, per la biodisponiblità, un aumentando della solubilità e velocità di dissoluzione. Valutiamo una nuova tecnica di ingegneria delle particelle di scioglimento per sonocristallizazione per migliorare la solubilità, nonché la dissoluzione del farmaco e il suo effetto sulle proprietà cristalline del farmaco. Il presente studio indaga quindi la tecnica di scioglimento per sonocristallizazione per modificare la solubilità e le proprietà indesiderabili del Rosiglitazone, farmaco antidiabetico della categoria dei thiozolidione (BCS II) con la formazione di depressioni circolari poco profonde sulla superficie, per aumentare la solubilità. Il processo di scioglimento per sonocristallizazione è stato sviluppato per il Rosiglitazone. In questo esperimento il Rosiglitazone è stato versato in acqua deionizzata e contemporaneamente sottoposto a energia ad ultrasuoni per 20 min. con ampiezza all’ 80 %. Il prodotto ottenuto è stato valutato mediante microscopia elettronica a scansione (SEM), calorimetria differenziale a scansione (DSC), raggi X a diffrazione (XPRD), trasformata di Fourier con spettroscopia ad infrarossi (FTIR), solubilità e velocità di dissoluzione. Sono stati ottenuti agglomerati irregolari, con superficie porosa con diverso aspetto cristallino, che aumenta la solubilità e di dissoluzione. La FTIR mostra un comportamento termico del Rosiglitazone non trattato e trattato senza alcuna differenza significativa, picchi di bassa intensità del Rosiglitazone trattato sono stati notati in XPRD con cambiamenti cambiamenti favorevoli delle proprietà fisico-chimiche del Rosiglitazone. L’uso della tecnica scioglimento con sonocristallizazione è una tecnica promettente un migliore flusso e migliore solubilità e dissoluzione.
Introduction
The poor solubility and low dissolution rate of poorly water soluble drug in the aqueous gastro- intestinal fluid often cause insufficient bioavailability. Especially for class-II substances according to the Biopharmaceutics Classification System (BCS) the bioavailability may be enhanced by increasing the solubility and dissolution rate of the drug in the gastro-intestinal fluid [1]. Any drug to be absorbed must be present in the form of an aqueous solution at the site of absorption [2–4].
The main possibilities for improving dissolution according to this analysis are to increase the surface area available for dissolution rate by decreasing the particle size of the solid compound and or by optimizing the wetting characteristics of the compound surface, to decrease the boundary layer thickness, to ensure sink condition for dissolution and last but definitely not least, to improve the apparent solubility of drug under physiologically relevant condition [5, 6]. As solubility and permeability is the deciding factor for the in vivo absorption of the drug, these can altered or modified by enhancement technique like the novel approach for particle size reduction on basis of crystallization by using ultrasound is sonocrystallization this utilizes ultrasound power characterized by a frequency range of 20–100 kHz for inducing crystallization its not only enhances the nucleation rate but also an effective means of size reduction and controlling size distribution of the active pharmaceutical ingredients (API) [7]. Most application used ultrasound in the range 20–5 kHz [8]. There are reports on application of ultrasonic (US) energy during crystallization i.e. sonocrystallization. US energy has been used to achieve nucleation at moderate super saturation during crystallization process or terminal treatment to achieve deagglomeration and to obtain crystal habbit [9].
Melt sono crystallization (MSC) is particle engineering technique are developing to modify the physicochemical and biopharmaceutical properties of drug Rosiglitazone-(RS)-5-{4-(2[methyl (pyridine-2-yl)amino]ethoxy benzyl]thiozolidione-2-4-dione, is oral hypoglycemic agent in the thiozolidinedione class of drug. It works as an insulin sensitizer, by binding to the pPAR receptor in fat cell and making the cells more responsive to insulin [10].
In the present study MSC a novel technique involving application of ultrasonic energy to the melt during crystallization has been reported. The effect of application of US energy on the property, of MSC Rosiglitazone was characterized by scanning electron microscopy (SEM), Differential scanning calorimetry (DSC), X-ray powder diffraction (XPRD), Fourier transform infrared spectroscopy(FTIR), and solubility and dissolution study.
Materials and methods
Material
Rosiglitazone was kindly, supplied by Cipla pharmaceutical Ltd. Mumbai, Pottasium dihydrogen phosphate and sodium hydroxide of analytical grade were purchase from Loba chemicals (Mumbai, India).
Method of preparation
The drug (2 g) was melted in vessel in paraffin oil bath. Molten mass was poured in vessel containing 20 ml deionized water and sonicated for 20 min. using probe sonicator (Chrome Tech ultrasonic) processor at pulse on off 1 s with 5–8 mm probe diameter and 80 %. The product obtained after solidification of disperse droplet was separate by filtration and dried at room temp.
Solubility determination
To evaluate increase in solubility of melt sono crystallization (MSC), Rosiglitazone saturation solubility carried out. An excess amount of MSC Rosiglitazone was added to 10 ml of distilled water maintained at 37 °C and shaken for more than 24 h. The solution were the centrifuge at 7,000 rpm for 10 min supernatant was suitably diluted and analyze by UV-spectrophotometer at 228 nm.
Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FT-IR) spectra of pure crystalline Rosiglitazone and MSC agglomerates were recorded on the sample prepared in KBr disks (2 mg sample in 200 mg KBr) using Shimadzu Fourier transformed infrared spectrophotometer. The scanning range was 500–4,000/cm. with a resolution of 4/cm.
Surface topography
Scanning electron microscopy of pure and MSC agglomerates were obtained using SEM- JSM6360 A JEOL JAPAN.
Differential scanning calorimetry (DSC)
The thermal analysis of pure Rosiglitazone and MSC Rosiglitazone agglomerates were carried out with a Metter Tolado DSC 60 (Japan), all powder samples were placed in sealed aluminum pans and heated at a rate 200 °C in the temp range 20–100 °C temp range under a nitrogen flow rate of 20 ml/min.
Powder X-ray diffraction
XPRD patterns were recorded using BRUKER-axs D8-ADVANCE, model generator powder X-ray diffraction patterns were traced for 2θ range 5–50 with reproducibility of 0.001 °C for Rosiglitazone and MSC Rosiglitazone. The position and intensities of diffraction peaks were considered for the identification and comparison of crystallinity of the drug and MSC of drug.
Dissolution study
The dissolution of pure drug and MSC agglomerates was studied using USP type II dissolution test apparatus TDT-08L with phosphate buffer pH-7.4 maintained at 37 ± 0.5 °C and stirred at 100 rpm samples were collected periodically and replaced with fresh dissolution medium after filtration through Whatman filter paper 40, concentration of Rosiglitazone was determine using spectrophotometer at 228 nm (Shimadzu 1800).
Results and discussion
Melt sono crystallization was designed for Rosiglitazone undergoes combination of melt solidification and ultrasonication, has advantages of melt solidified bonds and hardened surface, this hardened surface has enabled the particles to with stand high sonication shear and maintained integrity even in highly porous form [11].
Ultra sonication has been reported to cause spontaneous nucleation at relatively low degree of super saturation due to increase in number of collision similarly, ultrasonication enhanced collision in molecule of the melt favors nucleation rather than crystallization [12]. The crystallization induction time decreases significantly with increases in speed of agitation [13] this posses low energy shear caused complete crystallization in <30 s [14] thus, it may be conclude that MSC is promising technique to obtain porous, amorphous material with high stability. MSC process was designed for Rosiglitazone which undergoes slow and shears independent crystallization. During study, it was observe that the melt was immediately disperse into fine droplets and remained in the upper portion of vessel loner agglomerates were formed from the melt droplets at the top of liquid surface which received US energy [15] so that morphology of crystal influences pharmaceutical engineering and biopharmaceutical parameter as solubility and dissolution characteristic of drug sample [16].
Scanning electron microphotographs of Rosiglitazone and Rosiglitazone treated with US energy are shown in Fig. 1. The pure Rosiglitazone is in the form plate and needle shape (Fig. 1a, b) application of US energy to crystalline drug in suspension form resulted agglomeration of crystalline drug with number of shallow circular pits on the surface, reduction in particle size and surface roughness with porous nature. MSC of Rosiglitazone agglomerates shown in Fig. 1c. MSC Rosiglitazone agglomerates are irregular in shape with pores (Fig. 1d).
Fig. 1.
SEM of Rosiglitazone pure and MSC Rosiglitazone aPure drug at ×1,000, b Pure drug at ×2,000, c MSC Rosiglitazone at ×1,000, d MSC Rosiglitazone at ×3,000
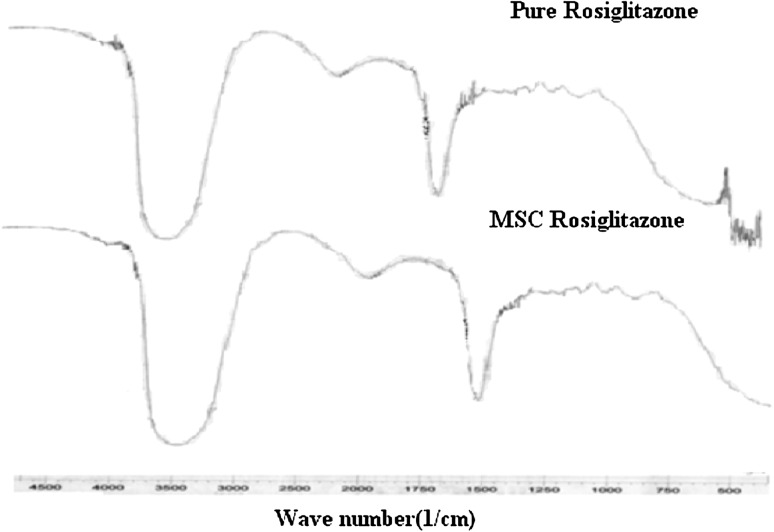
The FT-IR spectra of Rosiglitazone (Fig. 2) showed characteristics peaks of Rosiglitazone at 1,187/cm (C–N stretching aliphatic), 12,79/cm (C–N stretching aromatic) 1,655/cm (c=o stretching), 1,632/cm (N–H Bending amide) and 3,418/cm O–H aliphatic stretching 3,336/cm (N–H Stretching) were observed.
Fig. 2.
FTIR spectra of Rosiglitazone and MSC Rosiglitazone
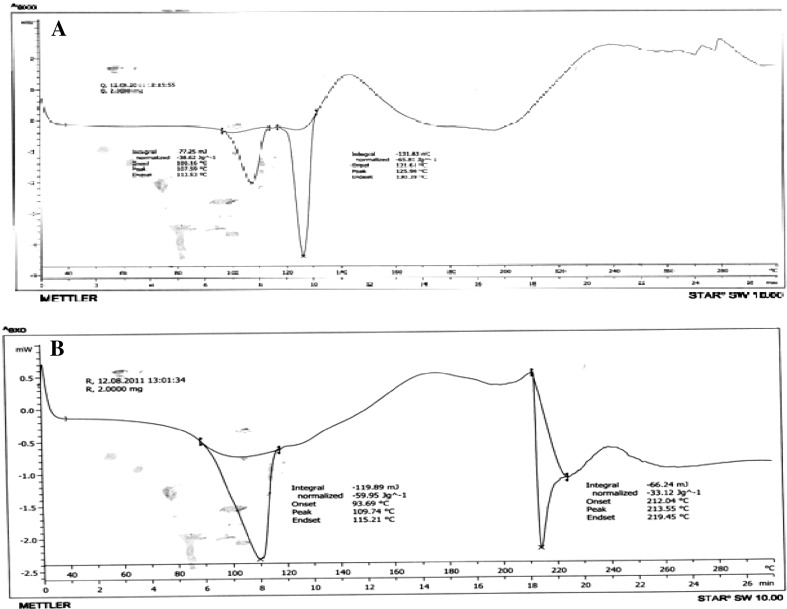
MSC samples showed spectrum as that of pure drug hence no chemical changes occur in MSC Rosiglitazone. The DSC curve of Rosiglitazone (Fig. 3a, b) was typical crystalline anhydrous substance with sharp melting endotherm (temp. onset = 100.16 °C temp peak = 107.59 °C) and end set 112.52 °C to the melting. The melting endotherm is sharp but asymmetric may be due to presence of different crystal structure these observation gives confirmation of changes in thermal properties of Rosiglitazone after MSC where broadening and asymmetry describe different crystals.
Fig. 3.
a DSC of Rosiglitazone. b DSC of melt sonocrystalized Rosiglitazone
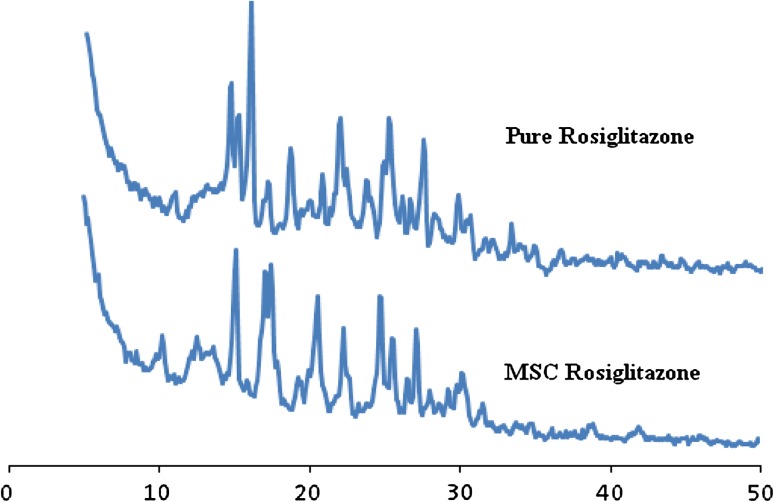
In the X-ray diffractogram of Rosiglitazone powder (Fig. 4) sharp peak at a diffraction angle 2θ 10.9, 14.6, 18.6, 21.9, 25.2, 29.7 are present and it suggest that the drug is present as crystalline material and maximum intensity peak shifted from 15.9 to 15.1 in pure Rosiglitazone diffractogram to MSC Rosiglitazone diffractogram.
Fig. 4.
XRPD of Rosiglitazone and MSC Rosiglitazone
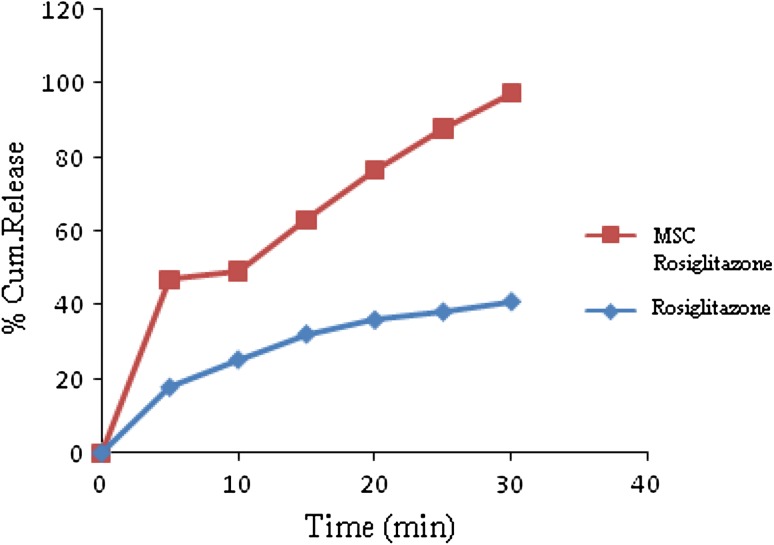
There was no significant change observed in the d spacing value as observed in SEM these include plates along with fine drug crystallites and some sintered crystals formed during processing [17]. The decrease in the intensity to the changes in crystal habbit of drug similarly the defects in the crystal structure produced due to ultrasonic energy may also be responsible for these changes [18]. Saturated solubility of pure Rosiglitazone was found to be 0.005 mg/ml where as for MSC Rosiglitazone agglomerate, it was found to be 0.345 mg/ml solubility of MSC Rosiglitazone has been significantly higher than the original powder it may be due to formation of agglomerates (Table 1). Dissolution profile of pure Rosiglitazone and MSC Rosiglitazone was shown in Table 1; Fig. 5 i.e. after 30 min Rosiglitazone pure 40.36 ± 0.40 and 97.48 ± 0.29 for MSC Rosiglitazone.
Table 1.
Solubility and % cumulative dissolution release of pure and MSC rosigliTazone
| Formulation code | Solubility mg/ml | % cum, drug release (15 min) | % cum drug release (30 min) |
|---|---|---|---|
| Rosiglitazone pure | 0.005 | 32.13 ± 0.10 | 40.36 ± 0.40 |
| MSC Rosiglitazone | 0.345 | 63.25 ± 0.58 | 97.48 ± 0.29 |
n = 3 for SD
Fig. 5.
Release profile of pure Rosiglitazone and MSC Rosiglitazone
Conclusion
Rosiglitazone agglomerates comprising of irregular in shape having rough surface area with pore by melt sono crystallization technique agglomeretates has shown no shallow circular pits on the surface there by increased solubility and dissolution profile.
Acknowledgments
Authors are thankful to Cipla pharmaceutical Ltd. Mumbai. for providing gift of drug sample, we are grateful to Dean of research, Suresh Gyanvihar University, Jaipur for providing all facilities for this work.
Conflict of Interest
V Jagtap, G. Vidyasagar, S. C. Dvivedi declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). All patients provided written informed consent to enrolment in the study and to inclusion in this article of information that could potentially lead to their identification.
Human and Animal Studies
The study was conducted in accordance with all institutional and national guidelines for the care and use of laboratory animals.
References
- 1.Urbanetz NA. Stabilization of solid dispersion of nimodipine and polyethylene glycol 2000. Eur J Pharm Sci. 2006;28:67–76. doi: 10.1016/j.ejps.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Meyer MC. Bioavailability of drug and bioequivalence. Encyclopedia of pharmaceutical technology. New York: Marcel Dekker Inc; 1998. pp. 33–50. [Google Scholar]
- 3.Shargil L, Yu . Applied Biopharmaceutics. Norwalk: Applton-Century Croft; 1985. pp. 193–203. [Google Scholar]
- 4.Martin A (2003) Physical pharmacy, Lippincott Williams & Wilkins, A. Walters Kluwer Co, Philadelphia, 5: 410–418
- 5.Noyes AA, Whitney WR. The rate of solution of solid substances in their own solution. J Am Chem Soc. 1897;19:930–934. doi: 10.1021/ja02086a003. [DOI] [Google Scholar]
- 6.Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersion. Eur J Pharm Biopharm. 2000;50(1):47–60. doi: 10.1016/S0939-6411(00)00076-X. [DOI] [PubMed] [Google Scholar]
- 7.Shree B, Garima C, Arvind K Banral (2004) New trends in the crystallization of active pharmaceutical ingrendients, Bussinessbriefing:pharmagenesis 70–74
- 8.Crystallization process using ultrasound, US patent, 20020031577
- 9.Fini A, Rodrignez L, Cavallori C, et al. Fractal analysis of β cyclodextrin–indomethacin particle compacted by ultrasound. J Pharma Sci. 1997;86(11):1303–1309. doi: 10.1021/js960489n. [DOI] [PubMed] [Google Scholar]
- 10.Ajjan RA, Grant PJ. The cardiovascular safety of Rosiglitazone. Expert Opin Drug Saf. 2008;7(4):367–376. doi: 10.1517/14740338.7.4.367. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary PD, Uttekar PS. Melt-sonocrystallization: a novel particle engineering technique for solubility enhancement. Int J Pharm Tech Res. 2009;1(1):111–120. [Google Scholar]
- 12.Manish M, Harshal J, Anant P. Melt-sonocrystallization of ibuprofen: effect on crystal properties. Eur J Pharm Sci. 2005;25(1):41–48. doi: 10.1016/j.ejps.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Paradkar A, Maheshwari M, Ketkar AR, Chauhan B. Preparation and evaluation ibuprofen beads melt solidification technique. Inten J Pharma. 2003;255(1–2):33–42. doi: 10.1016/S0378-5173(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 14.Watanable T, Wakiyama N, Senna, et al. Stability of amorphous Indomethacin compound with silica. Int J Pharm. 2001;226(1–2):81–91. doi: 10.1016/S0378-5173(01)00776-1. [DOI] [PubMed] [Google Scholar]
- 15.Kim JW, Ulrich J. Prediction of degree of deformation and crystallization time of molten droplets in pastillation process. Int J Pharm. 2003;257(1–2):205–215. doi: 10.1016/S0378-5173(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 16.Kamel, El Amal H. Improvement of physicochemical and biopharmaceutical properties of flubiprofen using melt sono crystallization technique. Drug Dev Res. 2008;69(1):34–41. doi: 10.1002/ddr.20225. [DOI] [Google Scholar]
- 17.Garekani HA, Sadeghi F, Badiee A, Mostafa SA, Rajabi-Siahboomi AR. Crystal modification of ibuprofen and their physic mechanical. Drug Dev Ind Pharm. 2001;27:803–809. doi: 10.1081/DDC-100107243. [DOI] [PubMed] [Google Scholar]
- 18.Ostuka M, Matsumoto T, Kaneniwa N. Effect of environment temperature on polymorphic solid state transformation of Indomethacin during grinding. Chem Pharm Bull. 1986;34(4):1784–1793. doi: 10.1248/cpb.34.1784. [DOI] [PubMed] [Google Scholar]