Abstract
A patient with a pseudoaneurysm of the right renal artery underwent treatment with percutaneous approach. No complications were observed. Based on the experience described in this report, a percutaneous ultrasound guided approach can be proposed in selected patients. Renal insufficiency and allergic reactions are potential contraindications to angiography with conventional ionic iodinated contrast dye in patients who need endovascular stent-graft placement. Real-time contrast-enhanced ultrasound (CEUS) guided endovascular procedures may provide an alternative to overcome these limitations. We report an endovascular renal artery repair in a solitary kidney patient with an asymptomatic infrarenal aortic aneurysm and renal insufficiency due to phenacetin abuse. The precise placement of the stent-graft was performed with CEUS and intraprocedural angiographic fluoroscopy without the use of any nephrotoxic contrast dye. During follow-up, CEUS was used to exclude endoleaks, stent-graft failure or malposition.
Electronic supplementary material
The online version of this article (doi:10.1007/s40477-014-0063-z) contains supplementary material, which is available to authorized users.
Keywords: Renal artery pseudoaneurysm, Chronic renal failure, Ultrasound, Coil embolization, Phenacetine
Riassunto
Presentiamo il caso di una paziente portatrice di uno pseudoaneurisma dell’arteria renale destra trattato con un approccio percutaneo senza alcuna complicanza. Sulla base dell’esperienza descritta nel presente caso clinico l’approccio percutaneo eco guidato potrebbe essere indicato in pazienti selezionati. La presenza di insufficienza renale e la diatesi allergica rappresentano controindicazioni alla convenzionale manovra angiografica con mezzo di contrasto iodato nei pazienti candidati a manovre di posizionamento endovascolari. Le procedure endovascolari sotto guida ecografica con mezzo di contrasto (CEUS) possono rappresentare una valida alternativa in presenza dei suddetti limiti. Riportiamo il caso di un trattamento endovascolare di uno pseudoaneurisma dell’arteria renale in una paziente con rene unico acquisito destro, affetta inoltre da insufficienza renale cronica da abuso di fenacetina. La manovra di embolizzazione dello pseudoaneurisma, mediante il posizionamento di spirali, è stata eseguita mediante CEUS e angiografia intraprocedurale senza mezzo di contrasto nefrotossico. Durante il follow-up è stata utilizzata la CEUS per escludere endoleaks o malposizionamento delle spirali.
Electronic supplementary material
The online version of this article (doi:10.1007/s40477-014-0063-z) contains supplementary material, which is available to authorized users.
Introduction
Arterial pseudoaneurysms are generated by disruptions of the arterial wall with consequent extra-luminal flow into a chamber contained by adjacent tissue. Renal artery pseudoaneurysms are a recognized complication of abdominal trauma, nephro-urological procedures, such as renal biopsy, nephrostomy, percutaneous nephro-ureterolithotomy [1–4], but can also occur spontaneously in patients with vascular miopragia. The rupture of pseudoaneurysms is associated with a high mortality rate.
The standard treatment of pseudoaneurysms is based upon selective angiographic embolization with various agents (coils, stent-grafts, polymeric agents, etc.) [5]. We report the case of an alternative renal artery pseudoaneurysm treatment with percutaneous trans-hepatic ultrasound (US) guided coil embolization in a patient with a story of phenacetin abuse and prone to pseudoaneurysm development. Contrast-enhanced ultrasound (CEUS) and 3D CEUS were used for planning treatment, for monitoring the execution of the procedure and during follow-up.
Case report
A 67-year-old woman affected by chronic renal failure from phenacetin abuse (serum creatinine 4 mg/dl, CKD 4 according to NKF-KDOQI), hypertension, and with a previous left nephrectomy for kidney cancer, presented at our Division of Nephrology for the semi-annual surveillance.
One year before, an aorto-iliac aneurysm had been surgically treated by aneurysmectomy and graft placement. The procedure was complicated by right iliac artery thrombosis and the patient was submitted to aorto-bifemoral artery by-pass graft. At the same time, a contrast-enhanced abdominal CT revealed the presence of a right renal artery pseudoaneurysm (rRAP) of 2 cm of diameter.
The rRAP was treated with the insertion of a covered stent (GraftMaster mm 5 × 26, Abbott-Vascular, Illinois, USA) during selective renal arteriography via a brachial approach. The angiographic control after the maneuver revealed a partial exclusion of the pseudoaneurysm, so another stent (Graft Master mm 5 × 19) was placed with an overlapping procedure. The final control showed a complete exclusion of the rRAP. The procedure was performed without any complication and no change in serum creatinine occurred. One week later, the patient complained severe pain and swelling of the right arm, and both Colour Doppler ultrasound (CDUS) and CT revealed the presence of a 5 cm-long pseudoaneurysm of the right brachial artery, previously used as puncture site for the angiography; a conservative treatment was adopted consisting in a high compression bandaging.
The day after the discharge, the patient presented to the ER with acute right lumbar pain, oligo-anuria and acute worsening of renal dysfunction (serum creatinine 7.0 mg/dl). An acute right renal artery thrombosis was suspected by CDUS. The patient was submitted to a selective percutaneous renal arteriography via an omeral approach that confirmed the hypothesis and revealed a large homolateral meso-renal infarcted area. An intrarenal artery catheter was placed and urokinase injection was performed, followed by systemic heparin infusion. This therapeutic approach leaded to the partial re-canalization of the renal artery and to the improvement of the serum creatinine (4 mg/dl). Even if successful, this procedure was complicated by the development of pseudoaneurysms of the left omeral artery that have been treated with standard vascular surgery.
Three months later, during the surveillance of the rRAP previously treated, the CDUS showed an increase in diameter (from 2.5 cm, 15 days before, to 3.6 cm) and a partial turbulent flow in the site of the renal pseudoaneurysm, so a new endovascular procedure was planned to definitely exclude the rRAP. Due to the patient predisposition to post-catheterization pseudoaneurysm formation and to the recent acute renal failure after iodine contrast dye, in spite of the contrast-induced nephropathy prophylaxes, a novel percutaneous trans-hepatic US guided approach for coil embolization was proposed. The procedure was planned and guided using CEUS and 3D CEUS.
Before the procedure, the US examination confirmed the presence of a 3.6 cm pseudoaneurysm of the right renal artery (Fig. 1).
Fig. 1.
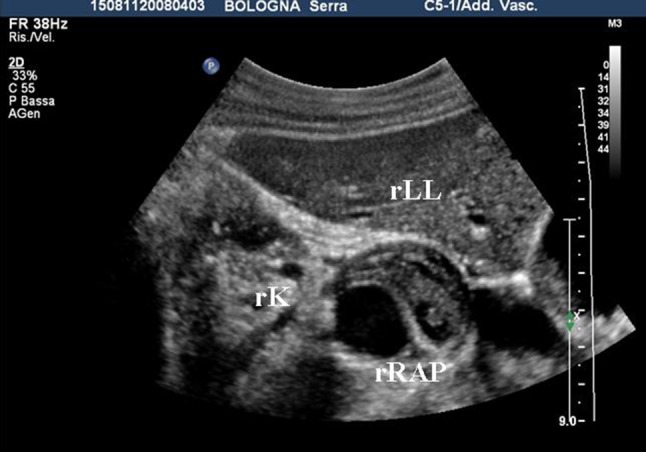
Renal artery pseudoaneurysm. Transverse US image shows a 3.6 cm renal artery pseudoaneurysm behind the right liver lobe. RLL right liver lobe, rRAP renal artery pseudoaneurysm, RK right kidney
The pseudoaneurysm was partially thrombosized with an anechoic portion of 2 cm of diameter in which both CDUS and CEUS revealed the presence of leak (Figs. 2, 3). The 3D CEUS permitted to evaluate the relationship between the aneurysm and the near structures.
Fig. 2.
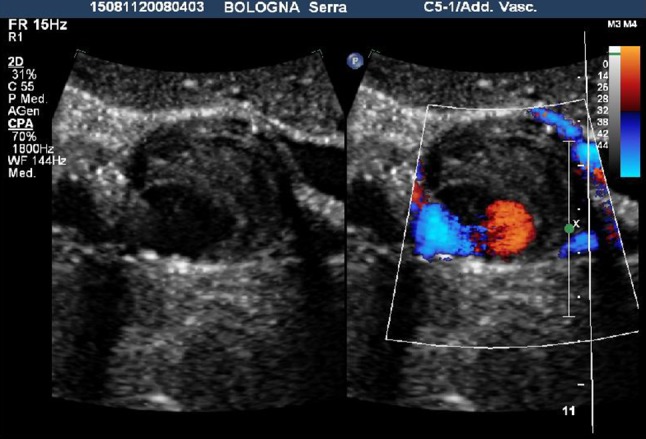
A transverse color Doppler scan revealed the presence of flow in the anechoic portion of the pseudoaneurysm with a partial thrombosis of the sac
Fig. 3.
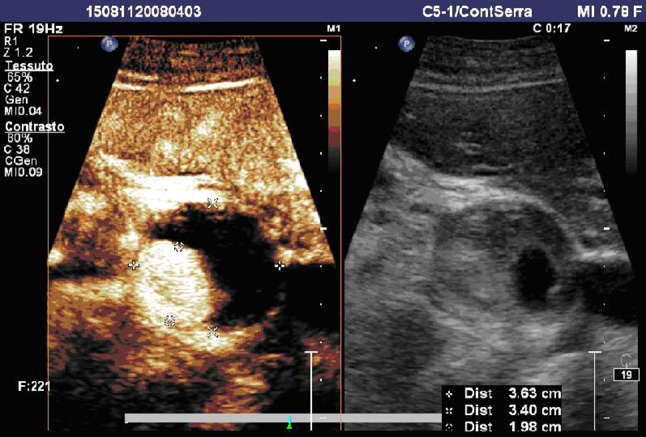
CEUS examination. In the arterial phase (17 s after the injection of SonoVue®), the flow into the pseudoaneurysm can be well visualized
The puncture site was scanned to determine the anatomy of the pseudoaneurysm, in particular the relationship among the flow lumen, the pseudoaneurysm neck and the artery was delineated. The renal artery and vein were confirmed to be patent. A written informed consent was obtained from the patient and alternative therapies were discussed, then the overlying skin was prepared with povidone iodine (Betadine®). The transducer was covered with a sterile sleeve. Using an attachable biopsy guide (CIVCO), an 18-gauge needle was advanced into the flow lumen (Fig. 4). Care was taken to avoid inadvertent puncture of the stent positioned into the renal artery. To maximize needle visualization during placement, we often had to turn off the CDUS component of the duplex imager and to use gray-scale imaging alone. Once the needle was positioned appropriately in the flow lumen, we restored CDUS imaging to monitor the effect of the injected coil on flow. After confirmation of the needle position in the flow lumen, numerous coils (Balt, Montmorency, France) of 6, 8 and 12 mm of diameter were inserted. In most cases, the injected coils were seen as an echoic “jet” emanating from the needle tip (Fig. 5). Once the coils were injected, the flow in the lumen was assessed with CDUS and CEUS for monitoring the development of occlusion. Once the aneurysm was thrombosed, the needle was removed. The characteristics of the main renal artery and intrarenal flow were documented before, during and after the procedure using CDUS and CEUS. The procedure had been well tolerated by the patient.
Fig. 4.
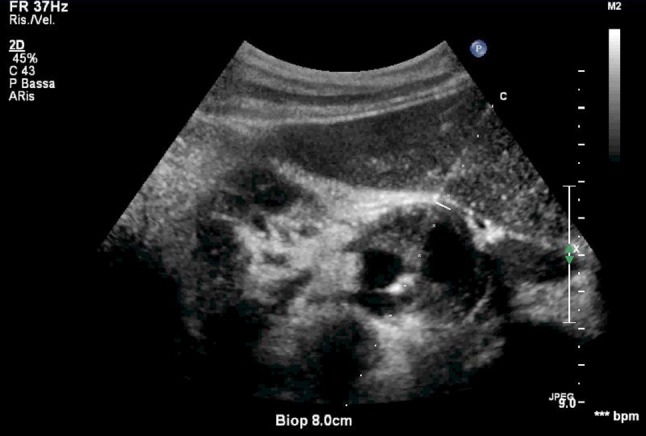
US examination: an 18-gauge needle advancing into the rRAP
Fig. 5.
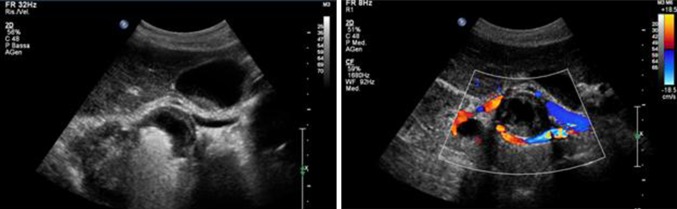
Coils insertion and rRAP exclusion
Follow-up CDUS and CEUS were performed the day after and showed a complete thrombosis of the aneurysm and the patency of the stent. One day later, another CEUS was performed and confirmed the patency of the right renal artery and the complete exclusion of the rRAP, without any systemic and local complications. Two days later, the patient was discharged.
Discussion
We described a case of US guided trans-hepatic embolization of a RAP. This procedure may be considered as an attractive alternative to surgery or traditional percutaneous trans-catheter approach in the elective management of RAPs in selected high-risk patients, such as those whose pseudoaneurysms are inaccessible trans-arterially.
Renal artery pseudoaneurysms are a well-described complication of nephro-urological surgery and procedures, such as renal biopsy, nephrostomy, percutaneous nephro-ureterolithotomy, and are also reported as spontaneous in patients with vascular disease. RAP is a potentially life-threatening condition and, when asymptomatic, is often difficult to diagnose [1–4].
In the past, surgical repair was considered the treatment of choice for post-catheterization pseudoaneurysms. Surgery requires prolonged recovery time and exposes the patient to several complications, such as bleeding, lymphocele and infections, without mentioning the risks of anesthesia. As a result of advances in ultrasonographic imaging, in 1991 US guided pseudoaneurysm compression was introduced [5] and surgical repair was almost abandoned. This procedure has a low rate of complications, but as a drawback, it is not well tolerated by the patient and sedation or intravenous analgesia is required. Nowadays, an alternative treatment for pseudoaneurysms is represented by selected angiographic trans-catheter coil embolization. However, because of the compromised arterial wall, angiography is often associated with complications such as dissection, thrombosis, hemorrhage and rupture, leading to high morbidity and mortality rates [6].
In our patient, due to the presence of an aorto-iliac by-pass graft, the brachial approach was chosen, and it was complicated by the formation of a pseudoaneurysm in the puncture site. Afterwards, to minimize the vascular complication previously occurred, a percutaneous US guided trans-hepatic approach was decided. The coil embolization with this novel approach was successful and complication-free.
Data from literature are exhaustive about vascular complications in hereditary connective disorders and include aortic and visceral aneurysms, pseudoaneurysms and dissection. For phenacetin abuse, capillary sclerosis with hyalinization and thickening of the vessel wall is a well-documented consequence [7, 8], but also large artery changes may occur in this condition. Our patient was prone to pseudoaneurysm formation after arterial catheterization, so we assumed that there was a condition of systemic vascular miopragia, maybe secondary to the long history of phenacetin assumption.
In literature, there are many studies concerning percutaneous US procedures for treating pseudoaneurysms (injection of thrombin, fibrin adhesive, glue or a detachable balloon) [9, 10]. To our knowledge, complications have been reported in two cases treated with thrombin injection. Both occurred in pseudoaneurysms that resulted from brachial artery puncture, and both were due to the embolization of the downstream arterial system. In one case, this followed inadvertent injection of the pseudoaneurysm neck. Compared with femoral artery pseudoaneurysms, those arising from the brachial artery tend to be smaller and to have a shorter neck, presumably because of the lesser thickness of the soft tissues in the arm than those in the groin.
Previous investigators demonstrated the efficacy and safety of an alternative approach to the repair of post-catheterization pseudoaneurysms using US guided thrombin injection [11–13]. Naidu et al. [14] reported a case of percutaneous embolization of a lumbar pseudoaneurysm in a patient with type IV Ehlers-Danlos syndrome using n-butyl cyanoacrylate and coils via a US guided lumbar approach.
In our case, percutaneous trans-hepatic US guided coil embolization was chosen, and it proved to be a safe and effective management for RAPs.
Conclusions
Traditionally, surgical repair has been the commonly accepted solution for the treatment of pseudoaneurysms. In the last decade, new repair procedures have been introduced, such as US guided compression, percutaneous US guided thrombin injection and angiographic coil embolization. Ultimately, a percutaneous US guided coil embolization has been proposed, even if further cases are needed to confirm the long-term results of this novel approach.
Our case report indicates that the percutaneous injection of coils with US guidance is effective and safe. However, in this procedure, familiarity with US guided techniques is essential. It is imperative that the needle tip is placed precisely in the flow lumen.
More studies are required to refine the indications, limitations and complications of this technique.
Electronic supplementary material
Conflict of interest
EF, CS, ADF, MM, EB, and AS declare no conflict of interest.
Informed consent
The patient provided written informed consent to enrolment in the study and to inclusion in this article of information that could potentially lead to her identification.
Human and Animal studies
The Study described in this article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Emiliana Ferramosca, Phone: +39-05-16362430, FAX: +39-05-16362511, Email: emiliana.ferramosca@aosp.bo.it.
Carla Serra, Phone: +39-05-16363163, Email: carla.serra@aosp.bo.it.
Antonio Di Felice, Phone: +39-05-16362430, Email: antonio.difelice@aosp.bo.it.
Marcora Mandreoli, Phone: +39-05-16362430, Email: marcora.mandreoli@ausl.imola.bo.it.
Eugenio Brunocilla, Phone: +39-05-16362747, Email: eugenio.brunocilla@aosp.bo.it.
Antonio Santoro, Phone: +39-05-1636 2430, Email: antonio.santoro@aosp.bo.it.
References
- 1.Singh D, Gill I. Renal artery pseudoaneurysm following laparoscopic partial nephrectomy. J Urol. 2005;174:2256–2259. doi: 10.1097/01.ju.0000181827.49239.8e. [DOI] [PubMed] [Google Scholar]
- 2.Albani JM, Novick AC. Renal artery pseudoaneurysm after partial nephrectomy: three case reports and a literature review. Urology. 2003;62:227–231. doi: 10.1016/S0090-4295(03)00364-9. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro EY, Hakimi AA, Hyams ES, Cynamon J, Stifelman M. Renal artery pseudoaneurysm following laparoscopic partial nephrectomy. Urology. 2009;74:819–823. doi: 10.1016/j.urology.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 4.Lee RS, Porter JR. Traumatic renal artery pseudoaneurysm: diagnosis and management techniques. J Trauma. 2003;55:972–978. doi: 10.1097/01.TA.0000032251.70194.65. [DOI] [PubMed] [Google Scholar]
- 5.Fellmeth BD, Roberts AC, Bookstein JJ, et al. Postangiographic femoral artery injuries: nonsurgical repair with US-guided compression. Radiology. 1991;178:671–675. doi: 10.1148/radiology.178.3.1994400. [DOI] [PubMed] [Google Scholar]
- 6.Fruhwirth J, Pascher O, Hauser H, Amann W. Local vascular complications after iatrogenic femoral artery puncture. Wien Klin Wochenschr. 1996;108:196–200. [PubMed] [Google Scholar]
- 7.Abrahams C, Van Tonder H, Hesse V. Abnormal vessels in the urinary tract following analgesic abuse in man. Arch Pathol Lab Med. 1976;100(12):630–631. [PubMed] [Google Scholar]
- 8.Abrahams C, Furman KI, Salant D. Dermal micro-angiopathy in patients with analgesic nephropathy. S Afr Med J. 1978;54(10):393–396. [PubMed] [Google Scholar]
- 9.Friedman SG, Pellerito JS, Scher L, et al. Ultrasound-guided thrombin injection is the treatment of choice for femoral pseudoaneurysms. Arch Surg. 2002;137(4):462–464. doi: 10.1001/archsurg.137.4.462. [DOI] [PubMed] [Google Scholar]
- 10.Khoury M, Rebecca A, Greene K, et al. Duplex scanning-guided thrombin injection for the treatment of iatrogenic pseudoaneurysms. J Vasc Surg. 2002;35(3):517–521. doi: 10.1067/mva.2002.120029. [DOI] [PubMed] [Google Scholar]
- 11.Cope C, Zit R. Coagulation of aneurysms by direct percutaneous thrombin injection. Am J Roentgenol. 1986;147:383–387. doi: 10.2214/ajr.147.2.383. [DOI] [PubMed] [Google Scholar]
- 12.Wixon CL, Philpott JM, Bogey WM, Jr, et al. Duplex-directed thrombin injection as a method to treat femoral artery pseudoaneurysms. J Am Coll Surg. 1998;187:464–466. doi: 10.1016/S1072-7515(98)00204-X. [DOI] [PubMed] [Google Scholar]
- 13.Kang SS, Labropoulos N, Mansour A, et al. Percutaneous ultrasound guided thrombin injection: a new method for treating postcatheterization femoral pseudoaneurysms. J Vasc Surg. 1998;27:1032–1038. doi: 10.1016/S0741-5214(98)70006-0. [DOI] [PubMed] [Google Scholar]
- 14.Naidu SG, Chong BW, Huettl EA, Stone WM. Percutaneous embolization of a lumbar pseudoaneurysm in a patient with type IV Ehlers–Danlos syndrome. J Vasc Surg. 2007;46(5):1036–1038. doi: 10.1016/j.jvs.2007.05.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


