Abstract
Purpose
The aim of this study is to assess the diagnostic efficacy and interobserver agreement of acoustic radiation force impulse (ARFI) elasticity imaging in differentiating thyroid nodules.
Methods
In our study, 74 consecutive patients (52 females, 22 males; age range 27–77 years, mean: 41 years) with 82 thyroid nodules (60 benign nodules, and 22 malignant) were examined by two radiologists with different experience. Patients underwent either cytology using fine needle aspiration cytology or thyroid surgery. The diagnostic performance of the two operators at ARFI with sensitivity, specificity, positive predictive and negative predictive value, and ROC curves was estimated. Inter-reader variability between the two operators was defined using Cohen’s k.
Results
According to receiver operating characteristics ROC curves (AUROC = 0.86 for observer 1; 0.81 for observer 2) sensitivity, specificity, PPV and NPV of reader 1 and 2 were respectively: 90, 75, 90.91 and 96.55 %; (cut-off value of shear wave: 2.455 m/s); 90, 72, 90 and 96.90 % (cut-off value shear wave: 2.365 m/s). Concordance between the two operators was good (k = 0.755).
Conclusions
This work is a feasibility study evaluating ARFI imaging. Its results suggest that ARFI imaging is a reproducible method which can be utilized with good diagnostic performance in the thyroid for discriminating benign and malignant nodules using the cut-off value of 2.455 m/s. However, larger studies are needed to validate this method.
Keywords: Acoustic radiation force impulse (ARFI), Elastography, Benign thyroid nodules, Malignant thyroid nodules, Ultrasound
Riassunto
Scopo
Lo scopo di questo studio è la valutazione della efficacia diagnostica e della concordanza inter-osservatore dell’imaging elastografico ARFI, per la differenziazione di noduli tiroidei.
Metodo
Nel nostro studio, 74 pazienti consecutivi (52 femmine, 22 maschi; range di età 22–77 anni, età media 41 anni) con 82 noduli tiroidei (60 noduli benigni e 22 maligni), sono stati esaminati da due radiologi con diverso livello di esperienza. I pazienti sono stati sottoposti o a citologia aspirativa con ago sottile (FNAC), o a chirurgia tiroidea. La performance diagnostica dei due operatori all’ ARFI è stata stimata con la sensibilità, la specificità, il valore predittivo positivo (VPP) e negativo (VPN), attraverso le curve ROC (receiver operating characteristics). La variabilità inter-osservatore tra i due operatori, è stata definita usando la kappa di Cohen.
Risultati
Secondo le curve ROC (AUROC = 0.86 per il primo osservatore; 0.81 per il secondo osservatore) la sensibilità, la specificità, il VPP e il VPN per gli osservatori 1 e 2, erano rispettivamente: 90, 75, 90,91, e 96.55 %; (valore medio di shear wave: 2.455 m/s); 90, 72, 90 e 96.90 % (valore medio di shear wave: 2.365 m/s). La concordanza tra I due operatori era buona (k = 0.755).
Conclusioni
Questo lavoro è uno studio preliminare che valuta l’imaging elastografico con Acustic Radiation Force Impulse. I risultati suggeriscono che l’ARFI è un metodo riproducibile che può essere utilizzato con buona performance diagnostica per la differenziazione di noduli benigni e maligni della tiroide, usando come valore di cut-off 2.455 m/s. Comunque, sono necessari studi con più ampia casistica di pazienti per la validazione di questa metodica.
Introduction
Thyroid nodules are very common with a prevalence of greater than 50 % after 60 years, however very few of them represent cancer (5–10 %) [1]. US examination is the preferred first line modality of imaging in evaluating thyroid nodules, providing detection of signs associated with malignant nature, such as microcalcifications, hypoechogenicity, irregular margins or absent halo, intranodular vascularization greater than perinodular, and deeper than wide shape [2]. Even though sensitivity and specificity can be improved by combining these parameters, no single suspicious US feature is sufficiently sensitive or specific for diagnosing malignancy and the overall performance of US is not satisfactory [3–6]. Currently, fine needle aspiration cytology (FNAC) is the standard procedure to determine malignancy and is the method with the highest specificity (60–98 %), but with a varying sensitivity of 54–90 % [7]. However, FNAC is invasive and about 10–20 % of biopsies yield inadequate results and need repetition.
A classical criterion of malignancy is hardness upon palpation and with the introduction of elastography, an assessment of tissue stiffness has become available. Qualitative and semi-quantitative elastosonography methods have been evaluated in a variety of studies on the thyroid with promising results [8–12]. A meta-analysis of eight studies, yielded a mean sensitivity of 92 % and mean specificity of 90 % [13]. However, there is a significant heterogeneity of performance of elastosonography in these studies due to the qualitative and semi-quantitative nature of methods. Increased reliance on nodule stiffness quantification is therefore needed to bring about a standardization of diagnostic criteria. Acoustic radiation force impulse technology (ARFI), already used in different fields, has also been applied to evaluate diffuse thyroid diseases, [14, 15], and recently to differentiate thyroid nodules such as in the study performed by Gu et al. [16].
The aim of our study was to assess the effectiveness of ARFI technology in the diagnostic evaluation of thyroid nodules and to assess the intraobserver and interobserver agreement of two operators with different experience as well.
Materials and methods
Patients
This study includes 74 consecutive patients (52 females, 22 males; age range 27–77 years, mean: 41 years) with 82 thyroid nodules examined by two radiologists (with different radiological experience, respectively 10 and 5 years). Informed consent was obtained from all patients and the study was performed in accordance with the ethical guidelines of the Helsinki Declaration and approved by the local ethics committee.
The study period was from September to October 2012. All patients presenting at our Radiology department, for work-up of thyroid nodules were evaluated for inclusion in the study. Inclusion criteria were the presence of a thyroid nodule ≥10 mm, FNAC and/or surgery planned at the time of ultrasound examination and finally performed. Exclusion criteria were nodules less than 1 cm, cystic lesions with little solid component, nodules with conspicuous calcifications, or nodules without final histological or cytological diagnosis. All patients received an ultrasound of the thyroid gland, including color-Doppler ultrasound, followed by ARFI evaluation. Cytology or post-surgical histopathological results were used as standard of reference method for the diagnosis of a benign or malignant thyroid nodule.
FNAC and histopathology
All included patients received either cytology using FNAC and/or histology from thyroid surgery. FNAC was performed with a 25-gauge needle attached to a 20 ml syringe. Adequacy of aspirates was defined according to the guidelines of the Papanicolaou society [17].
Acoustic radiation force impulse (ARFI) imaging
Acoustic radiation force impulse imaging (Virtual Touch™ Tissue Quantification, Siemens ACUSON S2000) involves targeting of an anatomic region to be interrogated for elastic properties with a region-of-interest (ROI) cursor while performing real-time B-mode imaging. The two kinds of imaging methods for ARFI include virtual touch tissue imaging (VTI) and virtual touch tissue quantification (VTQ) (Siemens Medical Solutions, Mountain View, CA, USA). They are used to assess qualitative and quantitative lesion stiffness, displayed on a dual screen with one side showing the B-mode image and the other side showing the elastic image. Patients were in a supine position with a fully extended neck and the measurements were performed with the patient in apnea to avoid movements that result in erroneous measured values. Tissue within a ROI (10 × 5 mm) is mechanically excited using short-duration (262 μs) acoustic pulses. The acoustic pulses generate localized tissue displacements within the ROI. Results are expressed in m/s with a measurement range of 0.5–4.9 m/s. All examinations were performed using a linear probe at 9–12 MHz, optimal for thyroid tissue evaluation. Two radiologists performed five measurements for each nodule obtaining a mean value of the shear wave velocity (denominated as virtual touch tissue quantification value or VTQ value), first with the ROI placed in the normal thyroid gland, (in the same lobe of the nodule, or in the other lobe in case of a too large nodule); thereafter, five measurements with the ROI placed within the thyroid nodule. If the measurement was not reliable, the signature “X.XX” was displayed on the screen and this was usually due to motion or breathing. Measurements in the normal gland were performed at the same depth as that of the nodule. For not altering the measurements, the operators did not include in the scan nodule degeneration phenomena such as macrocalcifications and tensive, diffuse cystic areas. Whereas in the thyroid gland, vascular structures were avoided. Each operator who performed ARFI measurements was blinded to all patients’ clinical data and to the results obtained by the other operator.
Statistical analysis
Data were collected prospectively and recorded by each radiologist in a computerized spreadsheet (Excel, Microsoft). Statistical analysis was carried out using SPSS statistical package, version 13.0 (SPSS Inc., Chicago, IL, USA). All measured data were presented as the mean ± SD. In order to assess the diagnostic performance of ARFI, the median value of the five measurements of the shear wave velocity of normal tissue and thyroid nodules was compared using the receiver operating characteristic (ROC) curve calculation.
Optimal cut-off values were chosen so that the sum of sensitivity and specificity would be the highest. Sensitivity, specificity, positive predictive and negative predictive value of ARFI were calculated. The P < 0.05 was considered statistically significant. Intra- and inter-reader variability for ARFI between the two operators was defined using Cohen’s k test (strength of agreement: <0.2 poor; 0.21–0.40 fair; 0.41–0.6 moderate; 0.61–0.80 good; 0.81–1.00 very good).
Results
The results obtained by histological examination after surgery revealed, on a total of 82 nodules analyzed, the presence of 22 malignant tumors (17 papillary carcinomas; 4 medullary carcinomas; 1 signet cell metastasis obtained after autopsy). The remaining 60 nodules included 48 nodules of benign hyperplasia; 4 inflammatory nodules (2 Graves–Basedow disease and 2 Hashimoto’s thyroiditis); 4 Plummer’s adenomas and 4 hemorrhagic cysts. The size of nodules ranged between 10 and 50 mm (mean 18.9 mm, SD: 10.08 mm) 39 of the 82 nodules were located in the right lobe of the gland; 36 nodules in the left lobe; 7 nodules were sited at the isthmus of the thyroid gland.
Virtual touch tissue quantification analysis
Measurements in the normal gland were performed with a ROI at the same depth as that of the nodule, and the depth of the measurements was between 1.0 and 3.1 cm. In 76 cases the ARFI measurements in the normal gland were performed at the same lobe as that of the nodule and in six cases, due to large dimensions of the nodule that occupied the whole lobe or almost all of it, the measurement of the normal gland was performed on the contralateral lobe.
Nodules that resulted benign at cytology and/or histology, in ARFI, had lower VTQ (shear wave velocity) values, with a mean of 2.141 ± 0.392 m/s, and showed no significant difference compared to the surrounding normal thyroid parenchyma (2.035 ± 0.518 m/s; P = 0.427). Among the benign nodules, the different groups that were formed according to the US features (hypoechoic versus isoechoic versus hyperechoic; vascularity pattern: absent versus perinodular versus peri- and intranodular; regular margin and thin halo versus irregular margins and thick halo), did not have any significant differences in ARFI elastic parameters (P > 0.045).
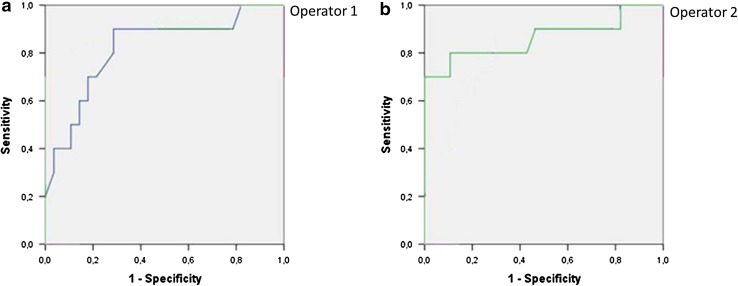
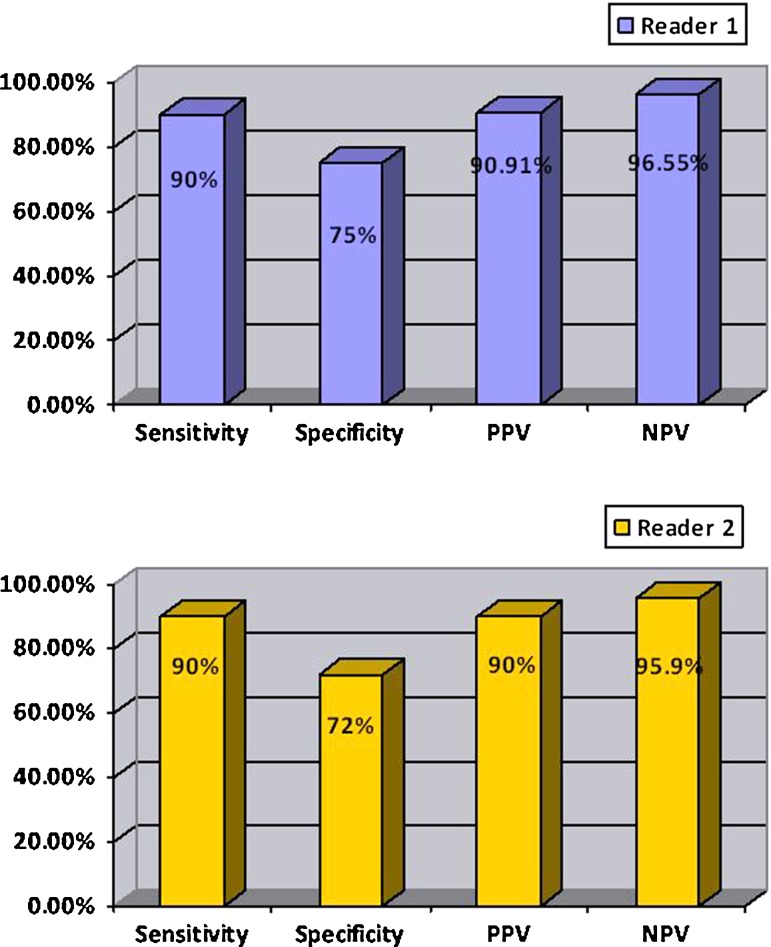
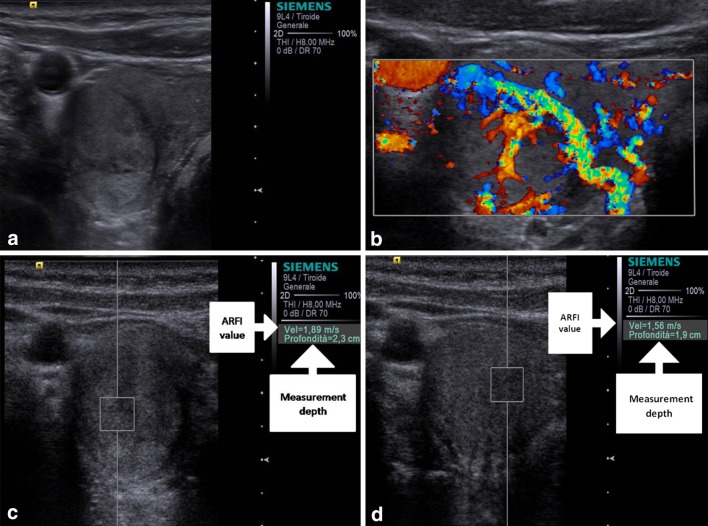
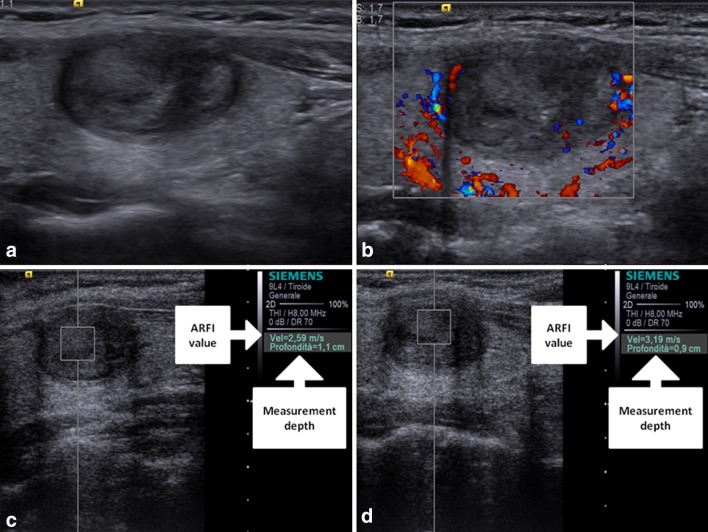
Whereas, the nodules that resulted malignant at histology had VTQ values with a mean of 3.751 ± 1.485 m/s, which was significantly higher than the value of benign nodules (2.141 ± 0.392 m/s; P < 0.001) and also higher than the value of the surrounding normal thyroid parenchyma (2.106 ± 0.491 m/s; P < 0.001). According to ROC curves (AUROC = 0.86 for Reader 1; 0.81 for Reader 2) sensitivity, and specificity for each reader were respectively 90 and 75 % for a cut-off value of shear wave of 2.455 m/s for Reader 1; 90 and 72 % for a cut-off value of shear wave of 2.365 m/s for Reader 2 (as shown in Figs. 1, 2; Table 1). The PPV and NPV for each reader were respectively 90.91 and 96.55 % for Reader 1; 90 and 95.90 % for Reader 2. Interobserver agreement was in the range considered good (k = 0.75). Intraobserver agreement was in the range considered good for benign nodules (k = 0.65 for more experienced reader and 0.63 for less experienced reader); and in the range considered good for malignant nodules (k = 0.65 for both readers). Figure 3 is an example of a benign nodule, and Fig. 4 of a malignant one.
Fig. 1.
ROC curves of ARFI for Reader 1 (AUROC = 0.86) (a) and for Reader 2 (AUROC = 0.81) (b) are showed
Fig. 2.
Values of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of ARFI for Reader 1 (a) and for Reader 2 (b) are reported in the histogram
Table 1.
The table shows the cut-off of both Readers for discriminating the nature of the nodules and their associated statistic values
| Mean cut-off ARFI | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|
| Reader 1 | 2.445 ± 0.10 m/s (P < 0.05) | 90 | 75 |
| Reader 2 | 2.365 ± 0.22 m/s (P < 0.05) | 90 | 72 |
Fig. 3.
Baseline US shows a well marginated, prevalently hyperechoic nodule with hypoechoic capsule (a) which at color-Doppler presented Pattern III and at ARFI 1.89 and 1.56 m/s of shear wave velocity, respectively, for operator 1 (c) and operator 2 (d). Histology after surgery demonstrated that the nodule was benign nodular hyperplasia
Fig. 4.
Baseline US shows a non-well marginated, prevalently hypoechoic nodule (a) which at color-Doppler presented perilesional with some tiny intralesional vascular signs and at ARFI 2.59 and 3.19 m/s of shear wave velocity, respectively, for operator 1 (c) and operator 2 (d). At FNAC the lesion was classified as Thy 3; histological examination after thyroidectomy demonstrated that the nodule was a papillary carcinoma
Discussion
The thyroid gland is a viable target for elastography. Real-time elastography (RTE) a qualitative method has been evaluated in a number of studies [8–13, 18–20]. A recent meta-analysis performed by Bojunga et al. [13] summarizes the results of several studies reporting a global sensitivity and specificity of 92 and 90 %, respectively, for RTE in the diagnosis of malignant thyroid nodules. Another recent study by Cantisani et al. [8] proved high accurate diagnostic value in the differentiation of thyroid nodules of semiquantitative elastosonography. In spite of good performance reported by these studies, thyroid US elastography has not yet gained widespread clinical use, with one of the major issues being low interobserver and intraobserver agreement. A study evaluating interobserver agreement both in data acquisition and scoring for thyroid US elastography was reported by Park et al. [21]. Three radiologists independently performed elastography data acquisition and scored on 52 malignant thyroid nodules. With external compression elastography, they found no interobserver agreement among the three radiologists. They attributed this lack of interobserver agreement in elastography mainly to the fact that the extent of compression influences both the elastography image and, consequently, elasticity score. Their results showed that the variability in elastography data acquisition is larger than that in scoring. On the other hand however, more recently Lim et al. [22] obtained good interobserver and intraobserver agreement with in vivo compression elastography.
Analyzing the state of the art of thyroid elastography, EFSUMB guidelines [23] (European Federation of Societies of Ultrasound in Medicine and Biology) conclude with the practical recommendations: “Elastography is an additional tool for thyroid lesion differentiation.” but the recommendation follows with: “Based on expert opinion, elastography may be used to guide follow-up of lesions negative for malignancy at FNAC”. Thus the limited use recommended shows the need for improvement of the modality and further thyroid validation.
Surpassing the free-hand compression method of RTE using a standardized acoustic pulse for compression, together with reliance on stiffness quantification could bring about improved performance and reproducibility. These are offered by the recent technologies enabling a quantitative determination of tissue elasticity such as the ARFI technology and shear wave elastography.
The principle of ARFI is that ultrasounds as mechanical waves, require a medium in which to spread. The wave propagation speed depends on the elastic properties of the medium and with the study of sound wave propagation speed in a medium, it is possible to assess the mechanical strain properties of the material [24, 25]. A vibration is transmitted to the tissue and it propagates as longitudinal compressional waves, and also as transverse vibrations known as shear waves. The velocity at which shear waves travel through tissue is proportional to the square root of the shear modulus (another measure of stiffness) of the tissue [26]. The velocity of the shear waves can be tracked by the small tissue displacements they cause using ultrasound methods similar to those used for normal elastography [27]. ARFI offers elasticity images as well as quantitative estimations of stiffness [21]. Integrated into a conventional ultrasonographic system, ARFI imaging can be performed during a standard examination. The target region is selected on a conventional B-mode image, and information is obtained in real time [28–30]. The quantification entails estimation of the shear wave velocity, which is measured in meters per second. The stiffer a tissue is, the greater is the shear wave velocity.
ARFI was applied to diffuse thyroid pathology evaluation with good results [14, 15]. Differently from the preliminary experience published by Friederich-Rust et al. [14] who used a curved array probe, more recently a linear probe at 9–12 MHz, optimal for thyroid tissue evaluation, is now available.
Gu et al. reported the first experience using ARFI to differentiate benign and malignant thyroid nodules. They evaluated 98 nodules and achieved sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accordance rate of 86.36, 93.42, 79.17, 95.95, and 91.84 %, respectively, based on the standard VTQ value (2.555 m/s). In total, 77.6 % (59 of 76) of the benign nodules showed softer and equal images in the VTI mode, and 77.3 % (17 of 22) of the malignant nodules showed stiffer images (P < 0.001). They concluded that ARFI imaging has high sensitivity and specificity in evaluating benign and malignant thyroid nodules and therefore a good diagnostic value in clinical applications [16]. More recently, Friedrich-Rust et al. [14] compared ARFI and RTE to differentiate benign from malignant thyroid nodules. They showed that the combination of RTE with ARFI imaging improved specificity for the diagnosis of malignant nodules from 72 (RTE alone) to 92 % but sensitivity was reduced from 76 to 58 %. Both the techniques achieved an excellent negative predictive value while the two methods together did not improve NPV.
In accordance with Gu et al. we achieved similar results, obtaining sensitivity, specificity, PPV and NPV of ARFI for reader 1 and 2 of 90, 75, 90.9 and 96.5 %, and (cut-off value of shear wave: 2.455 m/s); 90, 72, 90 and 95.90 % (cut-off value shear wave: 2.365 m/s), respectively. In addition, we tested the interobserver variability as well. We obtained good results, with a Cohen’s k value of 0.755, which is consistent with the result of the study of Lim et al. [22] (k = 0.77) and Merino et al. [31] (k = 0.82) using different elastographic techniques.
There were several limitations of this study. This was a pilot study with a limited sample size, especially with regard to malignant lesions and no follicular cancers; thus, the accuracy results are not definitive. Because of the limited patient population, detailed subgroup analysis could not be performed to determine whether specific combinations of CDUS criteria and ARFI can increase the diagnostic performance compared to CDUS alone. In the quantification model, the measure of the ROI is fixed: we used a 10 × 5 mm ROI and it cannot be modified. Nodules with cysts and calcifications had to be excluded due to the impossibility of placing the non-modifiable ROI inside the nodule in a cyst-free and calcifications-free portion of the nodule.
Conclusions
This work is a feasibility study evaluating ARFI imaging. Its results suggest that ARFI imaging is a reproducible method which can be utilized with good diagnostic performance in the thyroid for discriminating benign and malignant nodules using the cut-off value of 2.455 m/s. ARFI imaging of thyroid nodules seems to have the potentiality for differentiating benign nodules from suspicious ones, thus aiding in the decision to proceed to FNAC biopsies with greater confidence and efficiency. Benign nodules at ARFI can be managed via follow-up observations. Larger studies and multicentric ones are needed to validate this method and better define its utility.
Conflict of interest
The authors of this paper, Hektor Grazhdani, Vito Cantisani, Pietro Lodise, Giorgio Di Rocco, Maria Cristina Proietto, Eloisa Fioravanti, Antonello Rubini, Adriano Redler, declare that no fund or grant was received for this research and that there are no relationships/conditions/circumstances that present a potential conflict of interest.
Ethical standard statement
Informed consent was obtained from all patients and the study was performed in accordance with the ethical guidelines of the Helsinki Declaration and approved by the local ethics committee.
Footnotes
H. Grazhdani and V. Cantisani provided an equal contribution.
Contributor Information
Hektor Grazhdani, Phone: +39-3298183927, Email: ect70@hotmail.it.
Vito Cantisani, Phone: +39-3298183927, Email: vito.cantisani@uniroma1.it.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. Cancer statistics. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Trimboli P, Condorelli E, Catania A, Sorrenti S. Clinical and ultrasound parameters in the approach to thyroid nodules cytologically classified as indeterminate neoplasm. Diagn Cytopathol. 2009;37:783–785. doi: 10.1002/dc.21136. [DOI] [PubMed] [Google Scholar]
- 3.Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004;23:1455–1464. doi: 10.7863/jum.2004.23.11.1455. [DOI] [PubMed] [Google Scholar]
- 4.Wienke JR, Chong WK, Fielding JR, Zou KH, Mittelstaedt CA. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J Ultrasound Med. 2003;22:1027–1031. doi: 10.7863/jum.2003.22.10.1027. [DOI] [PubMed] [Google Scholar]
- 5.Sorrenti S, Trimboli P, Catania A, Ulisse S, De Antoni E, et al. Comparison of malignancy rate in thyroid nodules with cytology of indeterminate follicular or indeterminate Hürthle cell neoplasm. Thyroid. 2009;19:355–360. doi: 10.1089/thy.2008.0338. [DOI] [PubMed] [Google Scholar]
- 6.Trimboli P, Ulisse S, D’Alò M, Solari F, Fumarola A, et al. Analysis of clinical, ultrasound and colour flow-Doppler characteristics in predicting malignancy in follicular thyroid neoplasms. Clin Endocrinol. 2008;69:342–344. doi: 10.1111/j.1365-2265.2007.03158.x. [DOI] [PubMed] [Google Scholar]
- 7.Tee YY, Lowe AJ, Brand CA, Judson RT. Fine-needle aspiration may miss a third of all malignancny in palpable thyroid nodules: a comprehensive literature review. Ann Surg. 2007;246:714–720. doi: 10.1097/SLA.0b013e3180f61adc. [DOI] [PubMed] [Google Scholar]
- 8.Cantisani V, Grazhdani H, Ricci P et al (2013) Q-elastography of solid thyroid nodules: Assessment of diagnostic efficacy and inter observer variability in a large patient cohort. Eur Radiol (Epub ahead of print) [DOI] [PubMed]
- 9.Cantisani V, Lodise P, Grazhdani H et al (2013) Ultrasound elastograhpy in the evaluation of thyroid pathology. Current status. Eur J Radiol (Epub ahead of print) [DOI] [PubMed]
- 10.Ning CP, Jiang SQ, Zhang T, Sun LT, Liu YJ, et al. The value of strain ratio in differential diagnosis of thyroid solid nodules. Eur J Radiol. 2012;81:286–291. doi: 10.1016/j.ejrad.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Cantisani V, D’Andrea V, Biancari F, Medvedyeva O, Di Segni M, et al. Prospective evaluation of multiparametric ultrasound and quantitative elastosonography in the differential diagnosis of benign and malignant thyroid nodules. Eur J Radiol. 2012;81:2678–2683. doi: 10.1016/j.ejrad.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 12.Cantisani V, Ulisse S, Guaitoli E, De Vito C, Caruso R, et al. Q-elastography in the presurgical diagnosis of thyroid nodules with indeterminate cytology. PLoS One. 2012;7(11):e50725. doi: 10.1371/journal.pone.0050725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bojunga J, Herrmann E, Meyer G, Weber S, Zeuzem S, et al. Real-time elastography for the differentiation of benign and malignant thyroid nodules: a meta-analysis. Thyroid. 2010;20:1145–1150. doi: 10.1089/thy.2010.0079. [DOI] [PubMed] [Google Scholar]
- 14.Friederich-Rust M, Romenski O, Meyer G, Dauth N, Holzer K, et al. Acoustic radiation force impulse-imaging for the evaluation of the thyroid gland: a limited patient feasibility study. Ultrasonics. 2012;52:69–74. doi: 10.1016/j.ultras.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Sporea I, Vlad M, Bota S, Sirli RL, Popescu A, et al. Thyroid stiffness assessment by acoustic radiation force impulse elastography (ARFI) Ultraschall Med. 2011;32:281–285. doi: 10.1055/s-0029-1246048. [DOI] [PubMed] [Google Scholar]
- 16.Gu J, Du L, Bai M, Chen H, Jia X, et al. Preliminary study on the diagnostic value of acoustic radiation force impulse technology for differentiating between benign and malignant thyroid nodules. J Ultrasound Med. 2012;31:763–771. doi: 10.7863/jum.2012.31.5.763. [DOI] [PubMed] [Google Scholar]
- 17.(1996) Guidelines of the Papanicolaou Society of Cytopathology for the examination of fine-needle aspiration specimens from thyroid nodules. The Papanicolaou Society of Cytopathology task force on standards of practice. Mod Pathol 9:710–715 [PubMed]
- 18.Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, et al. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237:202–211. doi: 10.1148/radiol.2363041248. [DOI] [PubMed] [Google Scholar]
- 19.Wilson T, Chen Q, Zagzebski JA, Varghese T, Van Middlesworth L. Initial clinical experience imaging scatterer size and strain in thyroid nodules. J Ultrasound Med. 2006;25:1021–1029. doi: 10.7863/jum.2006.25.8.1021. [DOI] [PubMed] [Google Scholar]
- 20.Xing P, Wu L, Zhang G, Li S, Liu C, et al. Differentiation of benign from malignant thyroid lesions: calculation of the strain ratio on thyroid sonoelastography. J Ultrasound Med. 2011;30:663–669. doi: 10.7863/jum.2011.30.5.663. [DOI] [PubMed] [Google Scholar]
- 21.Park SH, Kim SJ, Kim EK, Kim MJ, Son EJ, Kwak JY. Interobserver agreement in assessing the sonographic and elastographic features of malignant thyroid nodules. AJR. 2009;193(5):W416–W423. doi: 10.2214/AJR.09.2541. [DOI] [PubMed] [Google Scholar]
- 22.Lim DJ, Luo S, Kim MH, Ko SH, Kim Y. Interobserver agreement and intraobserver reproducibility in thyroid ultrasound elastography. AJR. 2012;198:896–901. doi: 10.2214/AJR.11.7009. [DOI] [PubMed] [Google Scholar]
- 23.Cosgrove D, Piscaglia F, Bamber J et al (2013) EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: clinical applications. Ultraschall Med 34:238–53 (also free on line at http://www.efsumb.org) [DOI] [PubMed]
- 24.Shan B, Pelegri AA, Maleke C, Konofagou EE. A mechanical model to compute elastic modulus of tissue for harmonic motion imaging. J Biomech. 2008;41:2150–2158. doi: 10.1016/j.jbiomech.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Sumi C. Regularization of tissue shear modulus reconstruction using strain variance. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:297–307. doi: 10.1109/TUFFC.2008.649. [DOI] [PubMed] [Google Scholar]
- 26.Garra BS. Elastography: current status, future prospects, and making it work for you. Ultrasound Q. 2011;27:177–186. doi: 10.1097/RUQ.0b013e31822a2138. [DOI] [PubMed] [Google Scholar]
- 27.Nightingale K, Bentley R, Trahey G. Observations of tissue response to acoustic radiation force: opportunities for imaging. Ultrason Imaging. 2002;24:129–138. doi: 10.1177/016173460202400301. [DOI] [PubMed] [Google Scholar]
- 28.Nightingale KR, Palmeri ML, Nightingale RW, Trahey GE. On the feasibility of remote palpation using acoustic radiation force. J Acoust Soc Am. 2001;110:625–634. doi: 10.1121/1.1378344. [DOI] [PubMed] [Google Scholar]
- 29.Zhai L, Palmeri ML, Bouchard RR, Nightingale RW, Nightingale KR. An integrated indenter-ARFI imaging system for tissue stiffness quantification. Ultrason Imaging. 2008;30:95–111. doi: 10.1177/016173460803000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fahey BJ, Palmeri ML, Trahey GE. The impact of physiological motion on tissue tracking during radiation force imaging. Ultrasound Med Biol. 2007;33:1149–1166. doi: 10.1016/j.ultrasmedbio.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merino S, Arrazola J, Cárdenas A, Mendoza M, De Miguel P, et al. Utility and interobserver agreement of ultrasound elastography in detection of malignant thyroid nodules in clinical care. Am J Neuroradiol. 2011;32:2142–2148. doi: 10.3174/ajnr.A2716. [DOI] [PMC free article] [PubMed] [Google Scholar]