Abstract
Rationale
Treatment with estradiol, the primary estrogen produced by the ovaries, enhances hippocampus-dependent spatial memory and increases levels of hippocampal synaptic proteins in ovariectomized rats. Increasing evidence indicates that the ability of estradiol to impact the brain and behavior is dependent upon its interaction with insulin-like growth factor-1 (IGF-1).
Objectives
The goal of the current experiment was to test the hypothesis that the ability of estradiol to impact hippocampus-dependent memory and levels of hippocampal synaptic proteins is dependent on its interaction with IGF-1.
Methods
Adult rats were ovariectomized and implanted with estradiol or control capsules and trained on a radial-maze spatial memory task. After training, rats were implanted with intracerebroventricular cannulae attached to osmotic minipumps (flow rate 0.15 μl/hr). Half of each hormone treatment group received continuous delivery of JB1 (300 μg/ml), an IGF-1 receptor antagonist, and half received delivery of aCSF vehicle. Rats were tested on trials in the radial-arm maze during which delays were imposed between the 4th and 5th arm choices. Hippocampal levels of synaptic proteins were measured by western blotting.
Results
Estradiol treatment resulted in significantly enhanced memory. JB1 blocked that enhancement. Estradiol treatment resulted in significantly increased hippocampal levels of postsynaptic density protein 95 (PSD-95), spinophilin, and synaptophysin. JB1 blocked the estradiol-induced increase of PSD-95 and spinophilin and attenuated the increase of synaptophysin.
Conclusions
Results support a role for IGF-1 receptor activity in estradiol-induced enhancement of spatial memory that may be dependent on changes in synapse structure in the hippocampus brought upon by estradiol/IGF-1 interactions.
Keywords: estradiol, estrogen, IGF-1, hippocampus, learning, memory, synaptic proteins, PSD-95, spinophilin, synaptophysin
Introduction
The beneficial effects exerted by 17β-estradiol (estradiol) on cognitive function are hypothesized to be due in large part to its impact on the hippocampus, an area of the brain crucial for learning and memory (Bimonte-Nelson et al. 2010; Daniel 2006; Frick 2009; Korol 2004; Spencer et al. 2008). For example, estradiol-induced changes in hippocampal synaptic plasticity have been extensively documented. Changes in dendritic spine density are observed in the hippocampus throughout the estrous cycle of rats, whereby the highest dendritic spine density is correlated to when estradiol levels are at their greatest (Woolley et al. 1990). Additionally, ovariectomy decreases dendritic spine density in the CA1 region of the hippocampus, which can then be restored with systemic estradiol treatment (Gould et al. 1990; MacLusky et al. 2005; Woolley and McEwen 1992). Along with increases in spine and synapse density, estradiol increases hippocampal levels of the molecular components of the synapse, including the postsynaptic proteins postsynaptic density 95 (PSD-95) and spinophilin as well as the presynaptic protein synaptophysin (Brake et al. 2001; Sharma et al. 2007; Waters et al. 2009). As both learning (Leuner et al. 2003) and long-term potentiation (Engert and Bonhoeffer 1999; Muller et al. 2000), a putative synaptic mechanism of learning (Whitlock et al. 2006), are associated with increases in dendritic spine density, synaptic change in the hippocampus is a possible mechanism through which estradiol affects cognition (Daniel and Dohanich 2001; Sandstrom and Williams 2001).
Similar to estradiol, insulin-like growth factor-1 (IGF-1), a hormone produced both peripherally by the liver and locally in the brain (Trejo et al. 2007), is associated with enhanced cognitive function through complex mechanisms involving plasticity (Aleman and Torres-Aleman 2009; Shi et al. 2005). For example, when serum IGF-1 is low, the ratio of excitatory to inhibitory synapses in the hippocampus is decreased (Trejo et al., 2007). Additionally, like estradiol, IGF-1 impacts levels of pre- and postsynaptic proteins in the hippocampus (Cassilhas et al. 2012; Tropea et al. 2009). Furthermore, IGF-1 treatment enhances cognitive performance on spatial memory tasks and also increases hippocampal neurogenesis in both young and aged male mice and rats that are deficient in peripheral IGF-1 (Aberg et al. 2000; Lichtenwalner et al. 2001; Sonntag et al. 2005; Trejo et al. 2001). Therefore, a possible mechanism through which IGF-1 affects cognition is through regulation of hippocampal synapses.
A growing body of evidence indicates that IGF-1 and estradiol interactions are crucial to the ability of each to impact neuroprotection, neurogenesis and neuroplasticity (Cardona-Gomez et al. 2001; Garcia-Segura et al. 2010; Quesada and Micevych 2004). For example, in order for estradiol or IGF-1 treatment to have beneficial effects on neuronal survival in hypothalamic neurons, activation of both of their receptors is necessary, as antagonizing either blocks beneficial effects (Duenas et al. 1996). Additionally, IGF-1 receptor activation in the hypothalamus is needed for estradiol-induced plastic changes at synapses as well as the induction of sexual behavior in female rats (Etgen and Acosta-Martinez 2003). Like in the hypothalamus, interactions between IGF-1 signaling and estradiol are evident in the hippocampus. IGF-1- induced neurogenesis in the hippocampus is dependent on estrogen receptor signaling (Perez-Martin et al. 2003). Estradiol regulates hippocampal levels of IGF-1 receptor protein and mRNA (Cardona-Gomez et al. 2001) and IGF-1 binding protein-2 (Pechenino and Frick 2009), the most abundant IGF-1 binding protein in the brain. In our lab, we recently demonstrated that the cognitive enhancement exhibited by aging ovariectomized rats that had previous short-term treatment with estradiol in middle-age was blocked by antagonizing brain IGF-1 receptors beginning after the estradiol treatment had been terminated, results that support a putative role for IGF-1 in ligand-independent activation of estrogen receptor (Witty et al. 2013). To date, no studies have examined the role of IGF-1 receptor activation in the ability of ongoing estradiol treatment to impact learning and memory and hippocampal plasticity.
The goal of the current study was to test the hypothesis that the ability of estradiol to impact hippocampal dependent memory and levels of hippocampal synaptic proteins important for plasticity is dependent on its interaction with IGF-1. We infused JB1, an IGF-1 receptor antagonist, or vehicle into the lateral ventricles of ovariectomized rats, half of which were treated with chronic estradiol and half of which received control treatment. Spatial memory was assessed using a radial-maze task and levels of PSD-95, spinophilin, and synaptophysin in the hippocampus were measured by western blotting.
Methods
Subjects and Treatments
Forty female Long-Evans hooded rats, approximately 2 months of age, were purchased from Harlan Sprague-Dawley (Indianapolis, IN). Animal care was in accordance with guidelines set by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and all procedures were approved by the Institutional Animal Care and Use Committee of Tulane University. Rats were housed individually in a temperature controlled vivarium under a 12-h light/dark cycle (lights on at 7:00 a.m.). One week after arrival, all rats were ovariectomized while under anesthesia induced by injections of ketamine (100 mg/kg i.p., Bristol Laboratories, Syracuse, NY) and xylazine (7 mg/kg i.p., Miles Laboratories, Shawnee, KS). At the time of surgery, animals were implanted with 5 mm Silastic brand capsules (0.058-in i.d. and 0.077-in. o.d., Dow Corning, Midland, MI) containing either 25% 17β-estradiol (Sigma Chemical, St. Louis, MO) diluted with cholesterol or 100% cholesterol vehicle. We have reported previously that implants of these dimensions maintain blood plasma estradiol levels of 26–47 pg/ml, which fall within the physiological range of cycling female rats (Bohacek and Daniel 2007; Bohacek and Daniel 2010).
Maze Training
Two weeks after ovariectomy, rats were placed on diets to maintain body weights at 85–90% of pre-surgery weights and were trained to obtain food rewards (Froot Loops; Kellogg Co., Battle Creek, MI) from the arms of an elevated eight-arm radial maze purchased from Lafayette Instruments (Lafayette, IN). The maze consisted of black metal floors and clear acrylic walls with arms (10 cm wide × 70 cm long × 20 cm high) extending out from an octagonal center (33 cm across). The maze was located in the center of a 3 × 5 m room and raised approximately 1 m from the floor. Several extra-maze cues, including overhead fluorescent lights, desk, chairs, sink, and door, were visible from the maze. To begin a trial, a rat was placed in the center compartment in a pseudorandom orientation and had access to all eight arms. Arm choices were recorded by an observer seated in a fixed location approximately 1 m away from the maze. An arm choice was scored if the rat traversed half the length of an arm. Rats were allowed to choose arms in any order until all arms had been visited or 5 minutes elapsed. Errors were reentries into previously visited arms. Performance was assessed by the number of errors of the first eight arm choices. Each animal received one trial per day across 24 days of acquisition. At the end of the acquisition period, rats were averaging less than one error of the first eight arm choices. No differences in performance between control- and estradiol-treated animals were apparent.
Initiation of Drug Treatment
After acquisition of the radial maze task was completed, drug treatments were initiated. Rats were anesthetized with ketamine and xylazine and placed in a stereotaxic frame. An incision was made in the scalp and fascia that overlie the skull. A hole was drilled in the skull and cannulae (Brain Infusion Kits, Alzet; Cupertino, CA) were lowered through the hole to the appropriate depth (to the right lateral ventricle located −0.3 mm AP, +1.2 mm ML, and −4.5 mm DV) and anchored to the skull with screws and dental acrylic. Cannulae were connected to Alzet osmotic minipumps by vinyl tubing that delivered artificial cerebrospinal fluid (aCSF) vehicle (Tocris; Ellisville, MO) or JB1 (300 μg/ml) (Bachem; Torrance, CA), an IGF-1 receptor antagonist, diluted in vehicle at a rate of 0.15 μl/h. All pumps were implanted s.c. in the nape of the neck and cannulae were inserted after the pumps began pumping. Half of the cholesterol-treated rats received osmotic minipumps containing JB1 (CH + JB1 n = 10), and half received vehicle aCSF (CH + aCSF n = 10). Half of the estradiol-treated rats received osmotic minipumps containing JB1 (E + JB1, n = 10) and half received aCSF (E + aCSF, n = 10). To facilitate procedures, surgeries and subsequent behavior testing and sacrifice were staggered across three cohorts. All groups were represented in all cohorts.
Behavioral Testing
Rats were allowed approximately one week to recover from surgeries before being tested on the radial-arm maze. Delay trials were performed on the radial maze during which various delays were imposed between the fourth and fifth arm choices. Consequently, the animal had to remember over an extended period of time which arms had already been visited. After each fourth arm choice, the animal was removed from the maze and put in a holding cage in a separate room for various delays. Then the animal was returned to the maze until the four remaining, still baited arms, had been visited or until 5 min had elapsed. Arm choice accuracy was measured by the number of errors of the first four arm choices after the delay. Rats were given one day of habituation to a one-minute delay trial. Subsequently, two trials (one per day) were conducted for each of five delays (1 min, 30 min, 1 hr, 2 hrs and 4 hrs). During the course of testing, four rats (one from each group) had complications related to cannulae and/or mini-pump implantation and were excluded from the experiment.
Western Blotting
Western blotting procedures were used to determine the independent and interactive effects of exposure to estradiol and subsequent treatment with an IGF-1 antagonist on hippocampal levels of the postsynaptic proteins PSD-95 and spinophilin and the presynaptic protein synaptophysin.
Tissue Dissection and Processing
Rats used in behavioral testing were killed after completion of delay trials by decapitation under anesthesia induced by ketamine and xylazine. At this point, rats had been exposed to estradiol or cholesterol treatment for approximately two months and had been exposed to JB1 or vehicle treatment for approximately three weeks. The hippocampus from the left hemisphere was dissected on ice, quick-frozen on dry ice, and stored at −80 C until processing. Tissue was homogenized in 15 μl/mg lysis buffer containing 1mM EGTA, 1mM EDTA, 20 mM Tris, 1 mM sodium pyrophosphate tetrabasic decahydrate, 4 mM 4-nitrophenyl phosphate disodium salt hexahydrate, 0.1 μM microcystin, and 1% protease inhibitor cocktail (Sigma-Aldrich). Samples were centrifuged for 15 min at 1000 × g at 4 C, protein concentration of supernatants was determined (Bradford Protein Assay Kit; Pierce, Rockford, IL), and each sample was diluted 1:1 with Laemmli Sample Buffer (Bio-Rad; Hercules, CA) mixed with 350 mM D,L-dithiothreitol, boiled for 5 min, and stored at −80 C.
Electrophoresis and Immunostaining
For each sample, 20 μg of total protein were loaded and separated at 200 V on 10% SDS-PAGE gels (Bio-Rad) for 65 minutes (PSD-95, synaptophysin, spinophilin/neurabin-II). Molecular weight markers (Kaleidoscope; Bio-Rad) were included with each run. Proteins were transferred to nitrocellulose membranes at 100 V for 1 h. The membranes were cut to allow for simultaneous development of the protein of interest with the loading control, β-actin. The membranes were blocked with 5% nonfat dry milk in 0.1% Tween/1 x Tris-buffered saline (TTBS) at room temperature for 1 h. This was followed by incubation with primary antibody for PSD-95 (rabbit monoclonal, 1:1500; Millipore, Billerica, MA), synaptophysin (mouse monoclonal, 1:600; Sigma, St. Louis, MO), spinophilin or neurabin II (mouse monoclonal, 1:100; Santa Cruz; Santa Cruz, CA), and β-actin (mouse monoclonal, 1:15000; Santa Cruz; Santa Cruz, CA) overnight at 4 C in 1% nonfat dry milk-TTBS (PSD-95, synaptophysin, spinophilin) or TTBS (β-actin). Blots were washed three times for 15 min each with TTBS and incubated with 5% nonfat dry milk containing goat antirabbit IgG (Santa Cruz, Santa Cruz, CA; PSD-95, 1:5000) and goat antimouse IgG (Santa Cruz, Santa Cruz, CA; synaptophysin, 1:4000; spinophilin, 1:2000; β-actin, 1:10000). Blots were washed again for 15 min each and incubated for 5 min with the chemiluminescent substrate SuperSignal West Femto (Pierce) for PSD-95 or for 1 min with the chemiluminescent substrate ECL (Pierce) for synaptophysin, spinophilin, and β-actin. They were then exposed to film (Kodak Biomax MR) for varying durations to capture optimal signal intensity. Films were imaged using MCID 7.0 imaging software (Interfocus Imaging Ltd., Cambridge, England), and a single user-defined template was established for each blot to measure DxA for bands of interest. Mean values were calculated for the cholesterol-treated aCSF group (control group) for each blot. All values are expressed as a percentage relative to control per blot. Samples from each group were equally represented on each blot.
Cannulae Placement Confirmation
Right hemispheres from each rat were quick frozen on dry ice at the time of sacrifice. Coronal slices (20μM) of the brain were put on gelatinized slides, stained with Cresyl violet and analyzed with a Nikon light microscope. Correct cannula placement was verified in all animals.
Hormone Treatment Efficacy
At the time the rats were killed, uteri were extracted and 1-cm-long sections of the right uterine horns (cut at the base) were weighed to verify efficacy of hormone treatment. Additionally, proper removal of the ovaries and integrity of the implanted capsules were verified.
Statistical Analyses
Arm-choice accuracy data from each delay were averaged across the two days of testing and analyzed by three-way ANOVA (hormone x drug x delay) with repeated measures on delay. Western blotting data were analyzed with two-way ANOVA (hormone x drug). Posthoc tests (Fisher’s LSD, P < .05) were used to probe group differences following significant interactions of hormone x drug. A one-way ANOVA, with hormone group as the factor, was used to test for differences in uterine weight.
Results
Radial-Arm Maze
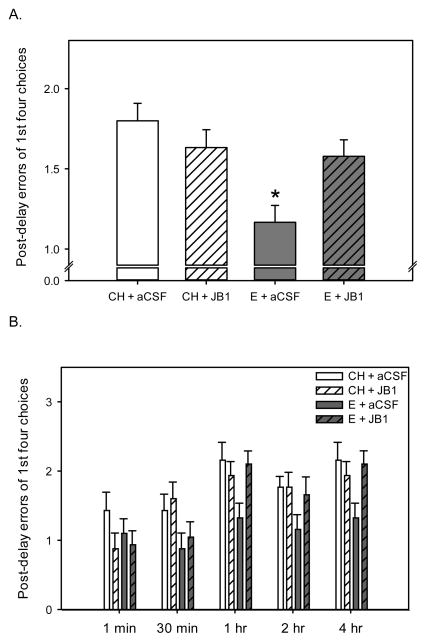
As illustrated in Fig. 1A, estradiol-treated rats that received aCSF infusions made significantly fewer errors during the post delay in the radial-arm maze across all delay trials than all other groups. There was a significant main effect of hormone (F(1,32) = 7.5, P=0.01). There was also a significant interactive effect of hormone x drug (F(1,32)= 5.3 P= 0.029), indicating the effect of estradiol treatment differed depending upon whether or not JB1 was administered. There was no main effect of drug. Post hoc analyses revealed that the rats that received estradiol treatment and control aCSF infusion (E + aCSF) had significantly better arm choice accuracy (fewer errors) than rats that received cholesterol control treatment and aCSF or JB1 infusions (CH + aCSF, CH + JB1) as well as rats that received estradiol treatment and JB1 infusions (E + JB1). Thus, chronic intracerebroventricular administration of JB1, an IGF-1 receptor antagonist, blocked the estradiol-induced enhancement in performance. There was a main effect of delay (F(1,32) = 54.4, P<0.001), but no interaction of delay with hormone or drug (Fig. 1B). Results indicate that activation of IGF-1 receptors is a necessary component in the ability of estradiol treatment to exert lasting cognitive benefits.
Fig 1.
Effects of estradiol and JB1, an IGF-1 receptor antagonist, on radial-arm maze performance in ovariectomized rats when delays were inserted between the 4th and 5th arm choices. Rats were ovariectomized and received subcutaneous implants containing estradiol (E) or cholesterol vehicle (CH). JB1 or aCSF vehicle was chronically delivered via intracerebroventricular infusion (n = 9 for all groups). (A) Mean number of errors (±SEM) across all delay trials. *P<0.05, E + aCSF vs. all other groups. (B) Mean number of errors (±SEM) at each delay.
Western Blots
PSD-95
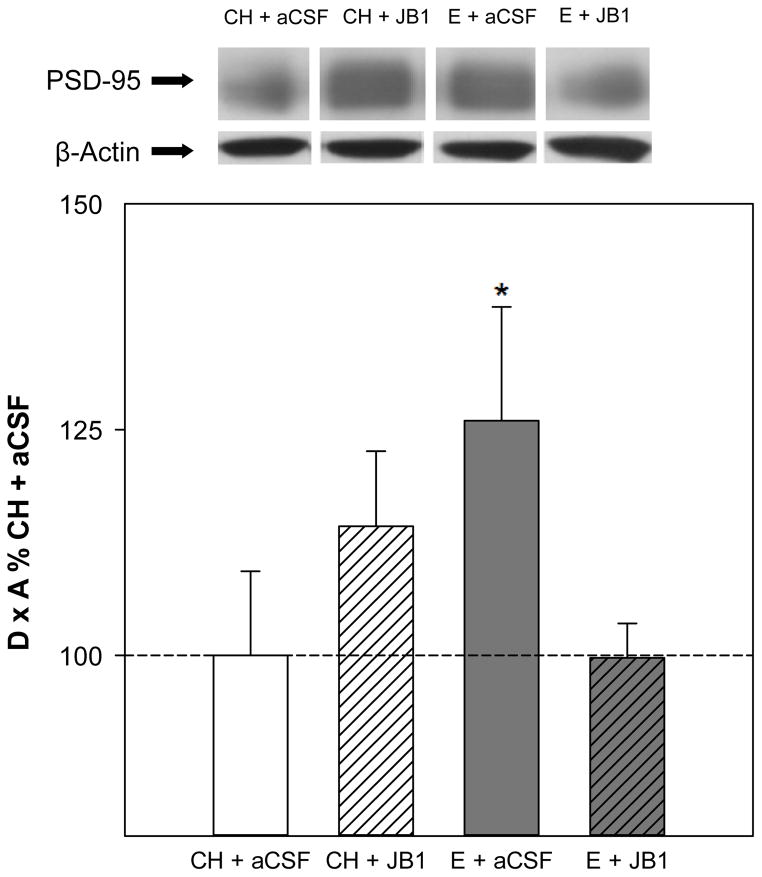
Western blots revealed a single band of PSD-95-like immunoreactivity at approximately 95 kDa. Analyses revealed no significant main effects of hormone or drug on protein levels of PSD-95 in the hippocampus. As shown in Fig. 2, there was a significant interactive effect of hormone x drug (F(1,32) = 5.0, P = .033), indicating that the ability of estradiol treatment to impact PSD-95 levels varies depending upon whether or not JB1 was administered. Post hoc analyses revealed the E + aCSF group had significantly higher levels of PSD-95 in the hippocampus than the CH + aCSF and the E + JB1 group. There were no effects of treatments on levels of β-actin, the loading control.
Fig 2.
Effects of estradiol and JB1, an IGF-1 receptor antagonist, on postsynaptic density protein 95 (PSD-95) levels in the hippocampus. Rats were ovariectomized and received subcutaneous implants containing estradiol (E) or cholesterol vehicle (CH). JB1 or aCSF vehicle was chronically delivered via intracerebroventricular infusion (n = 9 for all groups). Western blot data showing the effects of treatments on protein levels of PSD-95. Mean density x area (D x A) (±SEM) are expressed relative to CH + aCSF controls. *P < 0.05 vs. CH + aCSF and E + JB1. Representative blot images for PSD-95 and loading control β-actin are shown in insets above graph.
Spinophilin
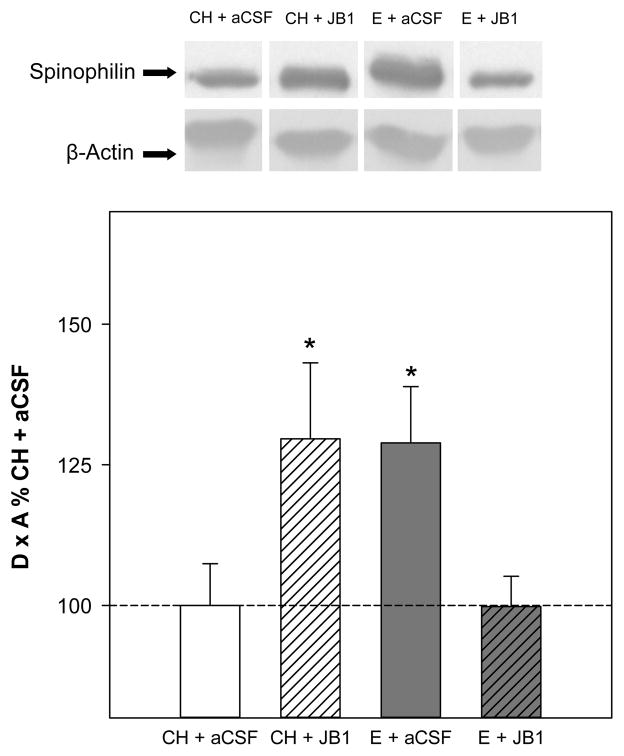
Western blots revealed a single band of spinophilin/neurabin-II-like immunoreactivity at approximately 140 kDa. Analyses revealed no significant main effects of hormone or drug on protein levels of spinophilin in the hippocampus. As shown in Fig. 3, there was a significant interactive effect of hormone x drug (F(1,32) = 9.3, P = .005), indicating that the ability of estradiol treatment to impact spinophilin levels varies depending upon whether or not JB1 was administered. Post hoc analyses revealed that the E + aCSF group and unexpectedly the CH + JB1 group had significantly higher levels of spinophilin in the hippocampus than the CH + aCSF and the E + JB1 group. There were no effects of treatments on levels of β-actin.
Fig 3.
Effects of estradiol and JB1, an IGF-1 receptor antagonist, on spinophilin levels in the hippocampus. Rats were ovariectomized and received subcutaneous implants containing estradiol (E) or cholesterol vehicle (CH). JB1 or aCSF vehicle was chronically delivered via intracerebroventricular infusion (n = 9 for all groups). Western blot data showing the effects of treatments on protein levels of spinophilin. Mean density x area (D x A) (±SEM) are expressed relative to CH + aCSF controls. **P < 0.05 vs. CH + aCSF and E + JB1. Representative blot images for spinophilin and the loading control β-actin are shown in insets above graph.
Synaptophysin
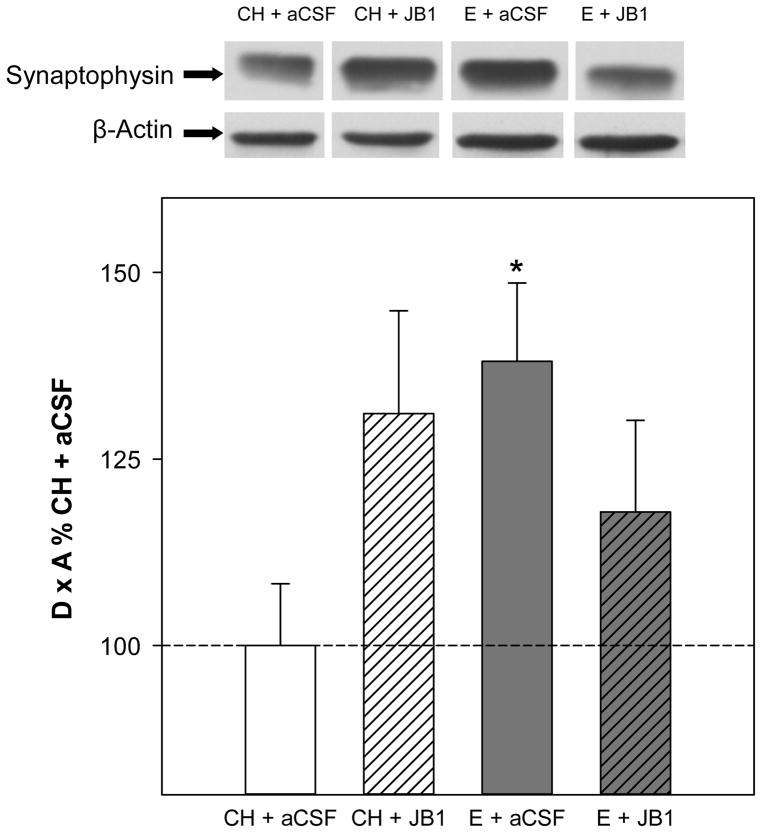
Western blots revealed a single band of synaptophysin-like immunoreactivity at approximately 38 kDa. Analyses revealed no significant main effects of hormone or drug on protein levels of synaptophysin in the hippocampus. As shown in Fig. 4, there was a significant interactive effect of hormone x drug (F(1,32) = 5.0, P = .032), indicating that the ability of estradiol treatment to impact synaptophysin levels varies depending upon whether or not JB1 was administered. Post hoc analyses revealed the E + aCSF group had significantly higher levels of synaptophysin in the hippocampus than the CH + aCSF. Interestingly, the CH + JB1 group showed a trend of an increase in (p=0.063) as compared to CH + aCSF. There were no effects of treatments on levels of β-actin.
Fig 4.
Effects of estradiol and JB1, an IGF-1 receptor antagonist, on synaptophysin levels in the hippocampus. Rats were ovariectomized and received subcutaneous implants containing estradiol (E) or cholesterol vehicle (CH). JB1 or aCSF vehicle was chronically delivered via intracerebroventricular infusion (n = 9 for all groups). Western blot data showing the effects of treatments on protein levels of synaptophysin. Mean density x area (D x A) (±SEM) are expressed relative to CH + aCSF controls. *P < 0.05 vs. CH + aCSF. Representative blot images for synaptophysin and the loading control β-actin are shown in insets above graph.
Hormone Treatment Efficacy
There was a significant difference between groups in uterine weight (F(1,35) =499.0, P = .033), indicating that hormone treatments were effective. Estradiol-treated rats had larger uteri (mean ± SEM; 84.7 ± 3.0 mg) than cholesterol-treated rats (13.0 ± 1.1 mg).
Discussion
The results of the present study provide behavioral and molecular evidence to support a role for IGF-1 receptor activation in estradiol-induced enhanced spatial memory in female rats. Intracerebroventricular infusions of an antagonist of IGF-1 receptor, JB1, blocked an estradiol-induced enhancement in performance on a hippocampal-dependent radial-maze task. Furthermore, JB1 infusion completely blocked an estradiol-induced increase in the postsynaptic proteins PSD-95 and spinophilin, and attenuated the estradiol-induced increase in the presynaptic protein synaptophysin. These results indicate that IGF-1 receptor activation is needed in order for chronic estradiol treatment to affect cognition and levels of synaptic proteins in the hippocampus. To our knowledge these results are the first to demonstrate the importance of IGF-1 receptor signaling in relation to the effects of chronic estradiol treatment on memory and synaptic plasticity in the hippocampus.
IGF-1 impacts estradiol signaling in the brain through multiple pathways. Estradiol exerts its effects through activation of nuclear estrogen receptors to regulate gene transcription as well as through activation of membrane associated receptors to rapidly affect intracellular signaling (Vasudevan and Pfaff 2008). IGF-1 receptor, a membrane associated tyrosine kinase receptor that is widely expressed in the brain, activates two main downstream signaling pathways, the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase (PI3K)/Akt pathways (Russo et al. 2005). IGF-1 initiated signaling via these pathways modulates estradiol-dependent estrogen receptor transcriptional activity (Mendez and Garcia-Segura 2006) and also can result in ligand-independent estrogen receptor transcription in the absence of estradiol (Kato et al. 1995). In addition to its influence on estrogen receptor-dependent transcription, IGF-1 can impact rapid estradiol signaling. Like IGF-1, estradiol rapidly activates the ERK/MAPK and PI3K/Akt pathways (Fan et al. 2010), providing potential mechanisms for crosstalk between the systems. Consistent with that idea is the demonstration of synergistic action of IGF-1 and estradiol on Akt activity (Cardona-Gomez et al. 2002). Further, estrogen receptor can physically interact with IGF-1 in an estradiol-dependent process (Mendez et al. 2003). Thus, multiple potential mechanisms exist whereby IGF-1 can impact estradiol effects on the brain and behavior.
Results of the current study demonstrate that JB1 is able to block both estradiol-induced changes in memory and in levels of hippocampal synaptic proteins. Estradiol (Spencer et al. 2008) and IGF-1 (Sonntag et al. 2000) impact the structure of synapses in the hippocampus to enhance synaptic efficacy through increases in dendritic spine density. This enhancement is associated with an increase in the postsynaptic density (PSD) that is critical for neurotransmission of NMDA and AMPA receptors (Toni et al. 1999) involved in learning and memory (Malenka and Bear 2004; Whitlock et al. 2006). This increase in PSD through synaptogenesis is correlated to higher levels of the postsynaptic protein, PSD-95 (El-Husseini et al. 2000). Both IGF-1 (Tropea et al. 2009) and estradiol (Brake et al. 2001; Waters et al. 2009) increase levels of hippocampal PSD-95, a scaffolding protein involved in excitatory neurotransmission and synaptic strength (Kim and Sheng 2004) that is crucial in learning and memory (Migaud et al. 1998). Furthermore, estradiol increases spinophilin, also called neurabin II, in the hippocampus, which is another postsynaptic protein that is used as a marker for dendritic spine density and can modulate excitatory neurotransmission (Brake et al. 2001). Although no studies have directly linked IGF-1 to spinophilin, IGF-1 treatment increases dendritic spine density in the hippocampus, which is associated with increases in spinophilin (Tropea et al. 2009). In addition to affecting postsynaptic proteins, IGF-1 (Cassilhas et al. 2012) and estradiol (Sharma et al. 2007) also influence presynaptic proteins such as synaptophysin, a component of synaptic vesicles that may determine efficacy and strength of a synapse. Whereas both IGF-1 and estradiol have previously been shown to independently impact hippocampal synaptic plasticity that may represent a neural correlate for learning and memory, our data are the first to demonstrate that estradiol effects are mediated by interactions with IGF-1 signaling.
The particular mechanisms by which IGF-1 interacts with estradiol signaling in the hippocampus to impact memory and levels of hippocampal synaptic proteins are as yet unclear. However, IGF-1 receptors and estrogen receptors are co-localized on hippocampal neurons (Cardona-Gomez et al. 2000) and cross-regulation between these systems is evident. For example, antagonism of brain estrogen receptor in ovariectomized rats resulted in downregulation of IGF-1 receptor protein and mRNA in the hippocampus (Cardona-Gomez et al. 2001). A functional consequence for their interaction is supported by results of a study examining mechanisms by which kainic acid-induced neuronal cell loss in the hippocampus of ovariectomized rats was prevented by either estradiol or IGF-1 administration (Azcoitia et al. 1999). Chronic ventricular infusion of JB1 or ICI, 182, 780, an estrogen receptor antagonist, blocked the beneficial effects of estradiol or IGF-1, respectively. Furthermore, antagonism of brain estrogen receptors blocked the ability of IGF-1 to induce neurogenesis in the dentate gyrus in ovariectomized rats that were or were not treated with estradiol (Perez-Martin et al. 2003). The present data, illustrating that activation of IGF-1 receptors is a necessary component in the ability of estradiol to induce synaptic plasticity in the hippocampus and to enhance hippocampus-dependent memory, add to the growing literature documenting interdependence of IGF-1, estradiol, their pathways and their receptors in the hippocampus.
In the current results, antagonizing brain IGF-1 receptors via JB1 treatment in the ovariectomized cholesterol control-treated animals unexpectedly resulted in significantly increased levels of spinophilin and a trend for an increase in synaptophysin as compared to aCSF vehicle treatment. These results are in contrast to the decreased levels of these proteins in the hippocampus resulting from JB1 treatment in the estradiol-treated animals. In spite of the increases in levels of spinophilin and synaptophysin induced by JB1 in the ovariectomized controls, there was no parallel JB1-induced memory enhancement. Interestingly, we observed a similar JB1-dependent increase of hippocampal levels of choline acetyltransferase in aging ovariectomized animals that had never received estradiol treatment without an associated increase in performance on a spatial memory task (Witty et al. 2013). The mechanism behind these divergent effects of JB1 is unknown, but the present results should be viewed in light of limitations associated with western blotting techniques. Our results do not provide information as to regional, cellular or subcellular localization of proteins impacted by treatments. Thus, caution should be applied when associating behavioral changes to changes in levels of synaptic proteins measured in whole hippocampus homogenate. Nevertheless, our results demonstrate the complexity of the effects of IGF-1 receptor activation on the brain and behavior that are not as yet completely understood (Piriz et al. 2011). Continued investigation into the effects of IGF-1 on the hippocampus and associated behaviors under different estrogenic conditions is warranted.
In summary, the results of the present study highlight for the first time the importance of IGF-1 receptor activation in mediating estradiol-induced changes in synaptic plasticity and associated changes in hippocampus-dependent memory. Although further investigation is required to determine mechanisms by which these systems interact in the hippocampus to affect plasticity and memory, these data indicate that IGF-1 signaling plays a key role in the ability to estradiol to exert its effects.
Acknowledgments
Grant Sponsor: National Science Foundation
Grant Number: 0951008
Grant Sponsor: National Institutes of Health
Grant Number: RO1AG041374
This work was supported by National Science Foundation Grant 0951008 and National Institutes of Health Grant R01AG041374 to JMD.
Footnotes
Conflicts of Interest: None
Reference List
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A, Torres-Aleman I. Circulating insulin-like growth factor I and cognitive function: neuromodulation throughout the lifespan. Prog Neurobiol. 2009;89:256–265. doi: 10.1016/j.pneurobio.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Neuroprotective effects of estradiol in the adult rat hippocampus: interaction with insulin-like growth factor-I signalling. J Neurosci Res. 1999;58:815–822. doi: 10.1002/(sici)1097-4547(19991215)58:6<815::aid-jnr8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Acosta JI, Talboom JS. Neuroscientists as cartographers: mapping the crossroads of gonadal hormones, memory and age using animal models. Molecules. 2010;15:6050–6105. doi: 10.3390/molecules15096050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Horm Behav. 2007;52:237–243. doi: 10.1016/j.yhbeh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. The beneficial effects of estradiol on attentional processes are dependent on timing of treatment initiation following ovariectomy in middle-aged rats. Psychoneuroendocrinology. 2010;35:694–705. doi: 10.1016/j.psyneuen.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, Greengard P, McEwen BS. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142:1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:751–760. doi: 10.1016/s0306-4522(00)00228-1. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, Doncarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection. Brain Res Brain Res Rev. 2001;37:320–334. doi: 10.1016/s0165-0173(01)00137-0. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-I in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Brain Res Mol Brain Res. 2002;107:80–88. doi: 10.1016/s0169-328x(02)00449-7. [DOI] [PubMed] [Google Scholar]
- Cassilhas RC, Lee KS, Fernandes J, Oliveira MG, Tufik S, Meeusen R, de Mello MT. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duenas M, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Interaction of insulin-like growth factor-I and estradiol signaling pathways on hypothalamic neuronal differentiation. Neuroscience. 1996;74:531–539. doi: 10.1016/0306-4522(96)00142-x. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Acosta-Martinez M. Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology. 2003;144:3828–3835. doi: 10.1210/en.2003-0157. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Arevalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog Brain Res. 2010;181:251–272. doi: 10.1016/S0079-6123(08)81014-X. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Brain Res Mol Brain Res. 2003;112:170–176. doi: 10.1016/s0169-328x(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Mendez P, Garcia-Segura LM. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology. 2006;147:3027–3039. doi: 10.1210/en.2005-1224. [DOI] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Muller D, Toni N, Buchs PA. Spine changes associated with long-term potentiation. Hippocampus. 2000;10:596–604. doi: 10.1002/1098-1063(2000)10:5<596::AID-HIPO10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Pechenino AS, Frick KM. The effects of acute 17beta-estradiol treatment on gene expression in the young female mouse hippocampus. Neurobiol Learn Mem. 2009;91:315–322. doi: 10.1016/j.nlm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Martin M, Azcoitia I, Trejo JL, Sierra A, Garcia-Segura LM. An antagonist of estrogen receptors blocks the induction of adult neurogenesis by insulin-like growth factor-I in the dentate gyrus of adult female rat. Eur J Neurosci. 2003;18:923–930. doi: 10.1046/j.1460-9568.2003.02830.x. [DOI] [PubMed] [Google Scholar]
- Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2011;46:96–99. doi: 10.1016/j.exger.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- Russo VC, Gluckman PD, Feldman EL, Werther GA. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev. 2005;26:916–943. doi: 10.1210/er.2004-0024. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Sharma K, Mehra RD, Dhar P, Vij U. Chronic exposure to estrogen and tamoxifen regulates synaptophysin and phosphorylated cAMP response element-binding (CREB) protein expression in CA1 of ovariectomized rat hippocampus. Brain Res. 2007;1132:10–19. doi: 10.1016/j.brainres.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in CA1 of rat hippocampus. Cereb Cortex. 2005;15:571–577. doi: 10.1093/cercor/bhh158. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Piriz J, Llorens-Martin MV, Fernandez AM, Bolos M, LeRoith D, Nunez A, Torres-Aleman I. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry. 2007;12:1118–1128. doi: 10.1038/sj.mp.4002076. [DOI] [PubMed] [Google Scholar]
- Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, Flannery R, Jaenisch R, Sur M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci U S A. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Witty CF, Gardella LP, Perez MC, Daniel JM. Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: a role for insulin-like growth factor-I. Endocrinology. 2013;154:842–852. doi: 10.1210/en.2012-1698. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]