Abstract
Cathepsin K (CatK) is one of the most potent mammalian collagenases. We showed previously the increased expression of CatK in human and animal atherosclerotic lesions. Here, we hypothesized that ablation of CatK mitigates injury-induced neointimal hyperplasia. Male wild-type (CatK+/+) and CatK-deficient (CatK−/−) mice underwent ligation or a combination of ligation and polyethylene cuff-replacement injuries to the right common carotid artery just proximal to its bifurcation, and they were then processed for morphological and biochemical studies at specific time points. On operative day 28, CatK−/− significantly reduced neointimal formation and neovessel formation in both single- and combination-injured arteries compared with the Cat K+/+ mice. At early time points, CatK−/− reduced the lesion macrophage contents and medial smooth muscle cell proliferation, the mRNA levels of monocyte chemoattractant protein-1, toll-like receptor-2, toll-like receptor-4, chemokine ligand-12, and the gelatinolytic activity related to matrix metalloproteinase-2/-9. An aorta-explant assay revealed that smooth muscle cell movement was impaired in the CatK−/− mice compared with the CatK+/+ mice. In addition, the smooth muscle cells and macrophages from CatK−/− mice had less invasive ability through a reconstituted basement membrane barrier. This vasculoprotective effect was mimicked by Cat inhibition with trans-epoxysuccinyl-L-leucylamido-{4-guanidino} butane (E64d). These results demonstrate an essential role of CatK in neointimal lesion formation in response to injury, possibly via the reduction of toll-like receptor-2/-4–mediated inflammation and smooth muscle cell proliferation, suggesting a novel therapeutic strategy for the control of endovascular treatment–related restenosis by regulating CatK activity.
Keywords: carotid arteries, cathepsin K, blood flow, vascular remodeling, artery injuries
The pathogenesis of atherosclerosis-based cardiovascular disease (CVD) involves extensive cardiovascular wall extracellular matrix remodeling, which requires the participation of proteases.1–3 Serine proteases and matrix metalloproteinases (MMPs) may participate directly in human and animal CVD.1 Like the members of the MMP family, most of the lysosomal cysteinyl cathepsins (Cats) have been shown to be regulatory proteases that are expressed in restricted tissues under physiological conditions but are induced in cardiovascular tissue or cells by growth factors and inflammatory cytokines.4–6 Human and animal atherosclerotic lesions are rich in inflammatory cells, including macrophages, neutrophils, and T cells.7,8 Experimental studies demonstrated that these cells produce inflammatory cytokines that enhance themselves and those of other vascular cell Cats.9,10 Previous work showed that vascular atherosclerotic plaques overexpress the elastolytic and collagenolytic Cats (CatS, CatK) but show relatively reduced expression of cystatin C, their endogenous inhibitor,10 suggesting a shift in the balance between Cats and their inhibitor that favors the remodeling of cardiovascular wall.
The research in the field of CVD has generated increasing interest in the family of toll-like receptors (TLRs).11–13 It was reported that human and animal atherosclerotic lesions had increased expressions of TLR2 and TLR4 proteins and genes.11 Edfeldt et al11 demonstrated that TLR1, TLR2, and TLR4 are highly expressed, particularly in endothelial cells and macrophages, and that TLR3 and TLR5, by comparison, are more weakly expressed. TLR2 and TLR4 play a critical a role in monocyte activation and in stimulating the release of inflammatory cytokines, chemokines, and several proteases, which are crucial processes in the progression of atherogenesis.12 Although a few lines of evidence suggest that the injury-related vascular repair process is regulated by a TLR4-dependent signaling pathway, the principle mechanisms remain largely unknown.14
Cats also appear to play nontraditional roles in CVD processes.2 For example, we demonstrated that active CatS colocalizes with integrin ανβ3 on the smooth muscle cell (SMC) surface, playing an important role in the invasive behavior of SMCs.15 Compared with collagenase MMP-1 and other Cats (CatS, CatL), CatK is capable of the forceful proteolysis of vascular major type I collagen component.16 Endovascular therapy–related restenosis is clinically different but shares many pathophysiologies with atherosclerosis, including extracellular matrix turnover, inflammatory cell infiltration, angiogenesis, and vascular cell proliferation.5,17
Genetic and pharmacological interventions to Cats have been shown to prevent CVD.9,18 There is a report that serum CatK levels were correlated with coronary plaque volumes in patients with coronary artery disease.19 Neointimal lesions induced by balloon injury contained significantly higher levels of CatK and CatS mRNAs and proteins than did control arteries,17 suggesting the involvement of Cats in restenosis pathogenesis, but a direct role of this class of proteases has never been proven. In the present study, we used CatK-deficient (CatK−/−) mice and an experimental carotid artery injury model to test whether this protease contributes directly to vascular repair–related restenosis.
Methods
An expanded Methods section is available in the online-only Data Supplement.
Mice
The male CatK−/−20 and wild-type (WT, CatK+/+) littermates used in this study were 8 weeks old and weighed between 21 and 25 g. All animal experiments were performed in accord with the guidelines on animal care of the Nagoya University Graduate School of Medicine.
Carotid Artery Injury Models
For the single injury model, the right common carotid artery of 9-week-old mice was ligated just proximal to their bifurcations as described (Figure 1A, top).3 For the combination injury, a polyethylene cuff (length, 2 mm; inside diameter, 0.580 mm; outside diameter, 0.965 mm; Becton Dickinson, Lincoln Park, NY) was applied just proximal to the ligated site (Figure 1A, bottom).21
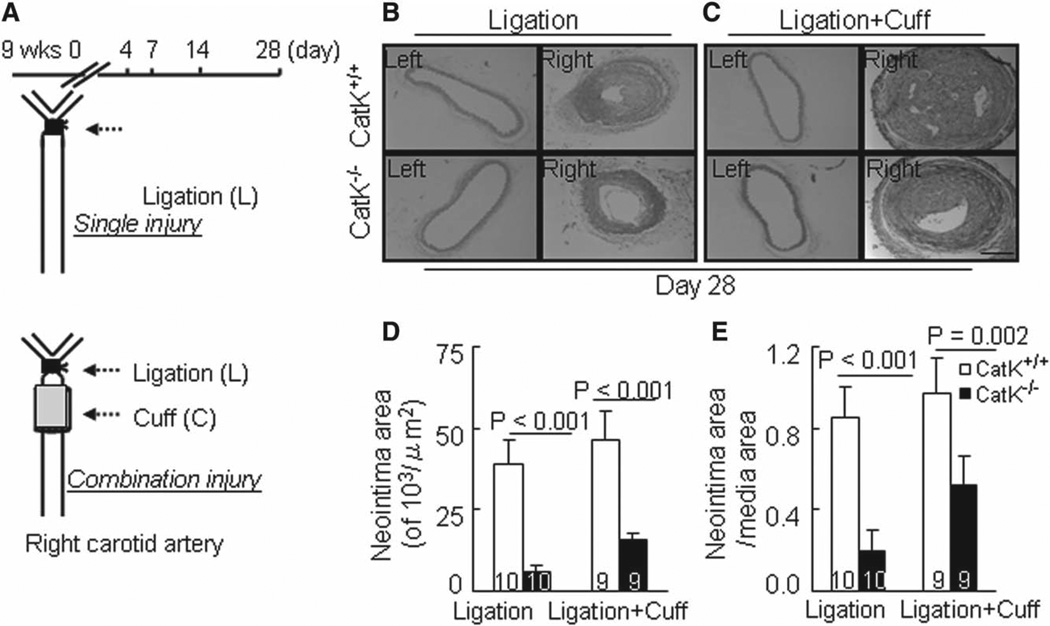
Figure 1.
Cathepsin K–deficient mice (CatK−/−) alleviates injury-induced neointimal formation. A, Schematic diagram of ligation and ligation+cuff surgeries in mouse right carotid artery. B and C, Representative hematoxylin and eosin staining images of right and left carotid arteries of wild-type (CatK+/+) and CatK−/− mice that received single or double injuries. D and E, Quantitative data showing the neointimal areas and the ratio of neointima area to media area in injured arteries of the 4 experimental groups. Results are mean±SEM. The number of animals used for each experiment is indicated in each bar. Scale bar, 100 µm.
Quantitative Real-Time Gene Expression Assay
Real-time gene expression assay was performed as previously described.22 The transcription of targeted genes was normalized to GAPDH. All experiments were performed in triplicate.
Statistical Analysis
Data are expressed as mean±SEM. Student t tests (for comparisons between 2 groups) or 1-way ANOVA (for comparison of 2 or 3 groups) followed by Tukey post hoc tests were used for statistical analyses. SPSS software version 17.0 (SPSS Inc, Chicago, IL) was used. A value of P<0.05 was considered significant.
Results
Reduced Neointimal Formation in CatK−/− Mice
As shown in Figure 1B and 1C, marked intimal lesion formation was observed in CatK+/+ mice at day 28 after the ligation+cuff procedure and to a lesser extent after ligation alone (Figure 1B and 1C). In contrast, much less intimal lesion formation was observed in CatK−/− mice at day 28 after both the single and double injury. Quantitative measurements revealed significantly lower levels of intimal areas in CatK−/− mice on day 28 compared with the CatK+/+ mice (Figure 1D), but no significant difference in media thickness was observed between the CatK+/+ and CatK−/− mice (single: 23.1±2.9 versus 21.3±3.1×103/µm2; combination: 24.9±2.6 versus 22.5±3.4×103/µm2; P>0.05). Therefore, the ratio of intimal to medial area was higher in CatK+/+ mice (Figure 1E).
The total cell numbers in the intima at day 28 were significantly higher in the single injury (289±28 versus 89±18) and combination injury CatK+/+ mice (378±37 versus 123±22; P<0.01 for each). There was no significant difference in the average circumference of the external elastic lamina between 2 genotypes at day 28 after the ligation (1757±149 versus 1701±163 µm) or ligation+cuff procedure (1829±103 versus 1699±113 µm; P>0.05). Immunostaining showed that the medial and intimal lesions contained mainly α-smooth muscle actin–positive cells in CatK+/+ and CatK−/− at indicated time points after the combination surgery (Figure S1A in the online-only Data Supplement). In addition, the data from quantitative analysis of picrosirius red staining demonstrated that CatK−/− mice had higher contents of collagen in either type of injured arteries than in that of control mice (Figure S3).
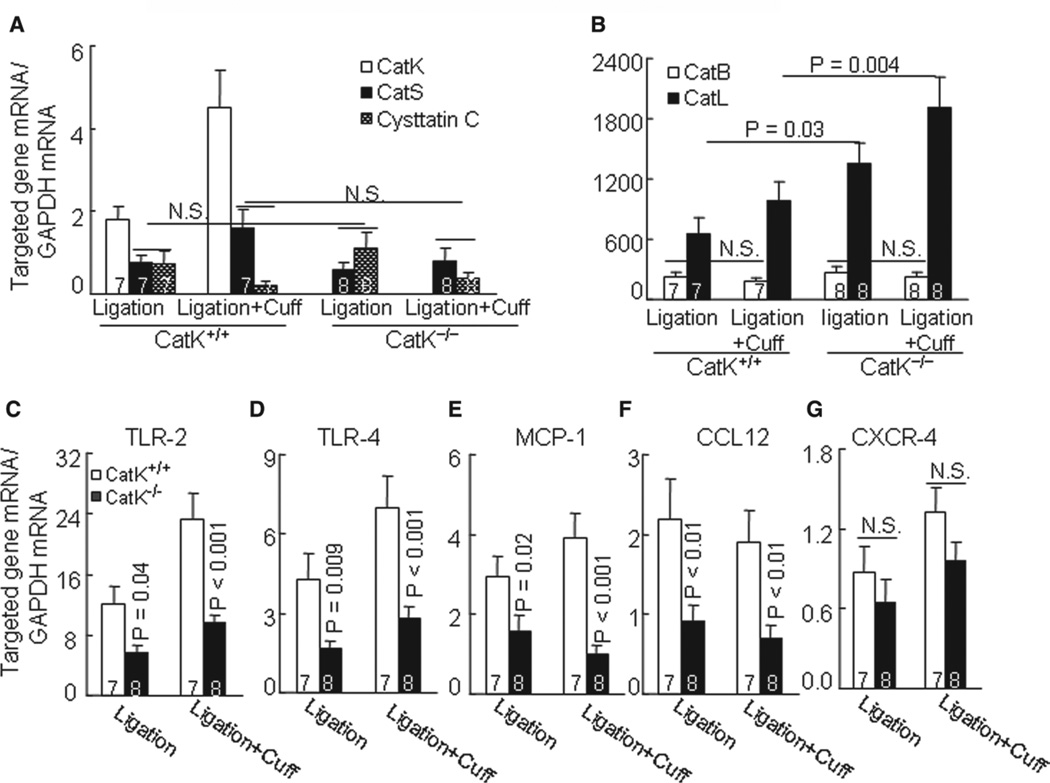
Injuries Induced Cat Expression in Carotid Arteries
At day 4 after ligation, the relative mRNA levels of CatS, CatK, CatL, CatB, and cystatin C were increased by 2.1-, 3.9-, 2.2-, 1.8-, and 1.4-fold over those of the uninjured control vessels of CatK+/+ mice. Similarly, the combination injury caused increases in these Cat genes by 3.9-, 6.2-, 3.4-, 2.6-, and 1.3-fold, respectively. However, there were no significant differences in the levels of CatS, CatB, or cystatin C genes in the injured arteries between the CatK+/+ and CatK−/− mice (Figure 2A and 2B). Similarly, we observed that CatK deficiency did not affect on their protein expressions.
Figure 2.
The levels of targeted genes in injured arteries of wild-type (CatK+/+) and Cathepsin K–deficient (CatK−/−) mice at day 4 after both injuries. A to G, Quantitative real-time polymerase chain reaction data for cathepsin family (CatS, CatK, CatB, CatL, and cystatin C), tolllike receptor-2 (TLR2), TLR4, monocyte chemoattractant protein-1 (MCP-1), chemokine (C–C motif) ligand-12 (CCL12), and C-X-C motif chemokine receptor-4 (CXCR4). Results are mean±SEM. The number of mice for each experimental group is indicated in the bar. NS indicates no significant difference.
As anticipated, we observed no gene and protein expression of CatK in injured carotid arteries from either type of injury of the CatK−/− mice (Figure 2A; Figure S1B). ELISA revealed that the plasma levels of CatK were increased in either type of injury of CatK+/+ mice (single: 18.2±2.1 versus 3.1±1.1 pmol/L; combination: 42.5±9.7 versus 3.1±1.1 pmol/L; P<0.05 for each). In contrast, CatL displayed a compensatory increase in gene and protein in the CatK−/− mice compared with the corresponding CatK+/+ (Figure 2B; Figure S4A). Next, we performed immunostaining to identify the cell source of CatK in injured arteries. As shown in Figure S1B, the positive staining signaling was quite pronounced in the media and neointima of combination-injured vessels from CatK+/+ mice on days 14 and 28, whereas no signal was detected in the whole arterial walls of CatK−/− mice. In addition, the in situ zymography showed gelatinolytic activity primarily in the neointimal and medial region in the injured arteries of CatK+/+ mice at 14 days after combination surgery, and this activity was suppressed by incubation with a specific CatK inhibitor (CatK-II) or nonspecific Cat inhibitor (E64; Figure S2).
Reduced Inflammatory Action in CatK−/− Mice
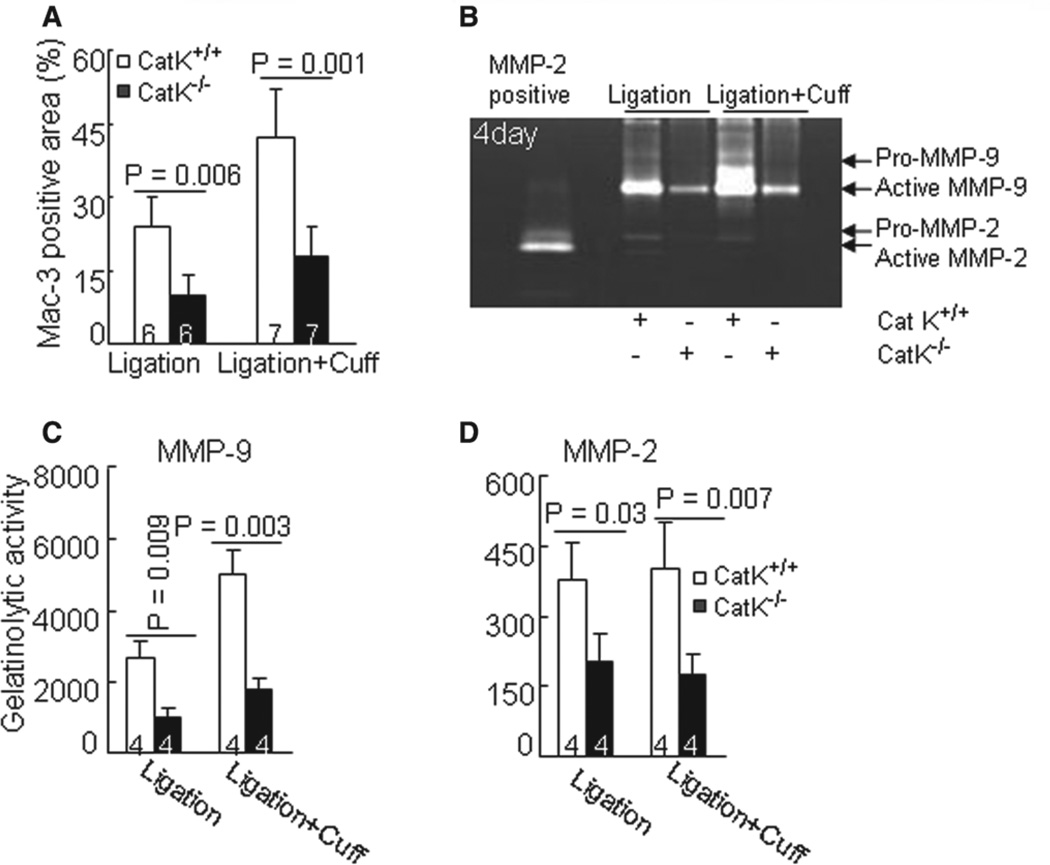
The macrophage activation–related release of inflammatory chemokines is an important hallmark of human and animal vascular repair and is mediated by a TLR signaling pathway in CVD.13 Here, we evaluated TLRs and inflammatory chemokine expressions. The quantitative polymerase chain reaction revealed that compared with the CatK+/+ mice, the atherosclerotic lesions in CatK−/− mice that received single or double injuries had lower levels of TLR2 and TLR4 as well as monocyte chemoattractant protein-1 and chemokine (C-C motif) ligand-12 genes, whereas C-X-C motif chemokine receptor-4 exhibited no significant difference (Figure 2C–2G). Similarly, immunostaining against macrophages showed that CatK deficiency markedly reduced the macrophage contents in both types of lesion (Figure 3A; Figure S4B).
Figure 3.
Cathepsin K–deficient mice (CatK−/−) reduced macrophage infiltration in response to injury. A, The Mac-3–positive staining areas of injured arteries were quantified and are expressed as the ratio (percentage) of the positively stained area to the entire lesion including adventitia, media, and neointima areas. B to D, Representative images of gelatin zymography and combined quantitative data for gelatinolytic activities of matrix metalloproteinase-2 (MMP-2) and MMP-9 in injured arteries at day 4 after surgeries. Results are mean±SEM. The number of mice for each experimental group is indicated in the bar. Scale bar, 100 µm.
Infiltrated macrophages were reported to be a major source of proteases (such as MMP-2, MMP-9, and MMP-12) that contribute to vascular repair.9,13 As compared with the CatK+/+ mice, the artery lesions of CatK−/− mice that received double injuries had lower levels of MMP-2, MMP-9, and MMP-12, as well as their tissue inhibitors of MMP (TIMP-1 and TIMP-2) proteins, whereas MMP-13 exhibited no difference (Figure S4C). Representative gelatin zymography bands of MMP-2 and MMP-9 activity are shown in Figure 3B. The quantitative analysis revealed that the gelatinolytic net activities of MMP-2 and MMP-9 were lower in the injured arteries of the CatK−/− mice that received both surgical injuries (Figure 3C–3D), supporting the concept that Cat deficiency impairs inflammatory action in response to injury.
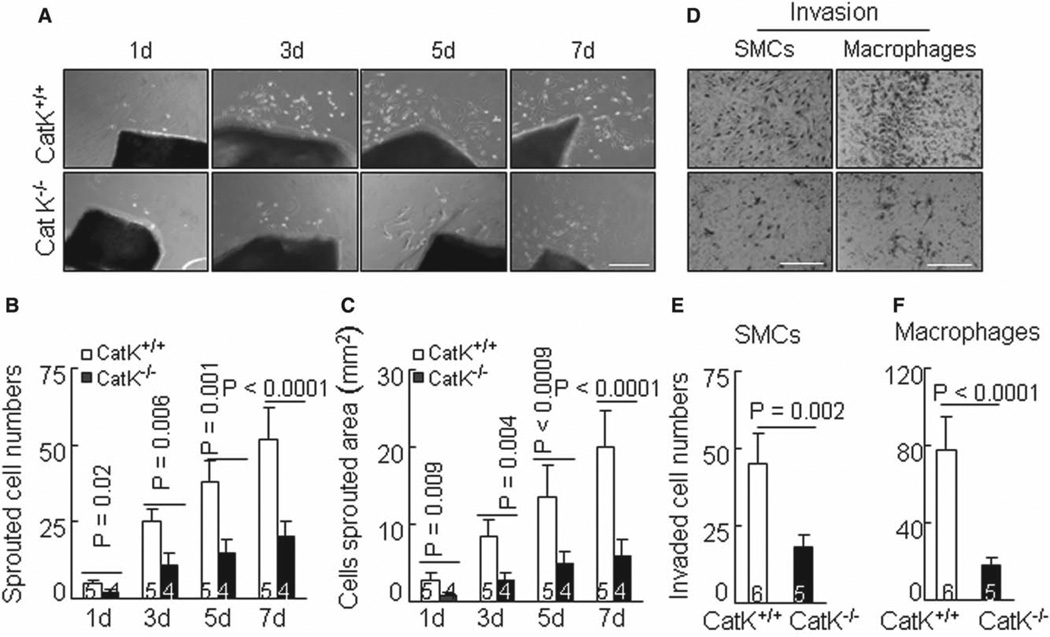
CatK Function in Vascular SMC Events
The photographs in Figure 4A show cell migration from the arterial explants of CatK+/+ mice observed at day 1. These migrating cells stained positive for α-smooth muscle actin (data not shown). Compared with the CatK+/+ explants, the sprouting cell numbers and areas were markedly decreased in the aorta explants of the CatK−/− mice during the follow-up period (Figure 4B and 4C). As shown in Figure S5, the phosphoinositide 3-kinase (PI3K) inhibitor LY294002 significantly suppressed SMC sprouting cell numbers. SMC ability was markedly diminished by CatK-II and by E64d. However, MMP inhibition with GM6001 had no effect on it (data not shown). To examine the ability of platelet-derived growth factor-BB–directed SMCs to cross a collagen-gel filter, we used several clones of cultured SMCs derived from either CatK+/+ (n=5) or CatK−/− (n=4) mice. The quantitative analysis revealed that SMC invasion through the collagen barrier was markedly reduced among the SMCs from CatK−/− mice (Figure 4D and 4E). Similarly, the macrophage invasion ability was also impaired in the CatK−/− group (Figure 4D and 4F).
Figure 4.
Cathepsin K–deficient mice (CatK−/−) impairs smooth muscle cell (SMC) migration and invasion ex vivo and in vitro. A to C, Representative images of SMC migration from arterial explants (A) and combined quantitative data for sprouted cell numbers (B) and area (C) at indicated culture time points are shown. D to F, A Transwell assay was applied to evaluate the cell invasion ability through the collagen barrier in the presence of a gradient of chemoattractant. Representative images of SMC or macrophage invasion and combined quantitative data for invaded cell numbers are shown. Results are mean±SEM. The number of mice for each experimental group is indicated in the bar. Scale bar, 100 µm.
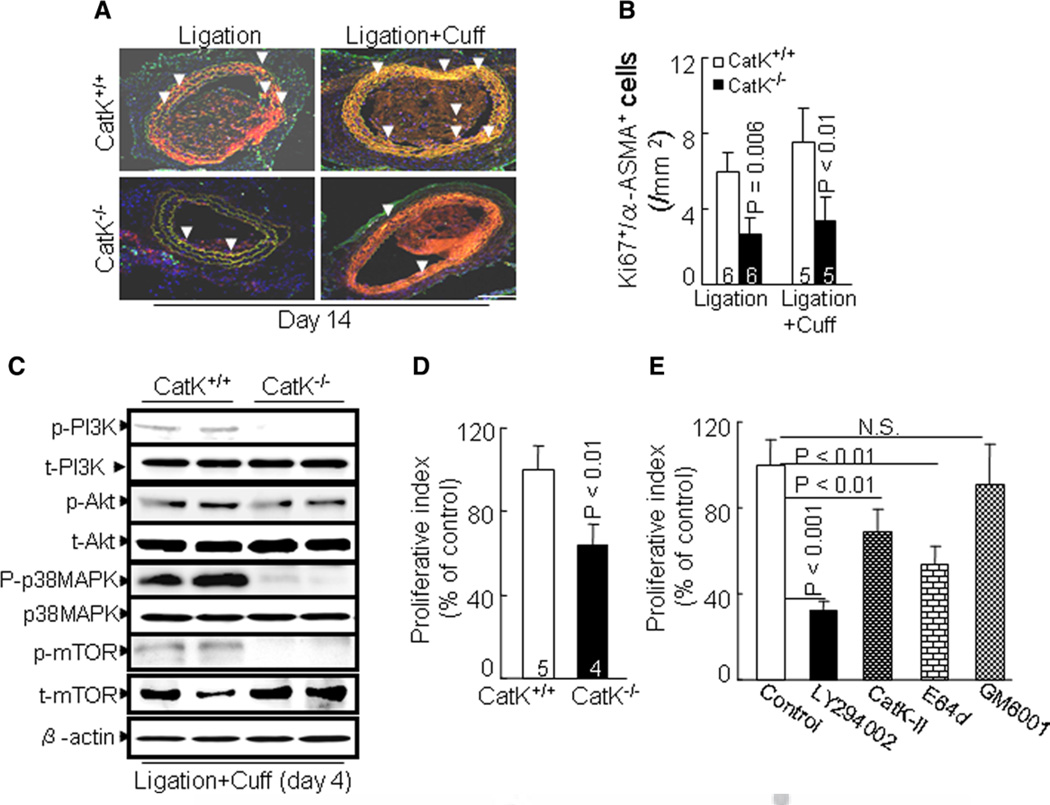
We next evaluated whether CatK activity is involved in the SMC proliferative response. At day 14 after both surgeries, we detected extensive double-positive immunostaining of Ki67- and α-smooth muscle actin for proliferative SMCs in the media of injured arteries of CatK+/+ mice (Figure 5A and 5B). The immunostaining of these cells was dramatically decreased in CatK−/− mice. Representative immunoblots revealed that the levels of phospho-PI3K, phospho-Akt, phospho-p38 mitogen–activated protein kinase (p38MAPK), and phospho-mammalian target of rapamycin (mTOR) proteins were lower in injured arteries of CatK−/− mice than in those of CatK+/+ mice (Figure 5C). In vitro, CatK deficiency also impaired the SMC proliferation ability induced by platelet-derived growth factor-BB (Figure 5D). As shown in Figure 5E, LY294002 significantly suppressed the platelet-derived growth factor-BB–induced SMC proliferation. The SMC proliferative action was diminished markedly by CatK-II and by E64d, whereas GM6001 had no effect.
Figure 5.
Cathepsin K–deficient mice (CatK−/−) impairs smooth muscle cell (SMC) proliferation in vivo and in vitro. A and B, Representative double Ki67/α-smooth muscle actin (ASMA) immunofluorescence image of media SMC proliferation and combined quantitative data for Ki67+/ASMA+ cells. The number of mice for each experimental group is indicated in the bar. Scale bar, 100 µm. C, Representative gel blots exhibiting the reductions of phosho-phosphoinositide 3-kinase (p-PI3K), phospho-p38 mitogen–activated protein kinase (p-p38MAPK), phospho-Akt (p-Akt), and phospho-mammalian target of rapamycin (p-mTOR) proteins in inured arteries of both genotype mice at day 4 after double injuries. Data represent observations from ≥3 independent arteries. D, Proliferative ability of aorta-derived SMC from wild-type (CatK+/+; 5 mice) and CatK−/− (6 mice) was assessed with a cell proliferation assay after 2 days of incubation with platelet-derived growth factor-BB. E, CatK+/+ aorta-derived SMCs were used in a proliferation assay in the presence or absence of a PI3K inhibitor LY294002, a specific CatK inhibitor II [1-(N-benzyloxycarbonyl-leucyl)-5-(N-Boc-phenylalanyl-leucyl)carbohydrazide] (CatK-II), a nonspecific Cat inhibitor [(l–3-trans-ethoxycarbonyloxirane-2-carbonyl)-l-leucine (3-methylbutyl) amide; E64d] and a nonspecific matrix metalloproteinase inhibitor GM6001, respectively. Results are mean±SEM of 4 independent experiments performed in triplicate.
Cat Inhibition Alleviates Vascular Remodeling
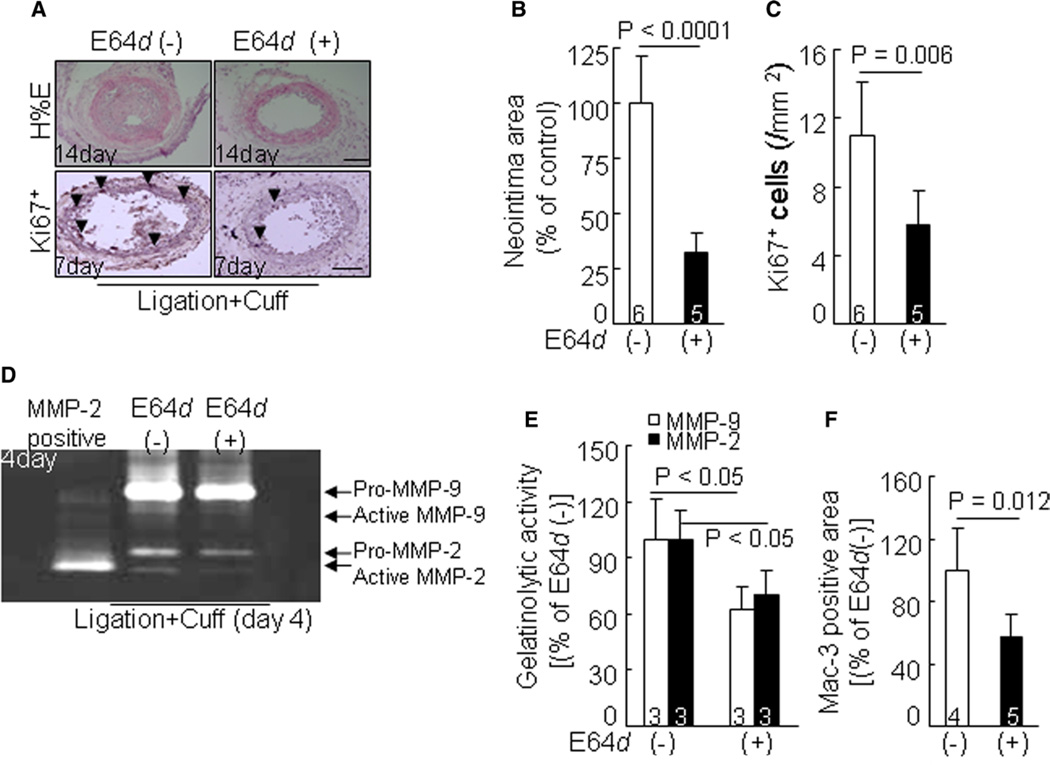
Compared with the mice treated with vehicle, Cat inhibition by E64d reduced not only neointimal formation but also medial cell proliferation in the combined-injured arteries of CatK+/+ mice, as determined by hematoxylin and eosin and Ki67 immunostaining (Figure 6A–6C). E64d decreased not only MMP-2 and MMP-9 expression and activation but also TIMP-1 and TIMP-2 expression (Figure S6A). It is consistent that E64d reduced MMP-2 and MMP-9 gelatinolytic activities (Figure 6D–6E). As anticipated, E64d also suppressed macrophage accumulation in arterial lesions (Figure 6F). In addition, the E64d-treated vessels had decreased expressions of TLR2 and TLR4 gene (TLR2: 24.5±4.1 versus 14.6±3.0; TLR4: 7.5±1.9 versus 3.0 versus 0.9; P<0.05). Moreover, E64d reduced the levels of phospho-PI3K, phospho-Akt, phospho-p38MAPK, and phospho-mTOR proteins (Figure S6B). E64d treatment also enhanced collagen volume of double-injured arteries (63.1±7.4 versus 44.2±5.3%; P<0.05).
Figure 6.
Evaluation of smooth muscle cell proliferation and neointima formation in injured arteries of wild-type (CatK+/+) mice treated with vehicle or E64d at indicated time points. A to C, Representative images of hematoxylin and eosin (H&E) staining or Ki67 immunostaining and combined quantitative data for the neointima area and medial Ki67+ cell at indicated time points. D to F, Representative images of gelatin zymography and combined quantitative data for gelatinolytic activities of matrix metalloproteinase (MMP)-2 and MMP-9 in injured arteries at day 4 after surgeries. Results are mean±SEM. The number of mice for each experimental group is indicated in the bar. Scale bar, 100 µm.
Reduced Neointimal Growth–Related Vasa Vasorum in CatK−/− Mice
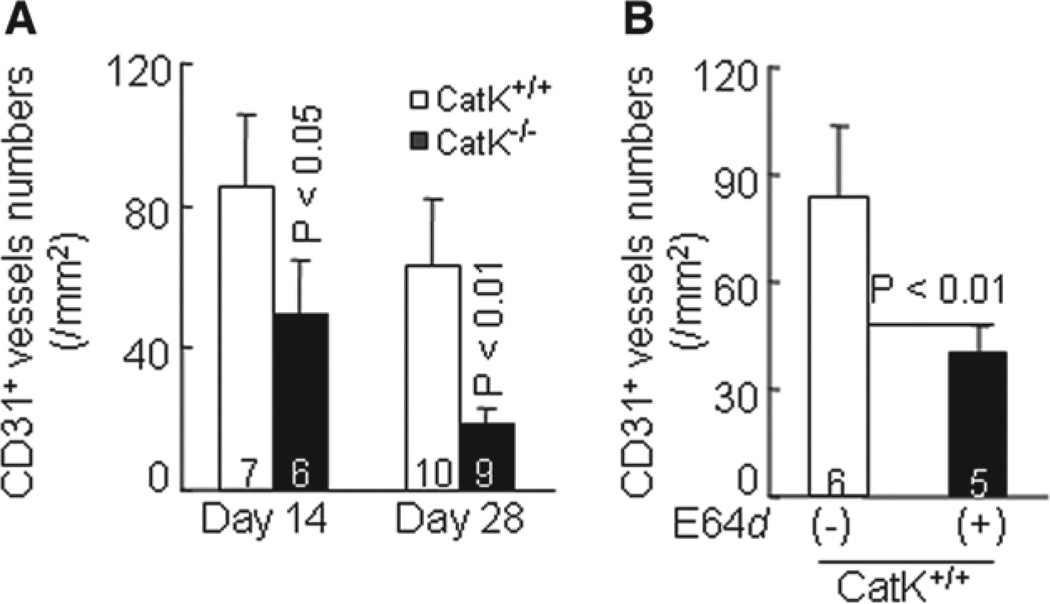
Increased vasa vasorum is associated with atherosclerotic plaque growth.9,23 Reduced neointimal growth in CatK−/− mice might be caused by impaired angiogenesis. To test this hypothesis, we immunostained carotid arterial sections with anti-CD31 antibody; we observed lower CD31+ microvessel numbers in lesions of CatK−/− mice than in those of CatK+/+ mice on days 14 and 28 after the double injury (Figure 7A; Figure S7A). Cat inhibition also reduced the microvessel density in arterial lesions (Figure 7B; Figure S7B). These data suggest that CatK activities in angiogenesis contributed to pathogenesis in this experimental intimal hyperplasia.
Figure 7.
Cathepsin K–deficient (CatK−/−) reduces neovessel density in neointima-related lesions. A, Quantitative measurement of neovessels from the entire lesion including adventitia and media in injured arteries of wild-type (CatK+/+) and CatK-deficient (CatK−/−) mice at days 14 and 28 after surgeries. B, Microvessel numbers were reduced in neointima-related lesions from CatK+/+ mice treated with trans-epoxysuccinyl-L-leucylamido-{4-guanidino} butane (E64d). Results are mean±SEM. The number of mice for each experimental group is indicated in the bar. Scale bar, 100 µm.
Discussion
Endovascular therapy–related vascular negative remodeling represents the leading cause of restenosis and cardiovascular events. Identifying novel targets to suppress maladaptive vascular remodeling will contribute to therapeutic strategies to preempt restenosis. The significant finding of our present work is that mice lacking the CatK gene are resistant to acute injury– induced intimal hyperplasia. At the molecular level, CatK deletion retards injury-induced TLR2/4 and their downstream inflammatory genes and PI3K/Akt-mediated-p38MAPK and -mTOR signaling activations. Pharmacological inhibition of Cats also results in vascular protective actions via the reduction of inflammation and SMC cell proliferation. In vitro, both CatK silencing and inhibition with a specific or nonspecific inhibitor attenuated SMC and macrophage functions (including migratory and proliferative abilities). To the best of our knowledge, this is the first study to report that genetic and pharmacological inhibitions of Cat confer vascular protection against acute injuries.
The ability of injury to increase CatK expression and activity probably contributed to the vascular repair under our experimental conditions. In agreement with a previous report that CatK silencing reduced atherosclerotic lesion formation,24 we observed that vascular repair and neointimal formation after injuries are mitigated by CatK−/−. Early in the formation of the thickened intima, as in atherosclerotic and neointimal lesions, SMCs must migrate from the tunica media into the developing intima.17 To elucidate the effect of CatK on SMC behaviors, we used ex vivo aortic explants and in vitro SMC invasion models. The SMC migration from the arterial explants reflects the intrinsic ability of cells to migrate and degrade the local extracellular matrix proteins.
Our present findings show that CatK−/− reduced the numbers of sprouted SMCs and areas from aortic explants. Cat inhibition also inhibits SMC sprouting. Moreover, CatK−/− impaired SMC invasiveness across the collagen-gel barrier. Based on these findings, we propose that CatK activity controls SMC behaviors to contribute to the vascular repair– related restenosis after injury. It was reported that patients with coronary artery disease and restenosis after endovascular therapy had higher levels of plasma CatK compared with patients without restenosis.19 Here, we have shown that pharmacological inhibition of Cats mitigated combination injury–induced intimal hyperplasia. Thus, the upregulation of CatK expression and activity could represent a common proteolytic mechanism in the vascular repair of acute injuries in CatK+/+ mice.
The engagement of TLRs on the cell surface by specific ligands leads to an increase in the expressions of proinflammatory cytokines and chemokines, such as monocyte chemoattractant protein-1 and interleukin-1β.11,12 Increased expression of TLR2 and TLR4 occurs in macrophages bordering the lipid core adjacent to the fibrous cap and in macrophages and SMCs in the shoulder regions of activated human atherosclerotic plaques.11 In the present study, arterial lesions of CatK−/− mice contain much lower levels of TLRs (TLR2, TLR4) and chemokines (chemokine [C-C motif] ligand-12, monocyte chemoattractant protein-1) genes compared with control CatK+/+ mice. CatK−/− retarded macrophage accumulation in injured arteries. Thus, CatK−/− seems to mitigate macrophage infiltration and activation and inflammatory chemokine expression in injury-stress states through its ability to reduce TLR2 and TLR4 expressions. A recent study demonstrated that genetic or pharmacological inhibition of CatS alleviated cardiac damage and fibrosis via inactivation of transforming growth factor-β–dependent mothers against decapentaplegic homolog-3 signaling.25 It was reported that this signaling pathway regulates TLR2 induction via the inhibition of p38MAPK activation,26 and that it modulates TLR4 expression and response in macrophages.27,28 Collectively, these observations and our finding that CatK silencing impaired p38MAPK activation in vivo indicate that inactivation of TL2/4 signaling by a negative regulator of p38MAPK might contribute to the anti-inflammatory action in mice lacking CatK (Figure S8).
Given the focus on the roles of a Cats/cystatin system in proliferative diseases in recent reviews,2,5 it is notable that CatK seems to be of particular importance for SMC proliferation in the vascular repair process. Sun et al7 showed that aneurysm lesions from CatL−/− mice contained fewer Ki+ 67 proliferating cells than did CatL+/+ mice. In contrast, cystatin C deficiency resulted in an increased Ki67+ proliferation index and epidermal hyperplasia, most likely attributed to an enhanced Cat activity in antagonizing the cell proliferation in K14-HPV16 transgenic mice.29 Our present results show that the lesions had lower Ki+ 67/α-smooth muscle actin+ proliferating cells in CatK−/− mice as compared with the control mice. E64d treatment also suppressed medial cell proliferation in the CatK+/+ mice. In vitro, CatK−/− suppressed the platelet-derived growth factor-induced SMC proliferation. Because injury induces CatK activation,17 we propose that CatK modulates intimal hyperplasia in a mechanical-stress state through its ability to activate SMC proliferative activity. PI3K/Akt-mTOR signaling has been accepted widely as an initiator of protein synthesis and cell growth. Our observations indicate that deficiency of CatK reduced the levels of phospho-PI3K, phospho-Akt, phospho-p38MAPK, and phospho-mTOR proteins in the arterial lesions. Our results also showed that the expressions of TLR2 and TLR4 genes were also decreased in injured arteries of CatK−/−. Several lines of investigation demonstrated that TLR2 and TLR4 are required in vascular SMC proliferation via MAPK activation.30,31 Saxena et al14 showed that the TLR4/MyD88 axis modulates a periadventitial collar injury–induced vascular repair process. Taken together, these findings suggest that attenuation of SMC proliferation by CatK deficiency via inactivation of TLR2/4-mediated PI3K/Akt-p38MAPK and PI3K/ Akt-mTOR signaling may represent a common mechanism underlying the reduction of injury-induced vascular remodeling and neointimal formation. It should be noted that CatK deletion compensatory enhanced injured-induced CatL gene and protein expression. CatL activity has been shown to control cell proliferation. This might possibly explain the minor difference in the SMC proliferation index noted on CatK-II and E64d treatment. Further studies will be needed to investigate this issue.
However, CatX has been shown to suppress the proliferation of mononuclear cells by the activation of CD11b/CD18, but it was also shown to increase the proliferation of T lymphocytes by the activation of CD11a/CD18.32 Another recent study demonstrated that the reduction of Cux1 processing by CatL−/− results in the accumulation of Cux1, the downregulation of p21/p27, and increased cell proliferation.33 Based on these observations, it will be worthwhile to explore the responsibility of individual Cats in cell proliferation in curtailing vascular positive remodeling and restenosis after common endovascular therapy.
Vasa vasorum provides paths for nourishment and inflammatory cell accumulation in the vascular repair process.5 Previous observations indicate that vasa vasorum can cause atherosclerotic plaque growth and instability in human and animal aortas.23 Further supporting this role, antiangiogenic endostatin was shown to suppress aorta intimal and adventitia neovessel formation and plaque growth in an apolipoprotein E–deficient mouse model.34 Previous study reported that deficiency of CatS reduced tumor-related angiogenesis.4 Here, we found that genetic and pharmacological inhibitions of CatK suppress adventitial and medial CD31+ neovessel density. Thus, attenuation of vasa vasorum by CatK inhibition may have a vasculoprotective effect that could mitigate vascular remodeling, the process often responsible for endovascular intervention–related restenosis.
In summary, our present findings suggest that targeting CatK represents an attractive therapeutic approach to curtail vascular negative remodeling and restenosis after endovascular interventions. Our data indicate that CatK−/−-mediated anti-inflammation is attributable to decreased inflammatory chemokine productions and macrophage activation via the reduction of TLR2/4 signaling. Moreover, the antiproliferative effect of CatK inhibition by genetic and pharmacological inhibition might be because of decreased TLR2/4-mediated PI3K/Akt-mTOR and PI3K/Akt-p38MAPK signaling activation.
Perspective
The expressions of CatK gene and protein increase in failing vasculature. CatK deletion alleviates acute injury–induced vascular repair. The pharmacological inhibition of Cats mimics the vascular protective effects of genetic CatK deletion. It seems that selective Cats and CatK inhibitors may have potential use in the treatment or control of restenosis after intravascular intervention therapies in CVD.
Supplementary Material
Novelty and Significance.
What Is New?
Cathepsin K (CatK) gene and protein levels increased in failing mouse carotid vasculature to injuries.
CatK deficiency attenuated injury-related inflammatory actions and medial smooth muscle cellular events (including migration, invasion, and proliferation) and mitigated vascular remodeling.
Cathepsin inhibitor l–3-trans-ethoxycarbonyloxirane-2-carbonyl)-l-leucine (3-methylbutyl) amide mimics CatK deficiency–mediated vasculoprotective actions.
What Is Relevant?
Acute mechanical injury–induced vascular negative remodeling and restenosis were alleviated by CatK deficiency.
Pharmacological inhibition of cathepsins mitigated vascular neointimal hyperplasia in response to acute mechanical stress via the reduction of inflammation and smooth muscle cell proliferation.
Summary
CatK is novel therapeutic target for controlling intravascular therapy–related restenosis.
Acknowledgments
Dr Hu researched data, statistical analysis, and wrote the first draft of the article. Drs Song and Li researched the morphological data and assisted with the carotid artery injury mouse models. A. Inoue, Dr Jiang, and Dr Kozawa researched the real-time polymerase chain reaction data and mouse genotyping. Dr Cheng designed the study and wrote the article. G.-P. Shi reviewed the article, contributed to the discussion, and provided transgenic mice. Dr Okumura reviewed/edited the article. Dr Kuzuya planned the study and edited the article.
Sources of Funding
This work was supported, in part, by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (no. 223900143 to Dr Kuzuya; no. 21590952 to Dr Cheng), the Japan Takeda Science Foundation (no. 26-007596 to Dr Cheng); and the Ministry of Education, Science, and Technology (no. 2012M3A9C6050507); and The Scientific Research Fund of the Chinese Ministry of Education (no. 82160068).
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.113.02141/-/DC1.
Disclosures
None.
References
- 1.Müller AL, Dhalla NS. Role of various proteases in cardiac remodeling and progression of heart failure. Heart Fail Rev. 2012;17:395–409. doi: 10.1007/s10741-011-9269-8. [DOI] [PubMed] [Google Scholar]
- 2.Cheng XW, Shi GP, Kuzuya M, Sasaki T, Okumura K, Murohara T. Role for cysteine protease cathepsins in heart disease: focus on biology and mechanisms with clinical implication. Circulation. 2012;125:1551–1562. doi: 10.1161/CIRCULATIONAHA.111.066712. [DOI] [PubMed] [Google Scholar]
- 3.Kuzuya M, Kanda S, Sasaki T, Tamaya-Mori N, Cheng XW, Itoh T, Itohara S, Iguchi A. Deficiency of gelatinase a suppresses smooth muscle cell invasion and development of experimental intimal hyperplasia. Circulation. 2003;108:1375–1381. doi: 10.1161/01.CIR.0000086463.15540.3C. [DOI] [PubMed] [Google Scholar]
- 4.Shi GP, Sukhova GK, Kuzuya M, Ye Q, Du J, Zhang Y, Pan JH, Lu ML, Cheng XW, Iguchi A, Perrey S, Lee AM, Chapman HA, Libby P. Deficiency of the cysteine protease cathepsin S impairs microvessel growth. Circ Res. 2003;92:493–500. doi: 10.1161/01.RES.0000060485.20318.96. [DOI] [PubMed] [Google Scholar]
- 5.Cheng XW, Huang Z, Kuzuya M, Okumura K, Murohara T. Cysteine protease cathepsins in atherosclerosis-based vascular disease and its complications. Hypertension. 2011;58:978–986. doi: 10.1161/HYPERTENSIONAHA.111.180935. [DOI] [PubMed] [Google Scholar]
- 6.Cheng XW, Obata K, Kuzuya M, et al. Elastolytic cathepsin induction/activation system exists in myocardium and is upregulated in hypertensive heart failure. Hypertension. 2006;48:979–987. doi: 10.1161/01.HYP.0000242331.99369.2f. [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Sukhova GK, Zhang J, Chen H, Sjöberg S, Libby P, Xiang M, Wang J, Peters C, Reinheckel T, Shi GP. Cathepsin L activity is essential to elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2011;31:2500–2508. doi: 10.1161/ATVBAHA.111.230201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T, Kuzuya M, Nakamura K, Cheng XW, Hayashi T, Song H, Hu L, Okumura K, Murohara T, Iguchi A, Sato K. AT1 blockade attenuates atherosclerotic plaque destabilization accompanied by the suppression of cathepsin S activity in apoE-deficient mice. Atherosclerosis. 2010;210:430–437. doi: 10.1016/j.atherosclerosis.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, Ridker PM, Libby P, Chapman HA. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191–1197. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 12.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 13.Cheng XW, Song H, Sasaki T, Hu L, Inoue A, Bando YK, Shi GP, Kuzuya M, Okumura K, Murohara T. Angiotensin type 1 receptor blocker reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Hypertension. 2011;57:981–989. doi: 10.1161/HYPERTENSIONAHA.110.168385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxena A, Rauch U, Berg KE, Andersson L, Hollender L, Carlsson AM, Gomez MF, Hultgårdh-Nilsson A, Nilsson J, Björkbacka H. The vascular repair process after injury of the carotid artery is regulated by IL-1RI and MyD88 signalling. Cardiovasc Res. 2011;91:350–357. doi: 10.1093/cvr/cvr075. [DOI] [PubMed] [Google Scholar]
- 15.Cheng XW, Kuzuya M, Nakamura K, Di Q, Liu Z, Sasaki T, Kanda S, Jin H, Shi GP, Murohara T, Yokota M, Iguchi A. Localization of cysteine protease, cathepsin S, to the surface of vascular smooth muscle cells by association with integrin alphanubeta3. Am J Pathol. 2006;168:685–694. doi: 10.2353/ajpath.2006.050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Hou WS, Brömme D. Collagenolytic activity of cathepsin K is specifically modulated by cartilage-resident chondroitin sulfates. Biochemistry. 2000;39:529–536. doi: 10.1021/bi992251u. [DOI] [PubMed] [Google Scholar]
- 17.Cheng XW, Kuzuya M, Sasaki T, Arakawa K, Kanda S, Sumi D, Koike T, Maeda K, Tamaya-Mori N, Shi GP, Saito N, Iguchi A. Increased expression of elastolytic cysteine proteases, cathepsins S and K, in the neointima of balloon-injured rat carotid arteries. Am J Pathol. 2004;164:243–251. doi: 10.1016/S0002-9440(10)63114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J, Bot I, de Nooijer R, Hoffman SJ, Stroup GB, Biessen EA, Benson GM, Groot PH, Van Eck M, Van Berkel TJ. Leucocyte cathepsin K affects atherosclerotic lesion composition and bone mineral density in low-density lipoprotein receptor deficient mice. Cardiovasc Res. 2009;81:278–285. doi: 10.1093/cvr/cvn311. [DOI] [PubMed] [Google Scholar]
- 19.Cheng XW, Kikuchi R, Ishii H, Yoshikawa D, Hu L, Takahashi R, Shibata R, Ikeda N, Kuzuya M, Okumura K, Murohara T. Circulating cathepsin K as a potential novel biomarker of coronary artery disease. Atherosclerosis. 2013;228:211–216. doi: 10.1016/j.atherosclerosis.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Kozawa E, Nishida Y, Cheng XW, Urakawa H, Arai E, Futamura N, Shi GP, Kuzuya M, Hu L, Sasaki T, Ishiguro N. Osteoarthritic change is delayed in a Ctsk-knockout mouse model of osteoarthritis. Arthritis Rheum. 2012;64:454–464. doi: 10.1002/art.33398. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki T, Kuzuya M, Nakamura K, Cheng XW, Shibata T, Sato K, Iguchi A. A simple method of plaque rupture induction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:1304–1309. doi: 10.1161/01.ATV.0000219687.71607.f7. [DOI] [PubMed] [Google Scholar]
- 22.Cheng XW, Kuzuya M, Nakamura K, et al. Mechanisms underlying the impairment of ischemia-induced neovascularization in matrix metalloproteinase 2-deficient mice. Circ Res. 2007;100:904–913. doi: 10.1161/01.RES.0000260801.12916.b5. [DOI] [PubMed] [Google Scholar]
- 23.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 24.Lutgens E, Lutgens SP, Faber BC, et al. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113:98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Wang J, Xiang MX, Lin Y, He A, Jin CN, Guan J, Sukhova GK, Libby P, Wang JA, Shi GP. Cathepsin S-mediated fibroblast trans-differentiation contributes to left ventricular remodelling after myocardial infarction. Cardiovasc Res. 2013;100:84–94. doi: 10.1093/cvr/cvt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikami F, Lim JH, Ishinaga H, Ha UH, Gu H, Koga T, Jono H, Kai H, Li JD. The transforming growth factor-beta-Smad3/4 signaling pathway acts as a positive regulator for TLR2 induction by bacteria via a dual mechanism involving functional cooperation with NF-kappaB and MAPK phosphatase 1-dependent negative cross-talk with p38 MAPK. J Biol Chem. 2006;281:22397–22408. doi: 10.1074/jbc.M602124200. [DOI] [PubMed] [Google Scholar]
- 27.St John AL, Abraham SN. Salmonella disrupts lymph node architecture by TLR4-mediated suppression of homeostatic chemokines. Nat Med. 2009;15:1259–1265. doi: 10.1038/nm.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCartney-Francis N, Jin W, Wahl SM. Aberrant Toll receptor expression and endotoxin hypersensitivity in mice lacking a functional TGF-beta 1 signaling pathway. J Immunol. 2004;172:3814–3821. doi: 10.4049/jimmunol.172.6.3814. [DOI] [PubMed] [Google Scholar]
- 29.Yu W, Liu J, Shi MA, Wang J, Xiang M, Kitamoto S, Wang B, Sukhova GK, Murphy GF, Orasanu G, Grubb A, Shi GP. Cystatin C deficiency promotes epidermal dysplasia in K14-HPV16 transgenic mice. PLoS One. 2010;5:e13973. doi: 10.1371/journal.pone.0013973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasu S, LaVerda D, Qureshi N, Golenbock DT, Beasley D. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ Res. 2001;89:244–250. doi: 10.1161/hh1501.094184. [DOI] [PubMed] [Google Scholar]
- 31.de Graaf R, Kloppenburg G, Kitslaar PJ, Bruggeman CA, Stassen F. Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through Toll-like receptors 2 and 4. Microbes Infect. 2006;8:1859–1865. doi: 10.1016/j.micinf.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 32.Obermajer N, Repnik U, Jevnikar Z, Turk B, Kreft M, Kos J. Cysteine protease cathepsin X modulates immune response via activation of beta2 integrins. Immunology. 2008;124:76–88. doi: 10.1111/j.1365-2567.2007.02740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcalay NI, Sharma M, Vassmer D, Chapman B, Paul B, Zhou J, Brantley JG, Wallace DP, Maser RL, Vanden Heuvel GB. Acceleration of polycystic kidney disease progression in cpk mice carrying a deletion in the homeodomain protein Cux1. Am J Physiol Renal Physiol. 2008;295:F1725–F1734. doi: 10.1152/ajprenal.90420.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.