Abstract
Rationale
Postpartum depression (PMD) occurs in roughly 10% of postpartum women and negatively impacts the mother and her offspring, but there are few placebo-controlled studies of antidepressant treatment in this population.
Objectives
To compare the selective serotonin reuptake inhibitor (SSRI) sertraline to placebo for treating PMD.
Methods
This was a single-center, 6-week, randomized double-blind placebo-controlled trial of sertraline with a one-week placebo lead-in. Participants (n=38) were women with depression onset within 3 months of delivery; a subset (n=27) met strict DSM-IV criteria for PMD (onset within 4 weeks of delivery). Participants were prescribed sertraline 50 mg or placebo daily, to a maximum of 200 mg/day. Primary outcome variables were the Hamilton Depression Rating Scale (HAM-D) and Clinical Global Impressions (CGI) scores, which were used to determine rates of response and remission.
Results
Sertraline produced a significantly greater response rate (59%) than placebo (26%) and a more than 2-fold increased remission rate (53% vs. 21%). Mixed models did not reveal significant group by time effects, although in the subset of women who met DSM-IV criteria, there was a statistically significant group by time effect for the HAM-D, Hamilton Anxiety Rating Scale (HAM-A) and CGI.
Conclusions
Women with PMD are more likely to have a remission of their depression with sertraline treatment, a finding that is more pronounced in women who have onset of depression within 4 weeks of childbirth. These data support the continued use of 4 weeks for the DSM-5 postpartum onset specifier for major depressive disorder.
Keywords: postpartum, puerperal, depression, sertraline, SSRI, pregnancy, women
Introduction
Postpartum major depression (PMD) is one of the most common complications of childbirth, occurring in roughly 10% of postpartum women (Gavin et al. 2005; Hübner-Liebermann et al. 2012). Symptoms mirror those of an episode of major depressive disorder (MDD), with the additional criterion that incident PMD has its onset within 4 weeks of childbirth and not during pregnancy or later in the puerperium (APA 2000).
PMD impairs functioning of the mother, which may affect her relationship with and ability to care for her infant (Murray et al. 1996; Reck et al. 2004; Reck et al. 2012; Kaplan et al. 2012). Left untreated, women who have experienced PMD are at increased risk for future depressive episodes, both puerperally and in general (Philipps and O’Hara 1991; Cooper and Murray 1995). While the prevalence of PMD is relatively high and exerts a negative impact upon the mother and her offspring, there are few placebo-controlled studies of antidepressant treatments, including selective serotonin reuptake inhibitors (SSRIs), in this population.
Of the studies on SSRI and other antidepressant treatment for PMD, methods vary and sample sizes are generally small. A recent open-label prospective study by Misri and colleagues (Misri et al. 2012) assessed escitalopram treatment in non-breastfeeding women (n = 15) with MDD symptom onset within 12 months of childbirth. The study found that 93% of women were responders, attaining at least a 50% reduction in Montgomery-Asberg Depression Rating Scale (MADRS) score. However, women showed variable response of anxiety symptoms. In a pragmatic, open-label randomized controlled trial, women with postpartum depression (n = 254) were randomized to antidepressant pharmacotherapy (general practitioner’s choice) or supportive therapy (“listening visits”) (Sharp et al. 2010). At four weeks, antidepressant medications showed twice the improvement rate compared to supportive therapy. However, after 18 weeks of treatment, there was no statistically significant difference between antidepressants and supportive therapy. This may have been affected by the fact that women receiving supportive therapy could also receive antidepressants after the initial four weeks. Among postpartum women with major depression and comorbid anxiety (n = 35) randomized to paroxetine monotherapy or paroxetine with cognitive-behavioral therapy (CBT), both groups showed significant improvement (Misri et al. 2004). Pilot data on eight women with MDD onset within three months of childbirth who took buproprion SR revealed 75% of the women had a 50% or greater decrease in Hamilton Rating Scale for Depression (HAM-D) score over eight weeks (Nonacs et al. 2005). Sertraline produced similar improvement in PMD symptoms compared with nortriptyline, although responders were more readily identified when they were on sertaline versus nortriptyline (Wisner et al. 2006). In sum, while these studies suggest that antidepressants may have efficacy in treating PMD, they are far from conclusive.
SSRIs represent a convenient and readily accessible form of treatment for women with PMD, and studies conducted by our group (Epperson et al. 1997; Epperson et al. 2001; Epperson et al. 2003) and others (Stowe et al. 2003; Davanzo et al. 2011) support the relative safety of maternal SSRI treatment during breastfeeding. At the time the present study was initiated, there were no placebo-controlled randomized clinical trials (RCTs) of any antidepressant in the treatment of PMD. In the interim, Yonkers and colleagues (Yonkers et al. 2008) reported such a study in which they found no significant difference between paroxetine and placebo in response rate, although there was a significantly greater proportion of remissions in the active medication vs. the placebo groups. Bloch and colleagues (2012) published a placebo-controlled RCT of sertraline add-on therapy to brief dynamic psychotherapy in women with postpartum depression (n = 42). Both treatment groups improved, and there was no significant benefit for sertraline over placebo.
We chose to study sertraline in the treatment of incident PMD as its short half-life (DeVane et al. 2002) would allow lactating women to time the “pumping and dumping” of their breast milk to correspond with peak drug levels, approximately eight to nine hours after medication administration (Stowe et al. 2003). Nursing infants’ sertraline levels are typically below the detection limit of most commercial laboratories and have little impact on peripheral measures of serotonin transporter blockade (Epperson et al. 1997; Epperson et al. 2001). We hypothesized that women randomized to sertraline would be more likely to achieve treatment response or symptom remission status than those randomized to placebo. Primary outcome variables were the HAM-D and Clinical Global Impressions (CGI) scores, which were used to determine rates of response and remission. Secondary exploratory analyses were change in HAM-D, Hamilton Anxiety Rating Scale (HAM-A), and Edinburgh Postnatal Depression Scale (EPDS) scores over time, and responder status in women meeting strict DSM-IV criteria for PMD.
Methods
Study Design
The study was a single-center, 6-week, randomized double-blind placebo-controlled trial of sertraline with a one-week placebo lead-in.
Subjects
Women were between ages 18 and 45 years old, and were recruited to a university-based women’s mental health clinical and research program if they (1) reported depression onset within the first 3 months of delivery, (2) were not taking a psychotropic medication for at least 5 weeks, and (3) had given birth to an infant without serious medical issues within the previous 12 months. Diagnosis of PMD was confirmed via the Structured Clinical Interview for DSM-IV (SCID) (First et al. 1995). Women were excluded if the SCID indicated onset of MDD during pregnancy. Participants were required to have a score of at least 18 and less than 32 on the 19-item HAM-D (Hamilton 1960), and to exhibit symptoms that were at least “moderate” in severity as defined by the severity of illness rating on the CGI scale (Guy 1976). Participants screening positive for thyroid disease were excluded unless their thyroid condition was stable. Other exclusions included a history of drug or alcohol dependence within the last 6 months or positive urine drug test during screening, past or present history of an Axis I psychotic disorder (including bipolar type I), presence of active suicidal ideation, any significant medical conditions or plan to become pregnant, or past failed trial of sertraline. Subjects were included only if they were English-speaking. Upon entering the study, subjects were permitted to take benzodiazepines as needed, but no other concomitant psychotropic medications were permitted. Subjects were recruited between 1994 and 2004 from the greater New Haven and southern Connecticut areas through local obstetrician-gynecologists, pediatricians, mental health professionals, PMD support groups and advertisements in local newspapers. Women who were interested and eligible for the study provided voluntary written informed consent for this study that was approved by the Yale University Human Investigation Committee. No changes to eligibility criteria or methods were made after trial commencement.
For the purposes of this study, PMD was defined as onset of MDD within three months of childbirth, rather than the 4 weeks stipulated by DSM-IV for MDD with postpartum onset. The primary reason for being more inclusive with our definition of incident PMD was to allow for analysis of treatment response by timing of onset with respect to childbirth and to broaden generalizability of our results, as onset of depression has been reported considerably later in the puerperium (Stuart et al. 1998).
Treatment Procedures
Participants underwent a one-week, single-blind placebo lead-in. After the lead-in, all subjects who continued to meet the inclusion criteria and had less than a 30% reduction in their HAM-D scores were randomized to a 6-week, double-blind trial of sertraline or placebo. A research pharmacist was responsible for creating a blinding table and distributing the study drug; all other study personnel remained blind to subject treatment status. Participants met weekly with a psychiatric nurse practitioner (DWO) or masters-level psychologist (KAC) to complete patient- and clinician-administered ratings and clinical assessment of symptoms and side effects for the duration of the study. Ratings included the EPDS (Cox et al. 1987), HAM-D, HAM-A (Hamilton 1959) and CGI. Weekly appointments were 50–60 minutes in duration and were conducted in a supportive therapeutic milieu in order to enhance compliance with drug treatment and to aid evaluation of the patient’s clinical condition. The study drug was prescribed initially as sertraline 50 mg or placebo daily. As tolerated, the dosage was increased by one capsule (50 mg) every 1–2 weeks until clinical remission was obtained, with a maximum of 4 capsules (200 mg) per day.
In order to be classified as a medication responder, subjects had a score of less than or equal to 10 on the HAM-D, at least a 50% decrease in HAM-D score from baseline, and a score of “much improved” or “very much improved” on the improvement scale of the CGI. All other subjects were considered non-responders. Participants who met the responder criteria and also had a HAM-D score equal to or less than 7 were considered to be in remission.
The study was powered to detect large effects for the comparison of the primary outcome variables (response and remission rates) between the two groups. In particular, 36 subjects approximately equally distributed among two groups provided at least 80% power to detect the following differences between proportions at a two-sided significance level of 0.05: 20% vs. 63%, 25% vs. 68% and 30% vs. 73%.
Statistical Methods
We compared the intent-to-treat groups on the following variables at baseline: parity, age of onset, duration of illness, baseline HAM-D, baseline HAM-A, baseline EPDS. Continuous variables were compared using two-sided t-tests, while categorical variables were compared using χ2 tests when expected cell counts were greater than 5 or Fisher exact tests (FET) otherwise. Log-transformation was applied to age of onset and duration of illness to better approximate normality in these variables. Our primary analysis was the comparison of response rates in the active and in the placebo group using χ2 tests in the intent-to-treat (ITT) sample (n = 36). We also compared remission rates in the active and placebo groups in the ITT sample. The same analyses were repeated in the evaluable sample (n = 33), consisting of participants with at least 3 post-randomization assessments. Analyses of responder and remitter status among women meeting strict DSM-IV criteria for PMD (onset within 4 weeks of delivery) were performed using Fisher’s exact tests in the ITT sample (n = 27) and in the evaluable sample (n = 24).
Secondary mixed models were fitted to evaluate the time trends in each of the outcome variables (HAM-D, HAM-A, EPDS). All mixed models included a treatment effect, a time effect (we considered linear and quadratic terms), treatment by time interaction and baseline measure as a covariate. Intercept and linear time were considered as random effects and subject was used as the clustering factor. We dropped higher order terms from the model when non-significant (for example, we dropped the treatment by quadratic time interaction). The effect of main interest in these analyses was the treatment by linear time interaction. All tests were conducted as two-tailed, with α = 0.05. Analyses were performed with SAS statistical software (SAS Version 9.2, Cary, NC).
Results
Enrollment
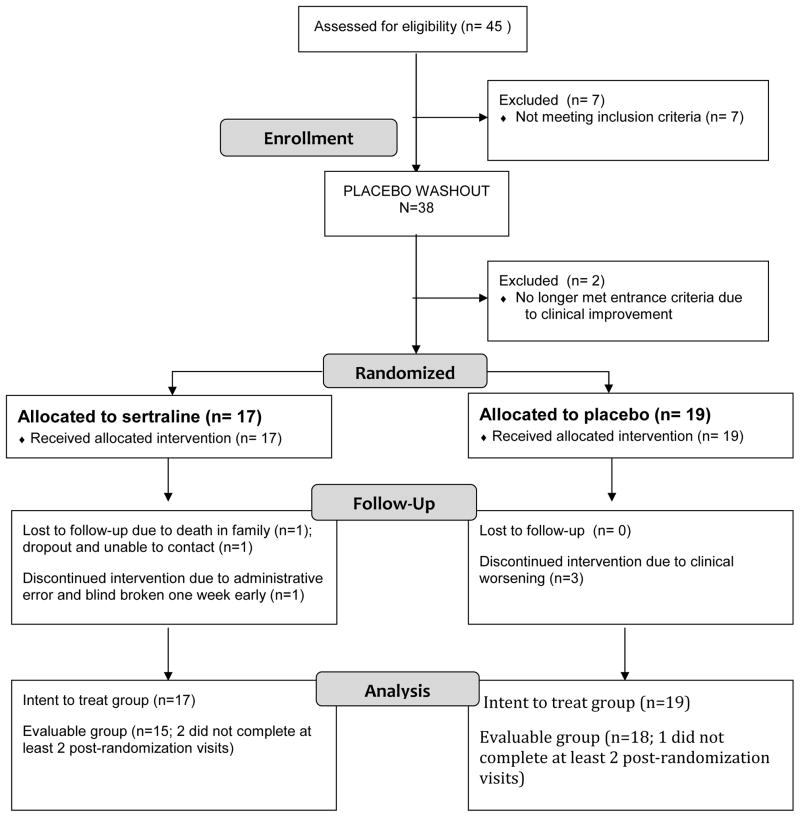
Eighty-five women contacted the clinic for information regarding the study or treatment for PMD in general; 40 of these were excluded by phone interview, the majority because they were already taking an antidepressant medication prescribed by their obstetrician, with those remaining either reporting other exclusions or declining to take medication. Forty-five women underwent in-office screening, of whom 7 failed to meet one or more study criteria. A sample of 38 women who met all study criteria underwent the placebo lead-in week. Two subjects demonstrated a greater than 30% decline in their HAM-D scores during the placebo lead-in and were removed from the study and followed clinically. A total of 17 women were randomized to the sertraline group and 19 were randomized to placebo, for a total of 36 women in the intent-to-treat group. Among those, 29 (81%) completed through week 7, 33 (92%) completed through week 4, and all 36 (100%) completed through week 2. Reasons for failure to complete the full seven weeks included clinical deterioration (n = 3, all in the placebo group), loss to follow-up (n = 3), and accidental unblinding (n = 1). This information is presented in CONSORT diagram form in Figure 3.
FIGURE 3. CONSORT Flow Diagram.
CONSORT diagram depicting participant flow through the study.
Patient Characteristics
Mean±SD age of participants was 30.8±4.0 years, with no between-group (sertraline vs placebo) differences in demographic variables (Table 1). Likewise, treatment groups were similar with respect to timing of onset of MDD, duration of illness and the number of weeks post-childbirth at the time of enrollment (Table 1). Baseline behavioral ratings were similar between groups (all ps >0.05), with the exception of significantly lower mean HAM-D scores in those randomized to sertraline vs. placebo (20.6±2.8 vs 23.2±3.9, t(34)= −2.26, p=0.03) (Table 1).
TABLE 1.
BASELINE PATIENT CHARACTERISTICS BY TREATMENT GROUP
| DEMOGRAPHICS | SERTRALINE (n = 17) | PLACEBO (n = 19) | STATISTIC |
|---|---|---|---|
| Age, years | 29.6 (4.0) | 31.7 (3.7) | t(34)=−1.6, p=0.11 |
| Race; n (%) | FET p=1.0 | ||
| Caucasian | 16 (94%) | 18 (95%) | |
| Hispanic | 1 (6%) | 1 (5%) | |
| Marital Status; n (%) | FET p=0.65 | ||
| Never Married | 3 (17.7%) | 1 (5.3%) | |
| Married | 13 (76.5%) | 17 (89.5%) | |
| Divorced | 1 (6%) | 1 (5.3%) | |
| Education, years | 14.4 (2.0) | 14.0 (1.2) | t(33)=0.75, p=0.46 |
| Parity | 1.9 (0.9) | 1.9 (0.9) | t(34)=0.15, p=0.88 |
| Breastfeeding; n (%) | 6 (35.3%) | 5 (26.3%) | χ2(1)=0.56, p=0.56 |
| Onset of illness, weeks postpartum | 3.9 (4.9) | 2.5 (3.0) | t(34)=1.1, p=0.30 |
| Duration of illness, weeks | 15.5 (13.6) | 13.5 (12.3) | t(34)=0.47, p=0.64 |
| Clinic presentation, weeks postpartum | 19.4 (12.0) | 15.4 (12.2) | t(34)=1.01, p=0.32 |
| BEHAVIORAL SCALES | SERTRALINE (n = 17) | PLACEBO (n = 19) | STATISTIC |
| Hamilton Depression | 20.6 (2.8) | 23.2 (3.9) | t(34)= −2.26, p=0.03 |
| Hamilton Anxiety | 21.3 (6.3) | 24.5 (5.8) | t(27)= −1.43, p=0.16 |
| Edinburgh Postnatal Depression | 18.8 (2.6) | 20.8 (5.7) | t(26.2)=−1.44, p=0.16 |
Legend: Subject characteristics are listed as mean and standard deviations unless otherwise indicated. FET=Fisher’s exact test.
Medication
The mean daily dose at week 7 was 100.0±54.0 mg for the sertraline group and 119.4±51.8 mg for the placebo group. Sertraline dose was not increased until the second post-randomization week and only if no clinical improvement was reported. Side effects were recorded in each subject’s weekly study visit case report form. In the sertraline group, three subjects reported side effects, including nausea (n = 3; 17.6%), headache (n = 1; 5.8%) and diarrhea (n = 1; 5.8%). One participant in the placebo group reported frequent diarrhea throughout the course of the study (n = 1; 5.3%). No participants stopped the study due to medication side effects, and there were no adverse events reported for the participants or for any breastfeeding (n = 6 sertraline, n = 5 placebo) infant. Concomitant medications included benzodiazepines (n = 11) and oral contraceptives (n = 1). Three subjects in the sertraline group and eight subjects in the placebo group used benzodiazepines as needed, but this difference was not significant ((χ2 (1)=1.9, p = 0.171).
Behavioral Outcomes
For our primary analysis of responder status in the ITT group, we found a significantly greater number of responders among those women randomized to sertraline (59%, 10/17) vs. those randomized to placebo (26%, 5/19) (χ2 (1)=3.9, p=0.05) (Figure 1a). The findings were similar in analyses conducted in the evaluable group, with 67% (10/15) of women in the sertraline group meeting responder criteria vs. 28% (5/18), of those in the placebo group (χ2 (1)=4.99, p=0.03) (Figure 1b).
FIGURE 1. RESPONDERS BY TREATMENT GROUP.
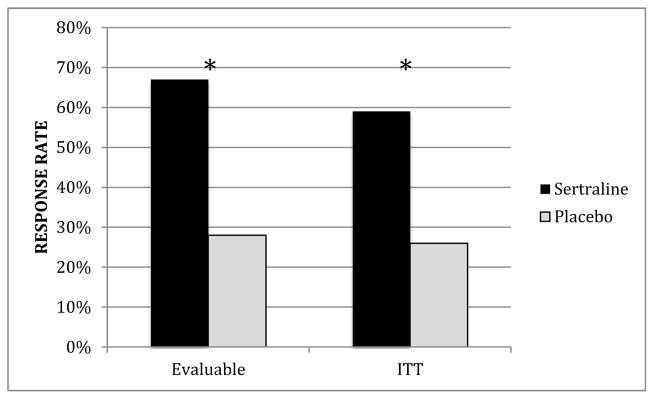
Response rates in intent to treat (ITT) and evaluable samples, by treatment group, represented as percentage. In the evaluable group, there was a significantly greater number of responders among those women randomized to sertraline (67%, 10/15) compared to those in the placebo group (28%, 5/18), (χ2 (1)=4.99, p=0.03). In the ITT group, there was a significantly greater number of responders among those women randomized to sertraline (59%, 10/17) vs. those randomized to placebo (26%, 5/19), (χ2 (1)=3.9, p=0.05).
The analysis of remitter status in the ITT group also revealed a significantly greater number of remitters among those women randomized to sertraline (53%, 9/17) vs. those randomized to placebo (21%, 4/19) (χ2 (1)=4.0, p=0.05). The results were similar in analyses conducted in the evaluable group, with 60% (9/15) of women in the sertraline group meeting remission criteria versus 22% (4/18) of those in the placebo group (χ2 (1)=4.89, p=0.03).
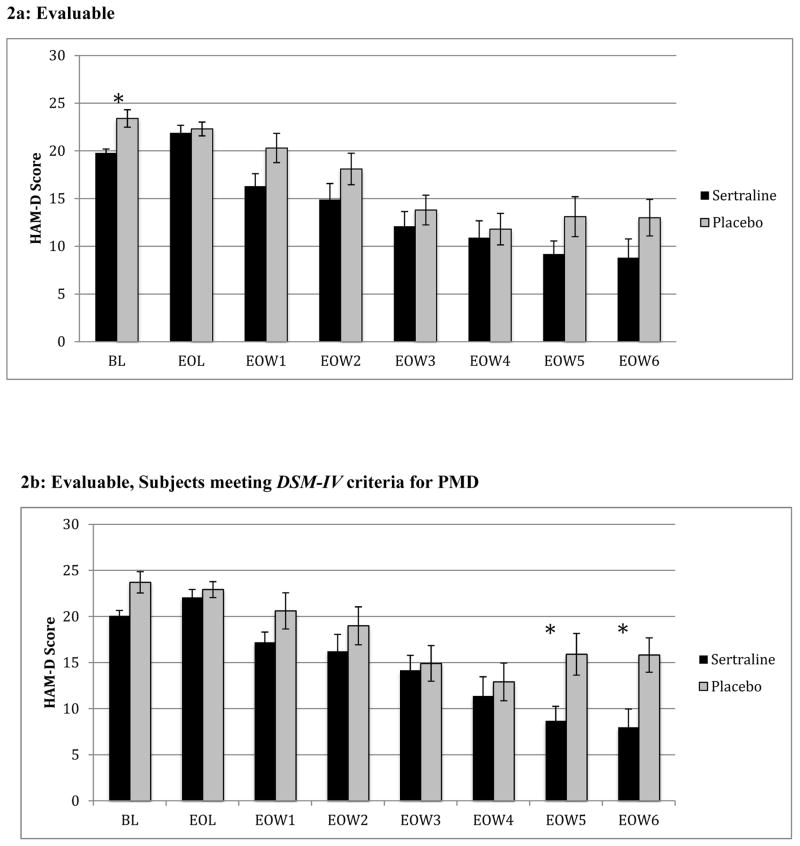
In the evaluable sample, mean HAM-D scores at end of placebo lead-in (EOL) were 21.9 (sertraline) and 22.3 (placebo), and at end of week 6 (EOW6) were 8.8 (sertraline) and 13.0 (placebo). These differences were not statistically significant (both p-values > 0.15). In the evaluable sample meeting strict DSM-IV criteria for PMD (onset of PMD within the first 4 weeks past giving birth), HAM-D scores at EOL were 22.1 (sertraline) and 22.9 (placebo), and at EOW6 were 8.0 (sertraline) and 15.8 (placebo) (Figure 2). Only the difference at EOW6 was statistically significant (p = 0.01). For our secondary analysis of change in HAM-D scores over time, mixed models did not reveal significant group by time effects (Table 2), although there was a trend for subjects randomized to sertraline to have a greater reduction in HAM-A scores from baseline to the end of Week 7 compared to subjects randomized to placebo (ITT group p=0.08; evaluable group p=0.07).
FIGURE 2. HAM-D Score by Week.
Mean HAM-D score with standard error from Baseline through study completion among evaluable subjects. 2a: In the full evaluable sample, there was no significant group x time effect (F(1,145)=1.95, p=0.17). Mean HAM-D scores at EOL were 21.9 (sertraline) and 22.3 (placebo) and at EOW6 were 8.8 (sertraline) and 13.0 (placebo). 2b: Among evaluable subjects meeting DSM-IV criteria for PMD, there was a statistically significant group x time effect (F(1,104)=6.87, p=0.01). Mean HAM-D scores at EOL were 22.1 (sertraline) and 22.9 (placebo) and at EOW6 were 8.0 (sertraline) and 15.8 (placebo). HAM-D - Hamilton Depression Rating Scale, EOL – End of Placebo Lead-In, EOW – End of Week, PMD – Postpartum Major Depression. Significant group differences by time point at two-sided 0.05 significance level are denoted by *.
TABLE 2.
MIXED MODELS ANALYSES OF BEHAVIORAL OUTCOMES IN THE INTENT-TO-TREAT AND EVALUABLE GROUPS
| ITT (n = 36) | Evaluable (n = 33) | |||
|---|---|---|---|---|
| HAM-D | F(1,145)=2.05, p=0.15 | β=0.60, SE=0.42 | F(1,145)=1.95, p=0.17 | β=0.59, SE=0.42 |
| HAM-A | F(1,111)=3.18, p=0.08 | β=0.92, SE=0.52 | F(1,111)=3.25, p=0.07 | β=0.93, SE=0.52 |
| EPDS | F(1,137)=0.43, p=0.51 | β=0.24, SE=0.37 | F(1,137)=0.42, p=0.52 | β=0.24, SE=0.37 |
Legend: F-statistics and p-values, betas and standard errors (SE) from mixed models analyses. HAM-D - Hamilton Depression Rating Scale; HAM-A - Hamilton Anxiety Rating Scale; EPDS - Edinburgh Postnatal Depression Scale. Independent variables were treatment, time (linear and quadratic term), treatment by time interaction and corresponding baseline measure as a covariate. That is, baseline HAM-D was a used as covariate for analyses of HAM-D, baseline HAM-A was used as a covariate for analyses of HAM-A and baseline EPDS was used as a covariate for analyses of EPDS.
The secondary analysis of responder status in women meeting strict DSM-IV criteria for PMD (onset of PMD within the first 4 weeks past giving birth) in the ITT sample (n = 27) showed a significantly greater number of responders among those women randomized to sertraline (50%, 6/12) vs. those randomized to placebo (6.7%, 1/15) (FET p=0.02). Findings in the evaluable sample (n = 24) were similar, with 60% (6/10) responders among those women randomized to sertraline vs. 7% (1/14) in those randomized to placebo (FET p=0.01). All women meeting strict DSM criteria for PMD who were responders were also remitters. Thus, secondary analysis among women meeting strict DSM criteria for PMD included the same subjects for responders and remitters. Mixed model analyses in the ITT and evaluable subsamples of women with onset of PMD within the first 4 weeks of giving birth revealed statistically significant group by time effects for all three outcome measures (Table 3). Women with PMD onset within 4 weeks of childbirth had a shorter duration of illness (M = 8.4 weeks) than women with later PMD onset (M = 16.9 weeks) that was not statistically significant (t(31) = −1.74, p = 0.09). They also had a greater incidence of past psychiatric diagnosis (8/27; 29.6%) than women with later PMD onset (1/9; 11.1%), but this was not statistically significant ((χ2 (2)=4.0, p = 0.2),
TABLE 3.
MIXED MODELS ANALYSES OF BEHAVIORAL OUTCOMES IN THE INTENT-TO-TREAT AND EVALUABLE GROUPS AMONG WOMEN WITH ILLNESS ONSET WITHIN FOUR WEEKS OF GIVING BIRTH
| ITT (n = 36) | Evaluable (n = 33) | |||
|---|---|---|---|---|
| HAM-D | F(1,104)=6.98, p=0.01 | β=1.18, SE=0.45 | F(1,104)=6.87, p=0.01 | β=1.18, SE=0.45 |
| HAM-A | F(1,87)=4.92, p=0.03 | β=1.19, SE=0.54 | F(1,87)=5.08, p=0.03 | β=1.22, SE=0.54 |
| EPDS | F(1,98)=4.16, p=0.04 | β=0.91, SE=0.45 | F(1,98)=4.16, p=0.04 | β=0.91, SE=0.45 |
Legend: F-statistics and p-values, betas and standard errors (SE) from mixed models analyses. HAM-D - Hamilton Depression Rating Scale; HAM-A - Hamilton Anxiety Rating Scale; EPDS - Edinburgh Postnatal Depression Scale. Independent variables were treatment, time (linear and quadratic term), treatment by time interaction and the corresponding baseline measure as a covariate. That is, baseline HAM-D was used as a covariate for analyses of HAM-D, baseline HAM-A was used as a covariate for analyses of HAM-A and baseline EPDS was used as a covariate for analyses of EPDS) .
Discussion
The primary purpose of this study was to examine whether sertraline alone was effective in the treatment of incident PMD and secondarily, whether timing of PMD onset contributed to treatment response. A placebo-controlled design was believed to be necessary given the special nature of the postpartum population with respect to incident depression, the hormonal milieu of the puerperium and the psychosocial factors that are unique to the postpartum experience. We observed that many women who called to inquire about the study had already been started on an SSRI by their obstetrician, as early as the 6-week postpartum visit. These individuals were without any formal psychiatric evaluation and there were no published placebo-controlled studies of an antidepressant in this population at that time. In contrast, the second most common reason women did not enroll in the study was the concern about taking a medication in the postnatal period, particularly while nursing their infant. As untreated maternal MDD can have deleterious consequences for the offspring (Murray et al. 1996; Reck et al. 2004; Reck et al. 2012; Kaplan et al. 2012), studies investigating the effectiveness and tolerability of antidepressants during the postnatal period are of vital importance.
With respect to the primary analysis of treatment response as a dichotomous variable, our findings differ from those of Yonkers and colleagues (2008), who have published the only placebo-controlled RCT of an antidepressant (paroxetine) in incident PMD. In that study, the placebo response rate was higher (31%) and the SSRI response rate (43%) less robust than what we observed in the present study. Notably, a placebo lead-in was not included in their study design and the drop-out rate was considerably larger (44%) than that in the present study (19%). Compared to the present study, response and remission rates were similar between active medication (70% and 65%, respectively) and placebo (55% and 50%, respectively) in a recently published study of sertraline in addition to an 8-week course of cognitive-behavioral therapy (CBT), underscoring the antidepressant efficacy of CBT (Bloch et al. 2012). While we observed a significant difference in treatment response between sertraline (59%) and placebo (26%), and a 2-fold increased rate of remission (53% vs. 21%) with active medication, the effect of SSRI treatment alone did not appear to be as large as that observed with combined psychotherapy and antidepressant medication (Bloch et al., 2012). These findings suggest that our study milieu was supportive, resulting in a low drop-out rate, but not psychotherapeutic in and of itself. With respect to drop-outs, it is important to highlight that the three women who decompensated during the course of this study were in the placebo group. The blind was broken early on one individual who developed suicidal ideation, another with mild paranoia and another with increasing anxiety and agitation. Weekly assessments allowed for timely detection of their clinical decline and all three responded well to open-label sertraline treatment.
An equally important finding that, to our knowledge, has not been previously reported is the impact of having PMD onset within 4 weeks of childbirth on antidepressant treatment response. Women who met strict DSM-IV criteria for PMD (onset within 4 weeks postpartum) showed a significantly greater response rate to sertraline than to placebo, in both the ITT and evaluable samples. Analyses in this subsample of women also revealed statistically significant group by time effects for all three outcome measures. There are a number of possible explanations for this. As the group with PMD onset within four weeks was larger than the group with later onset PMD, we were able to perform treatment comparisons within the former subgroup of subjects. Theoretically, this early-onset group is comprised of women for whom parturition may be a particularly potent trigger for depression. While women with PMD onset within four weeks of childbirth tended to have a shorter duration of illness and greater incidence of past psychiatric diagnosis than women with later PMD onset, the later-onset group was too small to allow for meaningful statistical comparisons.
We believe that the robustness of our findings in women who developed depression within the first month of delivery, a time that corresponds to profound reproductive and neurosteroid withdrawal, provides support for the validity of the 4-week onset specifier included in the DSM-IV. Progesterone’s neurosteroid metabolite allopregnanolone (ALLO), a positive allosteric modulator of the GABAA receptor, is higher during pregnancy but drops precipitously postpartum (Luisi et al. 2000; Turkmen et al. 2011). SSRIs increase ALLO production from 5α-dihydroprogesterone (5α-DHP), an aspect of SSRI treatment that is particularly relevant in new parturient women (Griffin and Mellon 1999). Estradiol, which is at its nadir within 72 hours after childbirth and remains low throughout the first month, particularly in lactating women, has potent effects on serotonin synthesis, degradation, pre- and postsynaptic receptors, and the transporter in brain regions implicated in mood regulation (Amin et al. 2005; Shanmugan and Epperson 2012).
Anxiety among postpartum women is an important clinical consideration, as it may influence healthcare utilization, breastfeeding duration, and parenting stress, among other factors (Misri et al. 2010; Paul et al. 2013). In the present study there was a trend for subjects taking sertraline to have a greater reduction in HAM-A scores compared to subjects randomized to placebo. While the present study was intended to examine SSRI treatment of depressive symptoms, future research might examine SSRI treatment for anxiety in the postpartum.
Psychosocial factors, such as social support and social role changes, are also important when considering PMD course and treatment response, and may be addressed in psychotherapy (Logsdon et al. 2003; Misri et al. 2012). Several previous studies have found psychotherapy (CBT and interpersonal therapy; IPT) alone or with antidepressant medications to be more effective than no psychotherapy, although not all studies were careful to limit enrollment to women with incident PMD and many included women with depression onset any time during the first postpartum year (Sockol et al. 2011). The earliest antidepressant study in PMD showed that fluoxetine out-performed placebo, and six CBT-derived counseling sessions provided greater benefit than one session, but the combination of fluoxetine and 6 sessions of CBT did not provide added benefit above either active intervention alone (Appleby et al. 1997). In a study of women with incident PMD randomized to sertraline or nortriptyline, there was no difference in final treatment response, although response occurred earlier with sertraline (Wisner et al. 2001).
The present study is constrained by several important limitations. The sample size was small for an RCT, although comparable to that of previous studies conducted in PMD (n = 42, Bloch et al., 2012; n = 31, Yonkers et al., 2008). Our drop-out rate was relatively low (19%). As the study progressed it became clear that women preferred to receive antidepressant treatment from their obstetrician (Yonkers et al. 2011), making recruitment increasingly difficult. As we did not obtain measures of symptom severity or demographics from the women who inquired about the study but opted not to enroll, the sample of women who completed the study may not be representative of the general postpartum population. Dose escalation was relatively slow over the 6 weeks of randomization and may have limited our ability to detect an impact of active treatment before study completion. Indeed, the HAM-D scores were becoming increasingly disparate between the active and placebo groups during the final two weeks of randomization (Figure 2). While we did not demonstrate a treatment x time effect for the HAM-D in the full sample, it is possible that given the increasing disparity in scores, a group x time effect might have become evident had the study continued for more than six weeks post-randomization. While it is possible that the group x time effect in women with PMD onset within four weeks of childbirth might have been an artifact of drop-out toward the end of the trial, leaving only the most ill women, only one subject in the sertraline group with PMD onset within four weeks dropped out, and three subjects in the placebo group with PMD onset within four weeks dropped out. Thus, it is unlikely that this is an artifact of drop-out of subjects with milder severity.
In summary, the postpartum is a unique psychosocial and biological context that may impact acceptability of antidepressant treatment, willingness to seek formal psychiatric care, and response to antidepressants modulating neurotransmitters that are also affected by ovarian hormones. The postpartum includes unique stressors, including parenting (Misri et al. 2010) and role changes (Logsdon et al. 2003), that may not be sufficiently addressed by antidepressant treatment alone. Indeed, psychosocial factors such as social support, role changes, or temperament may have important impacts on PMD course or treatment response. While not within the scope of the present study, these should be explored in future research. The postpartum period also includes profound biological changes, including fluctuation in neuromodulatory hormones such as progesterone (Turkmen et al. 2011), estrogen and neurosteroids, which may affect pharmacologic treatment response. Although the response rate observed in this RCT for the entire ITT group was similar to that for MDD in other populations (Kennedy et al. 2009; Thase et al. 2010), the restriction of disorder onset to within 4 weeks of delivery impacted treatment response and should be considered in future perinatal research. These data, together with those from the most recent RCT including both psychotherapy and SSRI medication (Bloch et al. 2012), suggest that antidepressant medication plus evidence-based psychotherapy provides the most optimal outcome, although SSRI alone leads to greater remission of symptoms than no treatment at all and is particularly effective in women with early-onset PMD.
Acknowledgments
Funding/Grants: The conduct and analysis of this research as well as manuscript preparation was funded in part by the following entities; Pfizer (New York NY), the National Institute of Mental Health [P50 MH099910 (CNE, LH, KC); K23 MH01830 (CNE)], and the National Institute of Drug Abuse [K24 DA03031 (CNE)]
Abbreviations
- 5α-DHP
5α-dihydroprogesterone
- ALLO
allopregnanolone
- CBT
cognitive-behavioral therapy
- CGI
Clinical Global Impressions
- CNS
central nervous system
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- FET
Fisher exact tests
- HAM-A
Hamilton Anxiety Rating Scale
- HAM-D
Hamilton Depression Rating Scale
- IPT
interpersonal therapy
- ITT
intent-to-treat
- 1H-MRS
proton magnetic resonance spectroscopy
- EPDS
Edinburgh Postnatal Depression Scale
- MDD
major depressive disorder
- PMD
postpartum major depression
- RCT
randomized clinical trial
- SCID
Structured Clinical Interview for DSM
- SERT
serotonin transporter
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Conflicts of Interest: Dr. Epperson has received research grant support from Pfizer, Eli Lilly, Shire, and Novartis, and honoraria from Pfizer, Eli Lilly, and Glaxo Smith Kline. Dr. Epperson or a family member holds stock in Johnson and Johnson, Pfizer, Merck and Company, Abbvie, and Abbott. Dr. Price has received research support from Medtronic, Neuronetics, NIH, HRSA, and Neosync; he has served on advisory panels for Abbott and AstraZeneca; and he has served as a consultant to Gerson Lehrman, Wiley, Springer, Qatar National Research Fund, and Abbott. All other authors declare that they have no conflicts of interest.
References
- Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: 2000. text rev. [Google Scholar]
- Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314:932–936. doi: 10.1136/bmj.314.7085.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Meiboom H, Lorberblatt M, et al. The effect of sertraline add-on to brief dynamic psychotherapy for the treatment of postpartum depression: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73:235–241. doi: 10.4088/JCP.11m07117. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Murray L. Course and recurrence of postnatal depression. Evidence for the specificity of the diagnostic concept. Br J Psychiatry. 1995;166:191–195. doi: 10.1192/bjp.166.2.191. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Davanzo R, Copertino M, De Cunto A, et al. Antidepressant drugs and breastfeeding: a review of the literature. Breastfeed Med. 2011;6:89–98. doi: 10.1089/bfm.2010.0019. [DOI] [PubMed] [Google Scholar]
- DeVane CL, Liston HL, Markowitz JS. Clinical pharmacokinetics of sertraline. Clin Pharmacokinet. 2002;41:1247–1266. doi: 10.2165/00003088-200241150-00002. [DOI] [PubMed] [Google Scholar]
- Epperson C, Anderson GM, McDougle CJ. Sertraline and breast-feeding. N Engl J Med. 1997;336:1189–1190. doi: 10.1056/NEJM199704173361615. [DOI] [PubMed] [Google Scholar]
- Epperson C, Czarkowski KA, Ward-O’Brien D, et al. Maternal sertraline treatment and serotonin transport in breast-feeding mother-infant pairs. Am J Psychiatry. 2001;158:1631–1637. doi: 10.1176/appi.ajp.158.10.1631. [DOI] [PubMed] [Google Scholar]
- Epperson C, Jatlow PI, Czarkowski K, Anderson GM. Maternal Fluoxetine Treatment in the Postpartum Period: Effects on Platelet Serotonin and Plasma Drug Levels in Breastfeeding Mother-Infant Pairs. Pediatrics. 2003;112:e425–e425. doi: 10.1542/peds.112.5.e425. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 1995. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P v 2.0) [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Mellon SH. Selective serotonin reuptake inhibitors directly alter activity of neurosteroidogenic enzymes. Proc Natl Acad Sci USA. 1999;96:13512–13517. doi: 10.1073/pnas.96.23.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; Rockville, MD: 1976. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatr. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner-Liebermann B, Hausner H, Wittmann M. Recognizing and treating peripartum depression. Dtsch Arztebl Int. 2012;109:419–424. doi: 10.3238/arztebl.2012.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan PS, Danko CM, Kalinka CJ, Cejka AM. A developmental decline in the learning-promoting effects of infant-directed speech for infants of mothers with chronically elevated symptoms of depression. Infant Behav Dev. 2012;35:369–379. doi: 10.1016/j.infbeh.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Andersen HF, Thase ME. Escitalopram in the treatment of major depressive disorder: a meta-analysis. Curr Med Res Opin. 2009;25:161–175. doi: 10.1185/03007990802622726. [DOI] [PubMed] [Google Scholar]
- Logsdon MC, Wisner K, Hanusa BH, Phillips A. Role functioning and symptom remission in women with postpartum depression after antidepressant treatment. Arch Psychiatr Nurs. 2003;17:276–283. doi: 10.1053/j.apnu.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85:2429–2433. doi: 10.1210/jcem.85.7.6675. [DOI] [PubMed] [Google Scholar]
- Misri S, Abizadeh J, Albert G, et al. Restoration of functionality in postpartum depressed mothers: an open-label study with escitalopram. J Clin Psychopharmacol. 2012;32:729–732. doi: 10.1097/JCP.0b013e31826867c9. [DOI] [PubMed] [Google Scholar]
- Misri S, Kendrick K, Oberlander TF, et al. Antenatal depression and anxiety affect postpartum parenting stress: a longitudinal, prospective study. Can J Psychiatry. 2010;55:222–228. doi: 10.1177/070674371005500405. [DOI] [PubMed] [Google Scholar]
- Misri S, Reebye P, Corral M, Milis L. The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry. 2004;65:1236–1241. doi: 10.4088/jcp.v65n0913. [DOI] [PubMed] [Google Scholar]
- Murray L, Hipwell A, Hooper R, et al. The cognitive development of 5-year-old children of postnatally depressed mothers. J Child Psychol Psychiatry. 1996;37:927–935. doi: 10.1111/j.1469-7610.1996.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Nonacs RM, Soares CN, Viguera AC, et al. Bupropion SR for the treatment of postpartum depression: a pilot study. Int J Neuropsychopharmacol. 2005;8:445–449. doi: 10.1017/S1461145705005079. [DOI] [PubMed] [Google Scholar]
- Paul IM, Downs DS, Schaefer EW, et al. Postpartum anxiety and maternal-infant health outcomes. Pediatrics. 2013;131:e1218–1224. doi: 10.1542/peds.2012-2147. [DOI] [PubMed] [Google Scholar]
- Philipps LH, O’Hara MW. Prospective study of postpartum depression: 4 1/2-year follow-up of women and children. J Abnorm Psychol. 1991;100:151–155. doi: 10.1037//0021-843x.100.2.151. [DOI] [PubMed] [Google Scholar]
- Reck C, Hunt A, Fuchs T, et al. Interactive regulation of affect in postpartum depressed mothers and their infants: an overview. Psychopathology. 2004;37:272–280. doi: 10.1159/000081983. [DOI] [PubMed] [Google Scholar]
- Reck C, Noe D, Gerstenlauer J, Stehle E. Effects of postpartum anxiety disorders and depression on maternal self-confidence. Infant Behav Dev. 2012;35:264–272. doi: 10.1016/j.infbeh.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: Towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Chew-Graham C, Tylee A, et al. A pragmatic randomised controlled trial to compare antidepressants with a community-based psychosocial intervention for the treatment of women with postnatal depression: the RESPOND trial. Health Technol Assess. 2010;14:iii–iv. ix–xi, 1–153. doi: 10.3310/hta14430. [DOI] [PubMed] [Google Scholar]
- Sockol LE, Epperson CN, Barber JP. A meta-analysis of treatments for perinatal depression. Clinical Psychology Review. 2011;31:839–849. doi: 10.1016/j.cpr.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe ZN, Hostetter AL, Owens MJ, et al. The pharmacokinetics of sertraline excretion into human breast milk: determinants of infant serum concentrations. J Clin Psychiatry. 2003;64:73–80. doi: 10.4088/jcp.v64n0114. [DOI] [PubMed] [Google Scholar]
- Stuart S, Couser G, Schilder K, et al. Postpartum anxiety and depression: onset and comorbidity in a community sample. J Nerv Ment Dis. 1998;186:420–424. doi: 10.1097/00005053-199807000-00006. [DOI] [PubMed] [Google Scholar]
- Thase ME, Nierenberg AA, Vrijland P, et al. Remission with mirtazapine and selective serotonin reuptake inhibitors: a meta-analysis of individual patient data from 15 controlled trials of acute phase treatment of major depression. Int Clin Psychopharmacol. 2010;25:189–198. doi: 10.1097/YIC.0b013e328330adb2. [DOI] [PubMed] [Google Scholar]
- Turkmen S, Backstrom T, Wahlstrom G, et al. Tolerance to allopregnanolone with focus on the GABA-A receptor. Br J Pharmacol. 2011;162:311–327. doi: 10.1111/j.1476-5381.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26:353–360. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Peindl KS, et al. Prevention of recurrent postpartum depression: a randomized clinical trial. J Clin Psychiatry. 2001;62:82–86. doi: 10.4088/jcp.v62n0202. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Lin H, Howell HB, et al. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J Clin Psychiatry. 2008;69:659–665. doi: 10.4088/jcp.v69n0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Vigod S, Ross LE. Diagnosis, pathophysiology, and management of mood disorders in pregnant and postpartum women. Obstet Gynecol. 2011;117:961–977. doi: 10.1097/AOG.0b013e31821187a7. [DOI] [PubMed] [Google Scholar]