Abstract
Avascular necrosis of the femoral head (AVN) is an increasingly common cause of musculoskeletal disability, and it poses a major diagnostic and therapeutic challenge. Although patients are initially asymptomatic, AVN usually progresses to joint destruction, requiring total hip replacement, usually before the fifth decade. Avascular necrosis is characterized by osseous cell death due to vascular compromise. Avascular necrosis of bone results generally from corticosteroid use, trauma, pancreatitis, alcoholism, radiation, sickle cell disease, infiltrative diseases (e.g. Gaucher’s disease), and Caisson disease. The most commonly affected site is the femoral head and patients usually present with hip and referred knee pain. The aim of diagnostic imaging procedures in avascular femoral head necrosis is to provide the patient with a stage-adapted therapy. Therefore, a differentiated diagnostic work-up is needed. Native radiography of the hip in two planes is still the first step. Over the past years, the diagnosis of femoral head necrosis has experienced tremendous improvement due to the use of MRI and CT scans. Because of these improvements the correct stage can be diagnosed early and the appropriate therapy can be initiated immediately. Today, MRI is the most sensitive diagnostic imaging procedure. CT scans can be particularly useful to exclude subchondral fractures. The use of bone scintigraphy is restricted to exceptional cases. In Europe, the ARCO classification of avascular femoral head necrosis has been widely accepted. In this overview, we describe the specific characteristics of the different diagnostic imaging procedures and illustrate them with appropriate imaging material.
Keywords: AVN, MRI, CT
Introduction
Avascular necrosis of the femoral head (AVN) is an increasingly common cause of musculoskeletal disability, and it poses a major diagnostic and therapeutic challenge. Although patients are initially asymptomatic, AVN usually progresses to joint destruction, usually before the fifth decade [1].
Femoral head AVN represents ischemic injury of femoral head. By convention, the term avascular (ischemic) necrosis generally is applied to areas of epiphyseal or subarticular involvement, whereas "bone infarct" usually is reserved for metaphyseal and diaphyseal involvement.
Avascular necrosis is characterized by osseous cell death due to vascular compromise [1]. Avascular necrosis of bone results generally from corticosteroid use, trauma, pancreatitis, alcoholism, radiation, sickle cell disease, infiltrative diseases (e.g. Gaucher’s disease), and Caisson disease [1,2]. The most commonly affected site is the femoral head and patients usually present with hip and referred knee pain.
Early detection of avascular necrosis of the femoral head allows conservative treatment to be effective, with alleviation of pain and preservation of normal joint function. Once subchondral fracture has occurred, more aggressive therapeutic measurements such as total joint replacement are necessary, with a significant increase in morbidity.
Noninvasive diagnostic tests used in detecting AVN include plain radiography, magnetic resonance imaging (MRI), computed tomography (CT), skeletal scintigraphy, and single photon emission computed tomography (SPECT). Since its introduction, magnetic resonance (MR) imaging has attracted the attention of investigators searching for a sensitive, noninvasive test for the early detection of AVN. The principal role of MR imaging is in establishing the diagnosis of AVN in symptomatic patients before radiographic changes become apparent. MR imaging can also depict early stages of AVN in high-risk, asymptomatic patients or in the asymptomatic contralateral hips of patients with known AVN.
Plain film radiography
Using plain film, the sensitivity for detecting early stages of the disease is as low as 41% [3]. Plain film does not detect stage 0 and 1 AVN. A delay of 1-5 years may occur between the onset of symptoms and the appearance of radiographic abnormalities. Normal radiographic findings do not necessarily mean that disease is not present. A staging system using radiographic findings has been developed by Ficat and Arlet and has been used widely for treating avascular necrosis [4]. This has been supplanted by the classification system of Steinberg et al, which incorporates MRI and scintigraphic findings [5].
In stage 0 (preclinical and preradiologic) avascular necrosis can be suggested only if it has already been diagnosed in the contralateral hip. Since the advent of MRI, stage 1 (preradiologic) is defined by normal findings on radiographs and positive findings on MRI or bone scintigraphy. Stage 1 represents the early resorptive stage. Late in this stage, plain radiographs may show minimal osteoporosis and/or blurring and poor definition of the bony trabeculae. Stage 2 (reparative) represents the reparative stage before flattening of the femoral head occurs. Stage 2 may extend for several months or longer. Demineralization now is evident. It may be generalized or patchy or appear in the form of small cysts within the femoral head. Patchy sclerosis appears after demineralization develops, usually in the superolateral aspect of the femoral head. In stage 3 (early collapse of the femoral head). A linear subcortical lucency, representing a fracture line, is present immediately beneath the articular cortex. It may extend into the articular cartilage at the superolateral aspect of the femoral head. This is termed the crescent sign (figure 1). Stage 4 (progressive degenerative disease) is represented by further flattening of the femoral head occurs with loss of its smooth convex contour (figure 2). Ultimately, the superior femoral fragment, representing the articular surface and the immediate subchondral bone, may become separated from the underlying femoral head or depressed and compacted into the femoral head. Severe collapse and destruction of the femoral head leads to progressive degenerative joint disease (DJD) with joint space narrowing, marginal osteophyte formation, and subchondral cyst formation.
Fig.1.

An anteroposterior pelvic radiography showing flattening of the superolateral aspect (the weightbearing portion) of the right femoral head. There is a zone of decreased density representing the crescent sign, indicating subchondral fracture (stage III)
Fig.2.
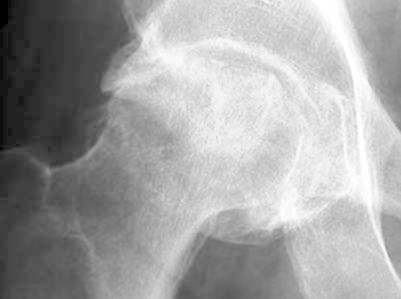
Anteroposterior radiographyc view of the pelvis shows flattening of the outer portion of the right femoral head from avascular necrosis, with adjacent joint space narrowing, juxta-articular sclerosis, and osteophytes representing degenerative joint disease (stage IV)
Magnetic resonance imaging (MRI)
Magnetic resonance imaging has recently emerged as the most sensitive, specific, and widely used diagnostic tool for avascular necrosis of femural head. In most reports, MRI can diagnose very early lesions with a greater than 90 percent specificity and sensitivity based on histology or eventual rogression [6,7]. Screening of asymptomatic, high-risk patients may enable early intervention. Imaging findings have been described and have been shown to correlate with the histologic changes within the marrow of the femural head.
In the early stages of the disease, there may not be any alteration of the normal signal intensity of the femural head. The first sign of AVN is nonspecific: diffuse areas of decreased signal intensity are seen in the normally high-signal-intensity fatty marrow on T1-weighted images [8]. This is thought to be due to edema within the marrow. Focal findings along the anterosuperior aspect of the femural head are more specific: low-signal-intensity bands or lines within the femoral head are seen surrounding the area that corresponds to ischemic bone on T1- and T2-weighted images. The band is thick on T1-weighted images and is thinner and accompanied by a second, innerband of high signal intensity on T2-weighted images. The appearance on T2-weighted images is known as the “double-line sign” and is considered highly specific for AVN. This band is believed to represent the reactive interface that separates normal marrow from infarcted marrow.The signal intensity of the central infarcted bone corresponds to areas of bone necrosis seen at histologic examination. High signal intensity on T1-weighted images and low signal intensity on T2-weighted images are seen within areas of necrosis when viable, fatty marrow is still present With prolonged ischemia and necrosis, the necrotic bone has a signal intensity pattern resembling that of fluid, with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Finally, when fibrosis and sclerosis of the involved bone occurs, it is reflected by low signal intensity on both T1- and T2-weighted images. Secondary signs and sequelae of AVN can also be seen at MR imaging. Joint effusion or cartilaginous thinning may be present. Progression of AVN leads to instability of the femural head with fragmentation and eventual collapse.
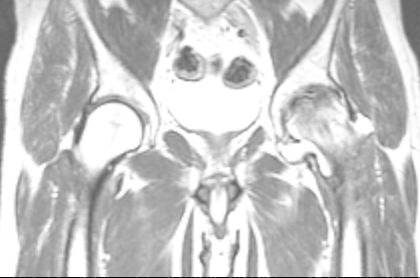
By comparing the appearance of hips with AVN on MR images and available radiographs, we can demonstrate a chronologic pattern of femoral head necrosis. In early AVN, repair and mechanical failure have not extended into the necrotic segment of bone. Thus, a class A (fatlike) MR appearance is observed (figure 3), with the preservation of normal fat signal except at the sclerotic reactive margin surrounding the lesion. This is similar to the “band pattern” described by Totty et al.[9], who noted this in their three cases with normal radionuclide examinations. When there inssufficient inflammation or vascular engorgement, or if subacute hemorrhage is present, a class B (bloodlike) result (figure 4,5), with highsignal intensity on long and short TR/TE images that is similar to subacute hemorrhage [10]. If enough inflammation, hyperemia, fibrosis, or sclerosis is present to decrease the lipid content of the femoral head, a class C (fluidlike) appearance will be observed (figure 4,5). This consists of low intensity with short TR/TE and high intensity with long TR/TE. Finally, if fibrosis and sclerosis predominate, a class D (fibrouslike) lesion will be seen with low signal intensity regardless of pulse sequence (figure 6,7).
Fig.3.
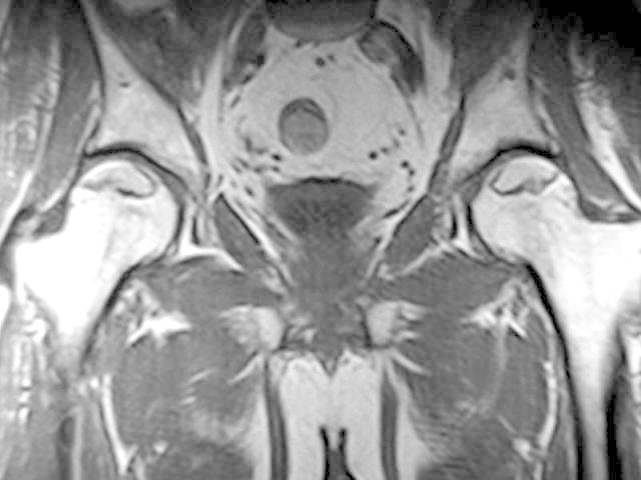
Coronal T1-weighted (T1W) MRI image of the pelvis in a patient with bilateral avascular necrosis of the femoral head shows increased signal within the superior aspect of the femoral head, representing fat, surrounded by a line of decresed signal, representing sclerotic reactive margin. This is an MRI class A (fatlike).
Fig.4,5.
Patient 39 years old with use of high dose of corticosteroids. Cor T1 and T2-weighted MRI image of the pelvis shows a stage B (blood-like) at the level of right femoral head with increased signal on T1W and T2W; AVN stage C (fluid-like) in left femoral head, with decreased signal intensity on T1W and increased signal on T2W.
Fig.4.

Fig.5.
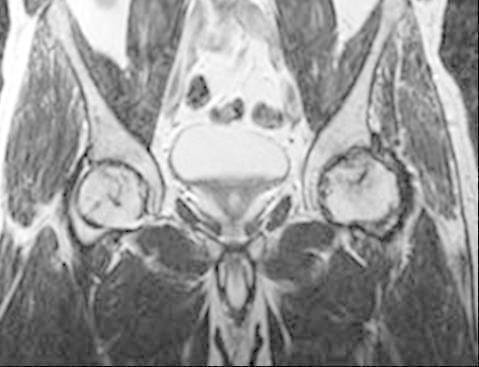
Fig.6,7.
Cor T1W and T2W MRI in a patient with AVN on the left femoral head with decresed signal intensity on T1W and T2W, representing a stage D (fibrous-like).
Fig.6.
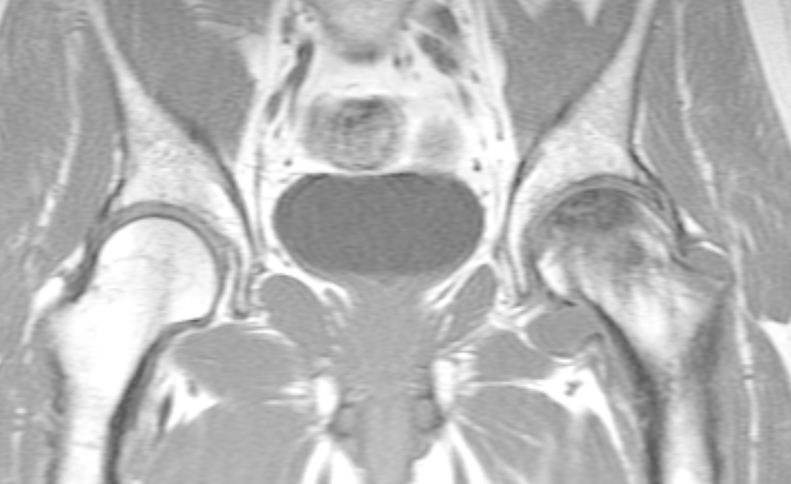
Fig.7.
Our characterization of the four MR imaging classes or intensity patterns as fatlike, bloodlike, and similar terms are intended as a device for remembering the patterns. For example, isointensity with fat (class A) is not proof that residual fat is present. It is also unknown whether our results will be duplicated at lower magnetic field strengths. Classes A (early) and D (late) AVN are most consistently correlated with radiographic staging. Lesions that are predominantly high in signal intensity with long TR/TE (classes B and C) represent a heterogeneous group, presumably consisting of lesions with proeminent inflammation, hyperemia, and/or hemorrhage, with variable lipid and fibrous contents. The frequent correspondence of the pattern of radionuclide uptake with the distribution of high MR signal intensity could be explained by hyperemia. Rupp et al., in a smaller series, found high intensity with long TR/TE to be correlated with an early stage seen radiographically [11]. The lack of exact correlation between MR image and radiographic staging reflects the different parameters these modalities are measuring; bone marrow changes indicated by MR imaging do not necessarily parallel the changes in bone depicted radiographically.
Single-photon emission computed tomography
Initially, SPECT images reflect vascular integrity. Early in the disease, SPECT scans may demonstrate an avascular focus; such findings are missed with MRI unless contrast is used. Collier found a sensitivity of 85% for SPECT [12]. With triple-head high-resolution SPECT, Lee et al reported a sensitivity of 97% [13].
A cold spot (photon-deficient region) within the femoral head is highly specific for AVN and is the earliest scintigraphic evidence of AVN (figure 8). This usually is seen 7-10 days after the ischemic event.Over a period of weeks to months, increased uptake representing revascularization and repair surrounds and eventually replaces the region of photopenia. The central region of photopenia with surrounding zone of increased uptake is termed the doughnut sign.
Fig.8.
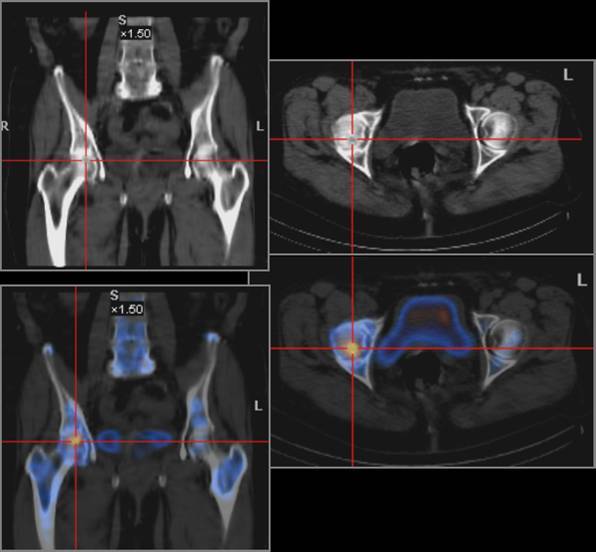
A small area of intense uptake at the level of RFH
SPECT provides images of the radioactivity within the target organ in 3 dimensions. With SPECT, overlying and underlying areas of radioactivity may be separated into sequential tomographic planes; this provides increased image contrast and improved lesion detection and localization, as compared with planar scintigraphy. A number of techniques, such as the use of multihead cameras with shorter acquisition times that improve resolution and increase sensitivity, have been advocated, but none has gained universal acceptance.
SPECT is used as an alternative to MRI when MRI cannot be performed or when the results of MRI are indeterminate [14, 15].
Planar scintigraphy
Collier reported a sensitivity of 55% with planar radionuclide imaging [12]. Initially, uptake is decreased in the perfusion and static phases, which represents the early ischemic event. Later, uptake is decreased within the femoral head in the perfusion phase and increased around the cold region in the static phase. The latter represents the reactive zone around the infarcted segment. The increased uptake from the reparative zone eventually replaces the photopenic region.Hungerford reported false-negative bone scans in the hips of 14 of 27 patients, 13 of whom had bilateral disease [16]. In patients with bilateral involvement, the uptake, although symmetric, really is increased bilaterally.
Bone scintigraphy equipped with a pinhole collimator has greater sensitivity for diagnosing AVN than bone scintigraphy using a high-resolution parallel-hole collimator [17,18]. The image obtained is magnified, allowing better visualization of small structures and improving detection of scintigraphic abnormalities.
Planar scintigraphic imaging using quantitative bone scan is a technique that provides physiologic data that cannot be obtained with other modalities, including MRI [19]. It allows quantification of uptake in the perfusion and static phases. It requires correct computer programming This technique is experimental and is not used widely in the clinical setting.
Computed tomography (CT)
The high spatial resolution and contrast resolution of CT allow analysis of morphologic features. The sensitivity of CT in detecting early AVN is 55%, which is similar to the sensitivity of planar nuclear medicine imaging [20,21]. CT is more appropriate in evaluating the extent of involvement, such as subchondral lucencies and sclerosis during the reparative stage, before the onset of femoral head collapse and superimposed degenerative disease.
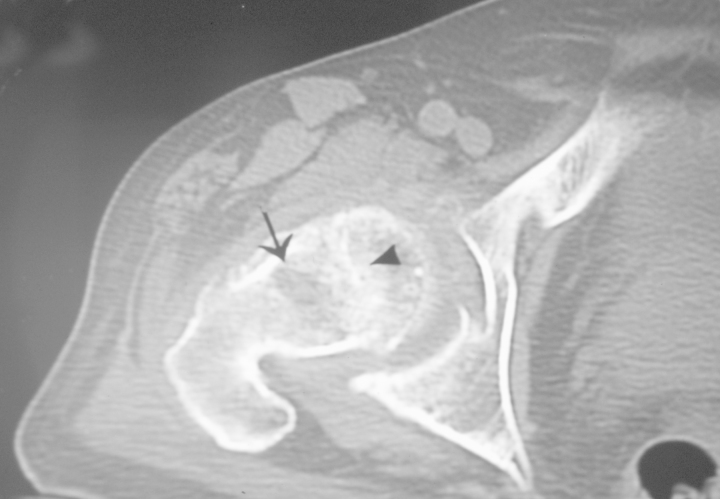
CT scans do not demonstrate the early vascular and marrow abnormalities that result in osteonecrosis [21].Osteoporosis is the first visible sign. Later, the central bony asterisk is distorted, appearing as clumping and fusion of the peripheral asterisk rays. Clumping appears as spots or as hyperdense "roads" of various width (figure 9). This represents changes in the sclerotic interface between necrotic and viable bone and is analogous to the line of low signal surrounding the necrotic bone seen on MRI images. Early signs are caused by microfractures resulting from reduced mechanical load of dead bone trabeculae, altering the shape of the asterisk. Signs also are related to new bone formation on the dead trabeculae. The lucent cystic region, representing the reparative zone, may be appreciated.
Figu.9.
Axial CT scan of a patient with avascular necrosis of the femoral head shows clumping and distortion of the central trabeculae representing the asterisk sign (arrowhead) and an adjacent low-density region (arrow) is representing the reparative zone
Unless the asterisk sign is appreciated, articular surface abnormalities may be interpreted as degenerative joint disease. The lucency within the reparative zone may be confused with malignancy, infection, insufficiency fracture, or plasma cell myeloma.
Although CT may delineate subtle alterations of bone density when plain radiograph findings are normal, MRI and SPECT scintigraphy are much more sensitive for evaluating early manifestations of the disease, such as bone marrow edema. CT scans are insensitive for detecting stage 0 and 1 AVN, but are excellent for detecting femoral head collapse, early degenerative joint disease (DJD), and the presence of loose bodies.
Despite many investigations into AVN, many issues remain unresolved. The pathogenesis in most cases is only speculative and may involve intravascular factors such as microemboli or extravascular factors such as increased interosseous pressure. MRI has emerged as the diagnostic test of choice for suspected early lesions, and radiographs should be used to diagnose and follow advanced lesions. Bone scanning can be useful for early diagnosis and CT scanning or tomography may help plan surgical procedures. The role of the functional exploration of bone is controversial.
In conclusion, diagnosing AVN as early as possible is imperative for a greater chance of success of conservative treatment.
References
- 1.Yochum T, Rowe L. Essentials of skeletal radiology. 2. Baltimore: Williams & Wilkins; 1996. pp. 260–263. [Google Scholar]
- 2.Tierney Jr. LM, McPhee SJ, Papadakis MA. Current Medical diagnosis and treatment. 36. Appleton & Lange; 1997. pp. 798–799. [Google Scholar]
- 3.Resnick D, Niwayama G. Osteonecrosis: diagnostic techniques, special situations and complications. In: Resnick D, editor. Diagnosis of Bone and Joint Disorders. 3. Philadelphia: WB Saunders Co; 1995. pp. 3495–3558. [Google Scholar]
- 4.Ficat RP, Arlet J. Necrosis of the femoral head. In: Hungerford DS, editor. Ischemia and Necrosis of Bone. 3. Philadelphia: Lippincott Williams & Wilkins; 1980. pp. 171–182. [Google Scholar]
- 5.Steinberg ME, Hayken GD. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed] [Google Scholar]
- 6.Bassett L, et al. MRI in the Early Diagnosis of Ischemic Necrosis of the Femoral Head. Clin. Ortho. 1987;214:237–248. [PubMed] [Google Scholar]
- 7.Beltran J., et al. emoral Head AVN: MR Imaging with Clinical-Pathological and Radionucide Correlation. Radiology. 1988;166:215–220. doi: 10.1148/radiology.166.1.3336682. [DOI] [PubMed] [Google Scholar]
- 8.Turner DA, Templeton AC, Seizer PM, et al. Femoral capital osteonecrosis: MR findings of diifusemarrow abnormalities without focal lesions. Radiology. 1989;171:135–140. doi: 10.1148/radiology.171.1.2928517. [DOI] [PubMed] [Google Scholar]
- 9.Totty WG, Murphy WA, Canz WI, Kumar B, Daum WJ, Siegel MA. Magnetic resonance imaging of the normal and ischemic femoral head. AJR. 1984;143:1273–1280. doi: 10.2214/ajr.143.6.1273. [DOI] [PubMed] [Google Scholar]
- 10.Comoni JM, Grossman RI, Goldberg HI, Zimmerman RA, Bilaniuk LT. Intracranial hematomas: imaging by high field MR. Radiology. 1985;157:87–93. doi: 10.1148/radiology.157.1.4034983. [DOI] [PubMed] [Google Scholar]
- 11.Rupp VN, Reiser M, Hipp H, et al. The diagnosis of bone necrosis by magnetic resonance (MR) tomography: possible early diagnosis. ROFO. 1985;142:131–137. doi: 10.1055/s-2008-1052618. [DOI] [PubMed] [Google Scholar]
- 12.Collier BD, Carrera GF, Johnson RP, et al. Detection of femoral head avascular necrosis in adults by SPECT. J Nucl Med. 1985;26:979–987. [PubMed] [Google Scholar]
- 13.Lee MH, Moon DH, Na HW. Diagnosis of femoral head avascular necrosis by triple head high resolution. J Nucl Med. 1992;33:936–941. [Google Scholar]
- 14.Collier BD, Krasnow AZ, Hellman RS. SPECT bone scanning. In: Collier BD, Fogelman I, Rosenthal L, editors. Skeletal Nuclear Medicine. 1. St. Louis: Mosby; 1996. [Google Scholar]
- 15.Murray IP. The role of SPECT in the evaluation of skeletal trauma. Ann Nucl Med. 1993;7(Feb):1–9. doi: 10.1007/BF03164786. [DOI] [PubMed] [Google Scholar]
- 16.Hungerford DS. Pathogenetic considerations in ischemic necrosis of bone. Can J Surg. 1981;24(Nov):583–587. [PubMed] [Google Scholar]
- 17.Hofman S, Staudennkerg A, Breitenseker M, et al. Diagnostic patterns for bone marrow edema syndrome and avascular necrosis of the femoral head in dynamic bone scintigraphy. Nucl Med Comm. 1997;18(12):1178–1188. doi: 10.1097/00006231-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Maillefert JF, Toubeau M, Piroth C, et al. Bone scintigraphy equipped with a pinhole collimator for diagnosis of avascular necrosis of the femoral head. Clin Rheumatol. 1997;16:372–377. doi: 10.1007/BF02242454. [DOI] [PubMed] [Google Scholar]
- 19.Naito M, Ogata K, Moriguchi H. Quantitative bone scanning of the hip. Comparison between the perfusion and static phases. Int Orthop. 1996;20:311–314. doi: 10.1007/s002640050084. [DOI] [PubMed] [Google Scholar]
- 20.Lee MJ, Corrigan J, Stack JP, et al. A comparison of modern imaging modalities in osteonecrosis of the femoral head. Clin Radiol. 1990;42:427–432. doi: 10.1016/s0009-9260(05)80900-6. [DOI] [PubMed] [Google Scholar]
- 21.Sarikaya I, Sarikaya A, Holder LE. The role of single photon emission computed tomography in bone imaging. emin Nucl Med. 2001;31:3–16. doi: 10.1053/snuc.2001.18736. [DOI] [PubMed] [Google Scholar]