Abstract
The purpose of this research was to analyze the influence of anemia on renal function in patients with chronic kidney disease. Were monitored for 12 months 165 patients with chronic kidney disease, 96 patients had anemia and a control group of 69 patients had no anemia. A value of hemoglobin under 120 g/L in women and under 130 g/L in men on admission defined anemia. Anemia was associated with a more severe renal damage, a lower residual diuresis, especially in the presence of other risk factors such as diabetes, inflammation or secondary hyperparathyroidism, so early diagnosis and correction of anemia is important for prognosis and evolution of these patients.
Keywords: anemia, chronic kidney disease, inflammation, glomerular filtration rate
Introduction
Anemia is a very common complication of chronic kidney disease (CKD) and is associated with severe adverse outcomes, such as deterioration in cardiac function, decreased cognition and mental acuity. Anemia may worsen renal function and reduce time to initiate renal replacement therapy by dialysis.
Matherial and Method
165 patients with CKD were included in the study. For all patients informed consent have been obtained. CKD was defined as an eGFR less than 60 mL/min/1.73 m2. GFR was estimated by Modification of Diet in Renal Disease formula. CKD classification was consistent with Kidney Disease Improving Global Outcomes (KDIGO) 2012: stage 1 CKD, the GFR is ≥ 90 ml/min/1.73 m2, stages 2 of CKD are defined by a GFR of 60–89 ml/min/1.73 m2, stage 3a 45–59 ml/min/1.73 m2, stage 3b 30-44 ml/min/1.73 m2, stage 4 15-29 ml/min/1.73 m2 and stage 5 was assessed as severe impairment of renal function and occurs when the GFR is under 15 ml/min/1.73 m2 or when patients require dialysis.
A group of 96 patients had anemia and a control group of 69 patients had no anemia.
A value of hemoglobin under 120 g/L in women and under 130 g/L in men on admission defined anemia.
We evaluated the following parameters: serum creatinine and GFR, residual diuresis, level of proteinuria, dialysis efficiency, the value of C-reactive protein and parathyroid hormone.
Results
Clinical and demographic characteristics of the patients studied are shown in table 1.
Table 1.
Clinical and demographic characteristics of studied patients
| Parameter | Patients without anemia | Patients with anemia | p |
| Mean age (years) | 59,09 ± 13,23 | 58,33 ±15,16 | 0,740 |
| Female (n, %) | 19 (27,5 %) | 53 (55,2 %) | 0,0001 |
| Smoking (n, %) | 30 (43,5 %) | 25 (26 %) | 0,015 |
| Alcohol(n, %) | 15 (21,7 %) | 11 (11,5 %) | 0,059 |
| Body Mass Index - BMI (Kg/m2) | 28,70 ± 5,77 | 24,82 ± 5,33 | 0,0001 |
| Dislipidemia (n, %) | 47 (68,1 %) | 53 (55,2 %) | 0,065 |
| Hypertension (n, %) | 63 (91,3 %) | 84 (87,5 %) | 0,305 |
| Diabetes mellitus (n, %) | 18 (26,1 %) | 32 (33,3 %) | 0,204 |
| Diuresis (ml) | 1636,96 ± 918,10 | 1201,04 ± 882,99 | 0,002 |
| GFR (ml/min/1,73 m2) | 35,74 ± 14,86 | 16,93 ± 13,21 | 0,0001 |
In the presence of anemia, renal damage was more severe: serum creatinine level was higher in patients with anemia compared with those without anemia (6,24 ± 3,49 mg/dl versus 3,54 ± 2,79 mg/dl, 95% CI: 1,69 – 3,70, p = 0,0001) and GFR was significantly lower in patients with anemia compared with those without anemia (16,93±13,21 ml/min/1,73 m2 versus 35,74±14,86 ml/min/1,73m2, 95% CI: -24,273- -13,349, p=0,0001).
Serum hemoglobin was positively statistically significant correlated with GFR value (Pearson coefficient r = 0.515, p = 0.0001) (Fig. 1) and negative also statistically significant with serum creatinine (Pearson coefficient r = -0.543, p = 0.0001) (Fig. 2).
Fig. 1.
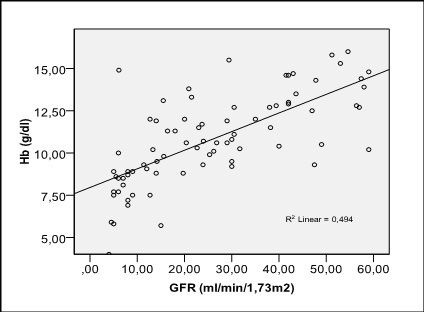
Correlation of hemoglobin with the level of GFR
Fig. 2.
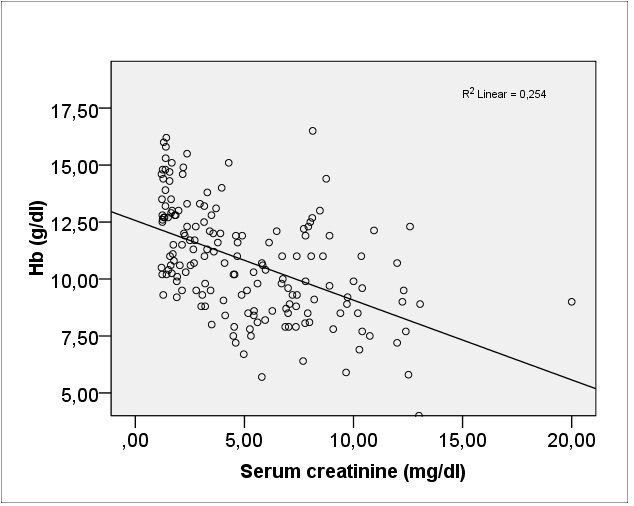
Correlation of hemoglobin with serum creatinine
By logistic regression was analyzed the influence of anemia on the severity of BCR and was observed that the presence of anemia was associated with a 5,9 times higher risk of having a GFR below 15 ml/min/1, 73 m2 (OR = 5.907, 95% CI: 2.973 - 11.734, p = 0.0001) compared with patients without anemia. On the other hand, residual diuresis in patients with anemia was lower compared with that of patients without anemia (1201 ± 882,99 ml versus 1636,96 ± 918,10 ml, 95 % CI: - 715,71 – 156,11, p = 0,002). The presence of anemia was associated with a higher risk of having a diuresis under 1000 ml compared with patients without anemia (OR=1,475, 95 % CI: 1,143 – 1,904, p = 0,002) and between diuresis and Hb level there was a direct relationship statistically significant (r = 0.300, p = 0.0001) (Fig. 3).
Fig. 3.
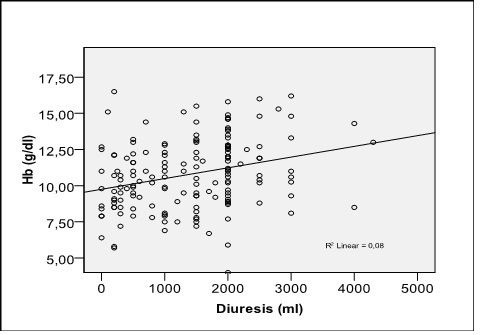
Correlation of hemoglobin with residual diuresis
Serum Hb was negatively correlated with proteinuria (r = -0,243, p=0,008) (Fig. 4).
Fig. 4.
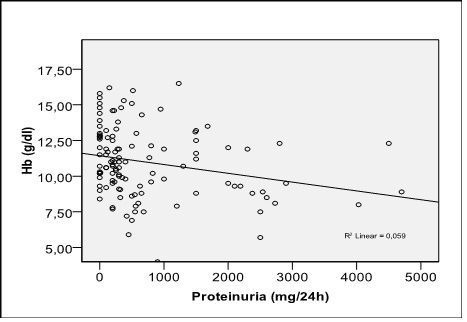
Correlation of hemoglobin with proteinuria
Erythropoietin (EPO) use for anemia correction within 12 months of monitoring was associated with improved GFR. Thus, GFR in patients with anemia treated with EPO increased from 15.77 ± 11,02 ml/min/1.73 m2 to 23.52 ± 12,22 ml/min/1.73 m2 (p = 0.010), while in untreated patients GFR did not differ significantly (19,11 ± 10,33 ml/min/1.73 m2 versus 19,28 ± 12,03 ml/min/1.73 m2, p=0,525).
Regarding the relationship between anemia and dialysis efficiency, assessed by measurement of Kt/V, analyzed by a multinomial logistic regression model, were not observed significant influence of anemia on dialysis efficiency (Exp(B) = 0,833, Wald = 0,075, p = 0,785). The value of Kt/ V was not significantly different in patients with anemia compared with those without anemia (1,86 ± 0,918 versus 1,99 ± 0,940, 95 % CI: -0,678 -0,414, p=0,630).
Association of anemia with DM in patients with CKD led to a more severe impairment of renal function. Thus GFR in patients without diabetes and without anemia was significantly higher compared with patients who had anemia and diabetes, too (35.67 ± 14.98 ml/min/1,73m2 versus 11.67 ± 6.00 ml/min/1.73 m2, p = 0.0001) or compared with those who had anemia but without diabetes (35.67 ± 14.98 ml/min/1,73m2 versus 19.48 ± 14.98 ml/min/1,73m2, p = 0.0001) (Fig. 5).
Fig. 5.
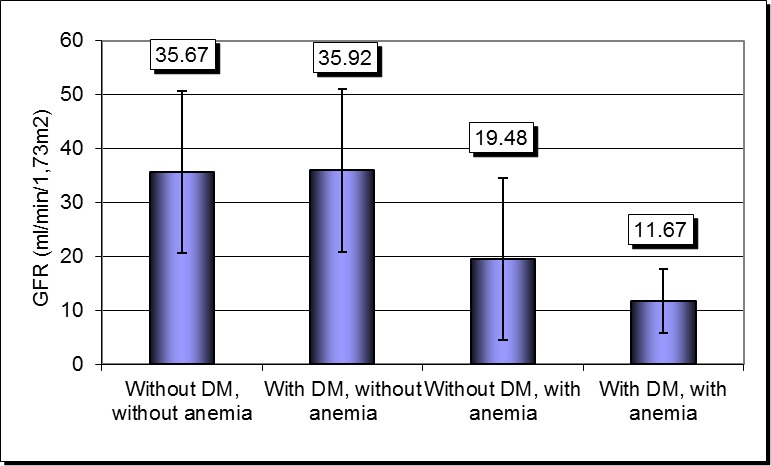
Severity of renal impairment according to the presence of anemia and diabetes
Conducted a binary logistic regression model, by backward method (conditional), in which the dependent variable was severe renal impairment (GFR less than 15 ml/min/1, 73 m2), and anemia was introduced as a covariate, the model being conducted separately on patients with CRP above 3 mg/dl, respectively below 3 mg/dL, we observed that: in patients with CRP less than 3 mg/dL anemia was associated with a 3.5 times higher risk of having a GFR below 15 ml/min/1,73 m2 (OR = 3.500, 95% CI: 0.86-14.13, p = 0.057) and in patients with CRP above 3 mg / dL anemia associated with a 5.5 times higher risk of having severe renal impairment (OR = 5.598, 95% CI: 2.37-13.17, p = 0.0001). GFR level was significantly lower in patients with anemia and CRP above 3 mg/dL compared with patients without anemia and CRP below 3 mg/dL (16.31 ± 17.09 ml/min/1,73 m2 versus 36,97 ± 14.78 ml/min/1,73 m2, p=0.0001). There were not statistically significant differences between those with anemia and CRP above 3 mg/dL compared with those who had anemia but CRP below 3 mg / dL (16,31 ± 17,09 ml/min/1,73 m2 versus 17,25 ± 10,90 ml/min/1,73 m2, p=0,252) or compared to those without anemia but CRP above 3 mg/dL (16.31 ± 17.09 ml/min/1,73 m2 versus 26.95 ± 13.45 ml/min/1,73 m2, p = 0.179) (Fig.6).
Fig. 6.
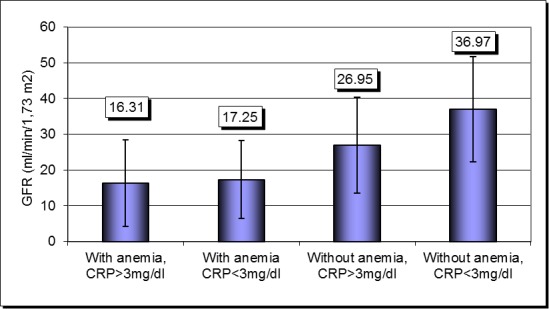
Severity of renal impairment according to the presence of anemia and inflammation
GFR decreased after 12 months of monitoring in patients with anemia and a CRP level above 3 mg / dL: from 16.31 ± 12.5 ml/min/1,73 m2 to 11.3 ± 7.3 ml/min /1.73 m2, p = 0.050. In patients without anemia and CRP less than 3 mg/dl, GFR did not differ significantly after 12 month (from 36.97 ± 12.4 ml/min/1,73 m2 to 39.2 ± 9.2 ml/min/1,73 m2, p=0.308).
Analyzing by logistic regression the influence of anemia on renal impairment in the presence of secondary HPT, it was observed that anemia was associated with a higher risk of developing severe kidney damage in the presence of secondary HPT compared with patients without secondary HPT (OR = 6.750, 95% CI: 0.964 to 47.269 p = 0.05 versus OR = 1.651, 95% CI: 0.578 to 4.518, p = 0.361). Simultaneous presence of anemia and secondary HPT was associated with a low GFR compared with patients without anemia and without secondary HPT (13.98 ± 11.49 ml/min/1,73 m2 versus 40.78 ± 12.74 ml/min/1,73 m2, p=0.022) or compared with patients who had only anemia (13.98 ± 11.49 ml/min/1,73 m2 versus 34.27 ± 8.79 ml/min/1,73 m2, p=0.032) or only secondary HPT (13.98 ± 11.49 ml/min/1,73 m2 versus 20.19 ± 9.15 ml/min/1,73 m2, p=0.088). In the absence of HPT, the GFR did not differ significantly with the presence of anemia (34.27 ± 8.79 ml/min/1,73 m2 versus 40.78 ± 12.74 ml/min/1,73 m2, p=0.177). The association of anemia with secondary HPT led to a significant decline in GFR within 12 months of monitoring from 13.98 ± 11.49 ml/min/1,73 m2 to 9.28 ± 9.2 ml/min/1,73 m2, p=0.041.
The presence of anemia was associated with increased hospitalization. Thus, between Hb level and number of days of hospitalization there was a statistically significant inverse correlation (r =- 0.365, p = 0.0001). Number of days of hospitalization was significantly higher in patients with anemia compared with those without anemia (15.33 ± 21.98 days versus 4.38 ± 9.89 days, 95% CI: 5.36-16.55, p = 0, 0001).
Discussion
Anemia is associated with a more severe impairment of renal function in this study and other published research [1]. Anemia severity correlates with the degree of renal function impairment [2] and there is evidence that low levels of Hb were associated with accelerated progression of CKD [3]. Introduction of EPO in therapy to these patients reduce transfusion requirements and improve quality of life of these patients [4]. In our study EPO administration resulted in improved GFR in patients with anemia and there are other studies that reporting an improvement in GFR decline after correcting anemia with EPO [5]. According with other published data, there was no difference in the progression rate of CKD in patients with higher values of Hb after EPO use, but was a higher rate of adverse events such as myocardial infarction, death, hospitalization [6]. In our study GFR was higher in patients without diabetes and without anemia compared with those who had anemia and diabetes, too. There are other published research which shows that the association of anemia and DM in patients with CKD had been shown to accelerate the progression of CKD, increasing cardiovascular morbidity and mortality, in association with a poor prognosis of these patients [7]. Anemia associated inflammation contributes to the decline in GFR, anemia secondary to hypoxia, causing inflammation and fibrosis and loss of capillaries [8]. In this study, anemia accompanied by inflammation was associated with a significantly higher risk of developing severe renal impairment. Secondary hyperparathyroidism is associated with hyporesponsiveness to EPO therapy [9] and vitamin D deficiency was associated with reduced hemoglobin levels in patients with CKD [10]. In our study the association of anemia with secondary HPT led to a significant decline in GFR within 12 months of monitoring. The presence of anemia was associated with a greater number of days of hospitalization (p = 0.0001). In a retrospective study demonstrated that patients with low Hb levels had the highest rate of hospitalization and several comorbidities [11].
Conclusions
Anemia, a frequent feature of CKD, play an important role in progression of renal impairment, probably due to hypoxia, especially in combination with other risk factors such as diabetes or inflammation. Thus early identification and correction of anemia is important for slowing the progression of CKD.
Glossary
ABBREVIATIONS
- Hb
hemoglobin
- DM
diabetes mellitus
- CKD
chronic kidney disease
- GFR
glomerular filtration rate
- HPT
hyperparathyroidism
- EPO
erythropoietin
References
- 1.Carrero J, Bárány P, Yilmaz M. Testosterone deficiency is a cause of anaemia and reduced responsiveness to erythropoiesis-stimulating agents in men with chronic kidney disease. Nephrology Dialysis Transplantation. 2012;27(2):709–715. doi: 10.1093/ndt/gfr288. [DOI] [PubMed] [Google Scholar]
- 2.Del Fabbro P, Luthi JC, Carrera E, et al. Anemia and chronic kidney disease are potential risk factors for mortality in stroke patients: a historic cohort study. BMC Nephrology. 2010 Oct 16;:11–27. doi: 10.1186/1471-2369-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovesdy C.P., Trivedi B.K., Kalantar-Zadeh K., et al. Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney International. 2006;69(3):560–564. doi: 10.1038/sj.ki.5000105. [DOI] [PubMed] [Google Scholar]
- 4.Ritz E, Laville M, Bilous RW, et al. Target level for hemoglobin correction in patients with diabetes and CKD: primary results of the Anemia Correction in Diabetes (ACORD) Study. American Journal of Kidney Disease. 2007;49:194–207. doi: 10.1053/j.ajkd.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg D. Outcomes of anaemia management in renal insufficiency and cardiac disease. Nephrology Dialysis Transplantation. 2003;18(Suppl.2):ii7–ii12. [PubMed] [Google Scholar]
- 6.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. New England Journal of Medicine. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 7.New JP, Aung T, Baker PG, et al. The high prevalence of unrecognized anaemia in patients with diabetes and chronic kidney disease: a population-based study. Diabetic Medicine. 2008;25:564–569. doi: 10.1111/j.1464-5491.2008.02424.x. [DOI] [PubMed] [Google Scholar]
- 8.Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney International. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- 9.Gaweda AE, Goldsmith LJ, Brier ME, et al. Iron, Inflammation, Dialysis Adequacy, Nutritional Status, and Hyperparathyroidism Modify Erythropoietic Response. Clin Journal of American Society of Nephrology. 2010 Apr;5(4):576–581. doi: 10.2215/CJN.04710709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel NM, Gutiérrez OM, Andress DL, et al. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney International. 2010;77:715–720. doi: 10.1038/ki.2009.551. [DOI] [PubMed] [Google Scholar]
- 11.Ebben JP, Gilbertson DT, Foley RN, et al. Hemoglobin level variability: associations with comorbidity, intercurrent events, and hospitalizations. Clinical Journal of the American Society Nephrology. 2006;1(6):1205–1210. doi: 10.2215/CJN.01110306. [DOI] [PubMed] [Google Scholar]


