Abstract
Prostate adenocarcinoma is frequently diagnosed on needle biopsies in early, organ-confined stages. New prognostic factors would help identifying at this stage patients at risk for unfavorable evolution, that would benefit from alternate therapy. This study aims to find correlations between the extent of neurocrine differentiation (NED), a feature commonly seen in prostate carcinoma, and known factors of disease evolution such as histological grade, malignant cell proliferation and serum PSA levels. Immunohistochemistry for choromogranin A and neuron-specific enaolase (NSE) was used to calculate expression scores in order to asses the extent of NED in prostate biopsies. Tumour proliferative activity was estimated by calculating percentages of Ki-67 immunoreactive cell nuclei. Results show that the presence of numerous clusters of chromogranin A positive cells is a feature that differentiate tumours with Gleason score 9 from those with a score of 6. Also, the same extended neuroendocrine differentiation is associated with high tumour proliferative activity. Multinomial regression analysis showed that high Ki indices, serum PSA values and NSE scores are predictive for moderately and poorly differentiated prostatic adenocarcinoma.
Keywords: Prostate adenocarcinoma, Neuroendocrine differentiation
Introduction
Text Prostate adenocarcinoma is one of the most frequent malignant diseases in men. Patients are stratified according to disease extension: organ-confined cancer (pT1, T2) benefits of definitive therapy (radical prostatectomy, radiotherapy, neoadjuvant therapy), while extraprostatic tumor extension or metastasis are an indication for hormone therapy. Androgen deprivation of malignant cells is well established for the treatment of metastatc disease, recurrence after radical prostatectomy or radiotherapy and as neoadjuvant therapy. However, 18 to 36 month after an initial response to hormone therapy, most of the prostate carcinomas swich to a hormone resistant phenotype, entering into a more aggressive and ultimately fatal stage of disease [1] .
Neuroendocrine (NE) cells are a distinct epithelial cell compartment of the normal human prostate gland. Their phenotype and range of endocrine secretion products are similar, but not identical to those of NE-like cells from prostate carcinoma. Neuroendocrine differentiation (NED) is seen in virtually all cases of prostatic carcinoma, mostly in a focal pattern; a number of studies pointed out that its extent is associated to hormone therapy refractory and aggressive disease. Yet neuroendocrine differentiation is included by the College of American Pathologists Consensus Statement 1999 in category III of prognostic and predictive factors (not sufficiently studied to demonstrate prognostic value) [2].
In prostate cancer extending beyond the organ limits, most of the studies pointed out a strog association between the extent of NE differentiation and aggressive disease, but there are conflicting conclusions regarding the value of NED as an independent prognostic factor. Increased NE differentiation was found to be associated with aggressive disease [3,4], Gleason score [5,6], anti-androgen thearpy failure [3] and survival [6,7] . However, McWilliam concluded that detection of neuroendocrine differentiation in conventional prostatic adenocarcinoma is not an independent indicator of prognosis [6] .
Only a few studies addressed the prognostic significance of NE differentiation in localized prostate cancer, and data collected on prostatic biopsies is even scarcer. Some results indicate that histological grade and NE differentiation seen in prostatectomy samples predicted progression in multivariate analysis [8] or disease specific survival [9] . NE differentiation was also considered an additional prognostic marker in radical prostatectomy samples [10] and a factor that significantly aggravate established adverse prognostic parameters such as nodal status, tumour stage, pretherapeutic PSA-level, and Gleason score [11]. However, other studies lead to diverging conclusions: the extent of neuroendocrine differentiation was not foud to be an independent prognostic factor for biochemical failure of therapy in multivariate analysis [12,13] .
Screening population at risk using serum PSA monitoring and clinical examination recently gained in frequency, leading to an increase in the proportion of low-grade adenocarcinoma that is diagnosed on prostate core biopsy. New prognostic factors would help identifying at this stage patients at risk for unfavorable evolution, that would benefit from alternate therapy. This study aims to find correlations between the extent of neurocrine differentiation and known factors of disease evolution such as histological grade, malignant cell proliferation and serum PSA levels.
Matherials and Method
43 patients were studied, 53 to 84 years-old (mean 69.7 years), after undergoing ultrasound-guided 6- or 10-core needle biopsy. 36 (83,7%) of the patients were referred for abnormal screening findings, 5 for symptoms of prostate hypertrophy and 2 for clinical findings consistent with bone metastatic disease. Serum PSA levels were tested using an automated immunoassay system (Abbott AxSYM) and ranged between 3.4 and 41.8 ng/mL (mean 17,43 ng/mL).
Tissues specimens were fixed in 4% buffered formalin for 24h and embedded in paraffin. Routine HE-stained sections were examined for diagnosis and tumours were graded according to the ISUP revised Gleason system [14] .
Immunohistochemistry assays were performed on 3 µm-thick sections using the LSAB technique (LSAB2 System, Dako, Glostrup, Denmark). Primary antibodies used to detect neuroendocrine cells were mouse monoclonal anti-human Chromogranin A (clone 5H7, Novocastra, Leica Microsystems) and anti-Neuron Specific Enolase (NSE, clone E27, Thermo Labvision). A different panel of antibodies was also used in order to confirm the histological diagnosis; it comprised anti-PSA (rabbit polyclonal, Dako), AMACR (P504S, rabbit polyclonal, Dako), HMW cytokeratin (mouse clone 34βe12, Dako) and p63 (mouse clone 4A4, BD Pharmingen) antibodies. Monoclonal mouse Ki-67 antibodies (clone MIB-1, Dako) were used to identify proliferating cells. Immune reactions were visualised using DAB as a substrate and slides were counterstained with Meyer’s haematoxylin.
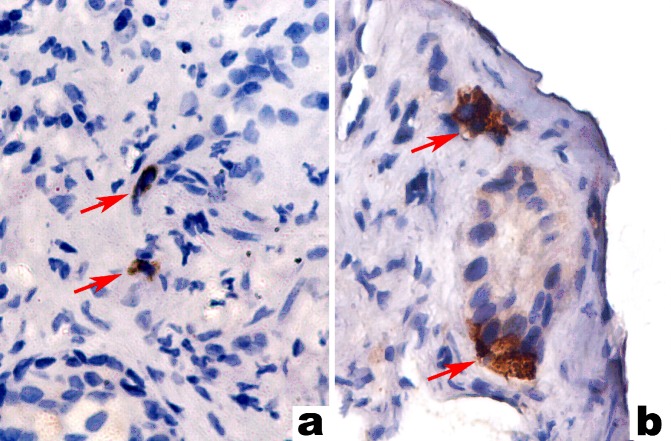
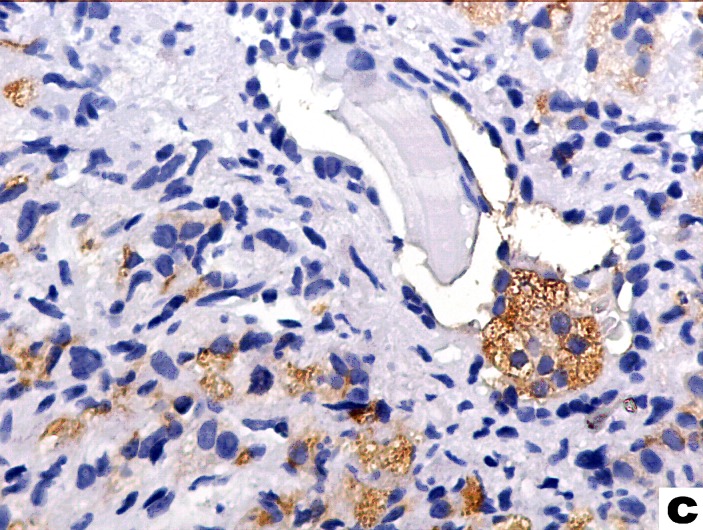
We estimated Chromogranin A expression by means of a semiquantitative score originally proposed by di Sant’Agnese [15]: 0 - no labelling, + for positive cells scatterd individually across the microscopic field, ++ for at least one CgA-positive cluster and +++ for numerous CgA-positive clusters present within the tumour (fig. 1). NSE reactivity was also scored using two cut-off levels: < 33%, 33% to 66%, and > 66% of tumour cells recognized by the antibody.To compensate the heterogenous distribution of positively stained cells, only tissue areas showing the highest number of stained cells were chosen for further analysis.
Fig. 1.
Examples of various degrees of tumour neuroendocrine differentiation with di Sant’Agnese scores of + (a), ++ (b) and +++ (c). Arrows point to chromogranin a-positive cells.
a,b.
c.
Images were recorded using a Nikon digital camera (DS-2M) mounted on a Nikon Eclipse E200 microscope. The public domain ImageJ software was used to perform color deconvolution of immunohistochemistry slides, to automate cell nuclei recognition and counting, to calculate percentages of Ki-67 positive nuclei and to estimate the number of cells expressing neuroendocrine markers.
Statistical data analysis included descriptive statistics, Kruskal Wallis tests of significant differences between groups followed by post-hoc Mann-Whitney analysis and ANOVA analysis for continuous variables. Finally, a multinomial logistic regression test was used to study interactions between the various factors we studied in predicting a histological grade of the tumour.
Results
39 of the 43 patients had localized disease (pT1-T2), while the reminder showed extraprostatic invasion into neighboring structures. Histological grading of the tumour resulted in classification of the patients into groups with Gleason scores of 6, 7, 8, and 9 comprising 9, 9, 15 and 10 cases respectively. Patients with a Gleason score of 9 had significantly higher serum PSA levels than any other group (Fisher-Hayer test above the studentized range critical values for all pairwise comparisions).
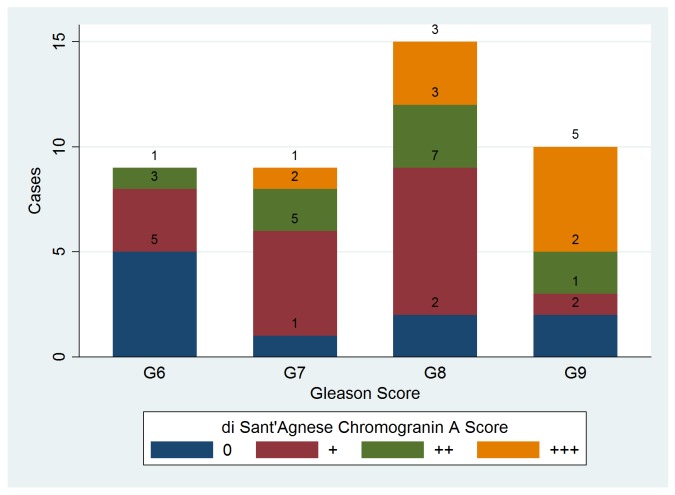
Chromogranin A expression defined by a score higher than 0 was seen in 33 of the 43 cases (76.4%), similar to percentages reported in other publications [16,17] . Distribution of ChrA scores in patients with different tumour grades is depicted in fig.2. Kruskal Wallis test showed that there are differences between groups medians (p=0.035 with 3 d.f.) and post-hoc testing revealed a significantly higher ChrA score for patiens with Gleason sum 9 than those with a sum of 6.
Fig. 2.
Chromogranin A di Sant’Agnese scores distribution in patients with different Gleason sums.
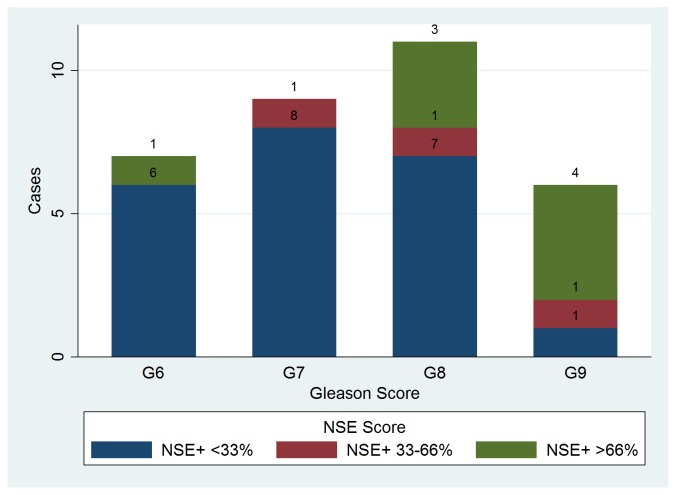
NSE expression in more than 33% of the malignant cells was noted in 11 cases (25.58% - fig.3), but it was less related to histological grade than ChrA positivity. The only difference that achieved statistical significance was seen when comparing Gleason 7 to Gleason 9 patients (KW test with ties p=0.0169, and Mann Whitney U statistics above the critical value in post-hoc testing). The scoring system using 33% as a cut-off for positivity was based on the observation that NSE reactivity, when present, is diffuse and seen in generally large numbers of tumour cells; thus, in order to avoid false-positive errors, we rasied the threshold value.
Fig. 3.
Neuron-specific enolase (NSE) scores distribution in patients with different Gleason sums.
We also noticed that NSE expression patterns are different when comparing NE cells in normal vs. malignant glands. In benign glands NSE positivity is more intense, has a granular cytoplasmic pattern and is present in individual cells of the basal layer, while large proportions of weaker, diffusely NSE-positive tumour cells are frequently seen within malignant glands. These differences are not seen when analysing ChrA staining patterns.
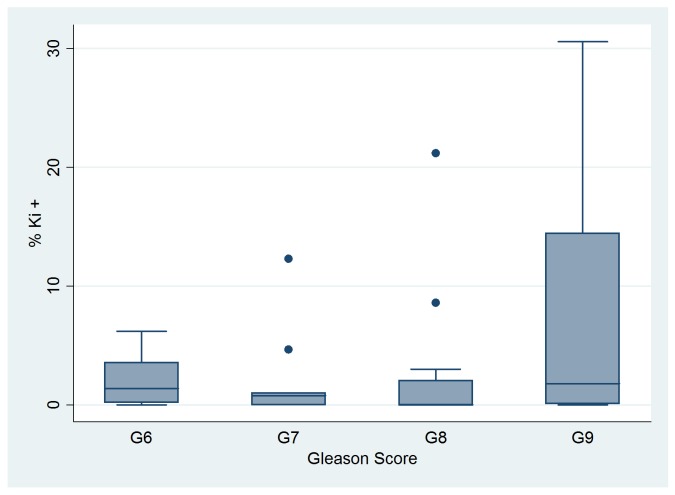
The mean percentage of nuclei positive for Ki-67 was 3.72% for all patients, with a range of 0% to 30.56% (standard deviation 7.31%). Descriptive statistics for Ki-67 expression in the different Gleason score groups are shown in fig.4. Tumour Ki-67 proliferative index was higher in patients with +++ ChrA scores than in any other patients (p value for the F statistic = 0.0396); this observation suggests a possible functional relation between NE and tumour cells, where the former cell type is able to stimulate malignant cell proliferation.
Fig. 4.
Median, 25th and 75 th percentiles, adjacent and outlier values of Ki-67 index within groups of patients with different Gleason scores.
We also performed a logistic multinominal regression test in order to identify wich of the factors we studied could be predictors of a less differentiated cancer and, subsequently, of a poor evolution. A Bayesian Information Criterion value was computed for different models and it showed that the most informative combination of factors included ChrA and NSE scores, Ki-67 labelling and preoperative serum PSA levels. Regression analysis achieved statistical significance (p = 0.0001, pseudoR2 correlation coefficient = 0.5258). Higher Ki indices, serum PSA values and NSE scores predict Gleason scores of 8 and 9 that define moderately and poorly differentiated prostatic adenocarcinoma (table 1)
Table 1.
Results of multinomial logistic regression test of di Sant’Agnese score (s_chr_1), NSE score (s_nse), Ki index (ki) and serum PSA values (psa) as predictors of Gleason score in prostatic adenocarcinoma patients.
| Multinomial logistic regression Log pseudolikelihood = -21.273141 |
Number of obs = 33 Wald chi2(12) = 40.87 Prob > chi2 = 0.0001 Pseudo R2 = 0.5258 |
|||||
|---|---|---|---|---|---|---|
| scrg | RR | Robust Std. Err. |
z | P>|z| | 95% Confidence Interval | |
| G6 | ||||||
| s_chr_1 | .1144512 | .1187574 | -2.09 | 0.037 | .0149759 | .8746784 |
| s_nse | 14.74063 | 18.68431 | 2.12 | 0.034 | 1.229095 | 176.7855 |
| ki | .9991047 | .1555536 | -0.01 | 0.995 | .736351 | 1.355617 |
| psa | .8478989 | .088444 | -1.58 | 0.114 | .6911235 | 1.040238 |
| G8 | ||||||
| s_chr_1 | 1.007175 | .8548987 | 0.01 | 0.993 | .1908087 | 5.31633 |
| s_nse | 128.7371 | 178.2471 | 3.51 | 0.000 | 8.533859 | 1942.057 |
| ki | .6181745 | .0914289 | -3.25 | 0.001 | .4626122 | .8260475 |
| psa | 1.309722 | .1056706 | 3.34 | 0.001 | 1.118157 | 1.534107 |
| G9 | ||||||
| s_chr_1 | 2.294587 | 2.325657 | 0.82 | 0.413 | .3147583 | 16.72753 |
| s_nse | 601.4446 | 963.6357 | 3.99 | 0.000 | 26.02549 | 13899.28 |
| ki | .6264741 | .0930901 | -3.15 | 0.002 | .4681877 | .8382745 |
| psa | 1.51875 | .1541763 | 4.12 | 0.000 | 1.244734 | 1.853089 |
(scrg==G7 is the base outcome)
RR – Relative Risk
Conclusions
Our aim was to study the possible correlation that exists between the extent of NE differentiation and cell proliferention on one hand, and histological grade on the other, due to the fact that Gleason score is one of the well-established predictors of prostate adenocarcinoma evolution and response to therapy. In radical prostatectomy, biopsy and trans-urethral resection, Gleason score is proven to be associated with higher risk of extraprostatic spreading, faster biochemical relapse, shorter survival and increased risk of rapid onset of hormone-refractory disease [1].
Our results suggest that the presence of numerous clusters of Chromogranin A positive cells is a feature that differentiate tumours with Gleason score 9 from those with a score of 6. Also, the same extended neuroendocrine differentiation is associated with high tumour proliferative activity. Multinomial regression analysis showed that high Ki indices, serum PSA values and NSE scores are predictive for moderately and poorly differentiated prostatic adenocarcinoma.
Thus, the links we found between NE differentiation characteristics and histological grade suggest that NED is a good candidate for a novel prognostic factor in needle biopsies. Unfortunately, we were not able to follow the patients included in this study, preventing us from analysing directly the influence of the neuroendocrine component on disease progression.
References
- 1.Shariat S.F., Karakiewicz P.I., Margulis V., Kattan M.W. Inventory of prostate cancer predictive tools. Curr. Opin. Urol. 2008;18(3):279–undefined. doi: 10.1097/MOU.0b013e3282f9b3e5. [DOI] [PubMed] [Google Scholar]
- 2.Bostwick D.G., Grignon D.J., Hammond M.E., Amin M.B., Cohen M., Crawford D., Gospadarowicz M., Kaplan R.S., Miller D.S., Montironi R., Pajak T.F., Pollack A., Srigley J.R., Yarbro J.W. Prognostic factors in prostate cancer, College of American Pathologists Consensus Statement. Arch. Pathol. Lab Med. 2000;124(7):995–undefined. doi: 10.5858/2000-124-0995-PFIPC. [DOI] [PubMed] [Google Scholar]
- 3.Taplin M.E., George D.J., Halabi S., Sanford B., Febbo P.G., Hennessy K.T., Mihos C.G., Vogelzang N.J., Small E.J., Kantoff P.W. Prognostic significance of plasma chromogranin a levels in patients with hormone-refractory prostate cancer treated in Cancer and Leukemia Group B 9480 study. Urology. 2005;66(2):386–undefined. doi: 10.1016/j.urology.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 4.Grobholz R., Griebe M., Sauer C.G., Michel M.S., Trojan L., Bleyl U. Influence of neuroendocrine tumor cells on proliferation in prostatic carcinoma. Hum Pathol. 2005;36(5):562–undefined. doi: 10.1016/j.humpath.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Berruti A., Mosca A., Tucci M., Terrone C., Torta M., Tarabuzzi R., Russo L., Cracco C., Bollito E., Scarpa R.M., Angeli A., Dogliotti L. Independent prognostic role of circulating chromogranin A in prostate cancer patients with hormone-refractory disease. Hum Pathol. 2005;12(1):109–undefined. doi: 10.1677/erc.1.00876. [DOI] [PubMed] [Google Scholar]
- 6.McWilliam L.J., Manson C., George N.J. Neuroendocrine differentiation and prognosis in prostatic adenocarcinoma. Br J Urol. 1997;80(2):287–undefined. doi: 10.1046/j.1464-410x.1997.00300.x. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya N., Suzuki H., Kawamura K., Imamoto T., Naya Y., Tochigi N., Kakuta Y., Yamaguchi K., Ishikura H., Ichikawa T. Neuroendocrine differentiation in stage D2 prostate cancers. Int J Urol. 2008;15(5):423–undefined. doi: 10.1111/j.1442-2042.2008.02015.x. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein M.H., Partin A.W., Veltri R.W., Epstein J.I. Neuroendocrine differentiation in prostate cancer: enhanced prediction of progression after radical prostatectomy. Hum Pathol. 1996;27(7):683–undefined. doi: 10.1016/s0046-8177(96)90398-6. [DOI] [PubMed] [Google Scholar]
- 9.Theodorescu D., Broder S.R., Boyd J.C., Mills S.E., Frierson H.F. Jr. Cathepsin D and chromogranin A as predictors of long term disease specific survival after radical prostatectomy for localized carcinoma of the prostate. Cancer. 1997;80(11):2109–undefined. [PubMed] [Google Scholar]
- 10.May M., Siegsmund M., Hammermann F., Loy V., Gunia S. Prognostic significance of proliferation activity and neuroendocrine differentiation to predict treatment failure after radical prostatectomy. Scand.J.Urol.Nephrol. 2007;41(5):375–undefined. doi: 10.1080/00365590701224445. [DOI] [PubMed] [Google Scholar]
- 11.Gunia S., Albrecht K., Koch S., Herrmann T., Ecke T., Loy V., Linke J., Siegsmund M., May M. Ki67 staining index and neuroendocrine differentiation aggravate adverse prognostic parameters in prostate cancer and are characterized by negligible inter-observer variability. World J.Urol. 2008;26(3):243–undefined. doi: 10.1007/s00345-008-0257-0. [DOI] [PubMed] [Google Scholar]
- 12.Revelos K., Petraki C., Scorilas A., Stefanakis S., Malovrouvas D., Alevizopoulos N., Kanellis G., Halapas A., Koutsilieris M. Correlation of androgen receptor status, neuroendocrine differentiation and angiogenesis with time-to-biochemical failure after radical prostatectomy in clinically localized prostate cancer. Anticancer Res. 2007;27(5B):3651–undefined. [PubMed] [Google Scholar]
- 13.Autorino R., Lamendola M.G., De Luca G., De Sio M., Giuliano F., M D.A., De Placido S., Conti P., Di Lorenzo G. Neuroendocrine immunophenotype as predictor of clinical recurrence in 110 patients with prostate cancer. Int J Immunopathol Pharmacol. 2007;20(4):765–undefined. doi: 10.1177/039463200702000412. [DOI] [PubMed] [Google Scholar]
- 14.Epstein J.I., Allsbrook W.C. Jr., Amin M.B., Egevad L.L. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29(9):1228–undefined. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 15.di Sant'Agnese P.A., de Mesy Jensen K.L. Neuroendocrine differentiation in prostatic carcinoma. Hum Pathol. 1987;18(8):849–undefined. doi: 10.1016/s0046-8177(87)80060-6. [DOI] [PubMed] [Google Scholar]
- 16.Abrahamsson P.A., Cockett A.T., di Sant'Agnese P.A. Prognostic significance of neuroendocrine differentiation in clinically localized prostatic carcinoma. Prostate Suppl. 1998;8:37–undefined. [PubMed] [Google Scholar]
- 17.Grobholz R., Griebe M., Sauer C.G., Michel M.S., Trojan L., Bleyl U. Influence of neuroendocrine tumor cells on proliferation in prostatic carcinoma. Hum Pathol. 2005;36(5):562–undefined. doi: 10.1016/j.humpath.2005.02.019. [DOI] [PubMed] [Google Scholar]