Abstract
Background
Our aim was to investigate the incidence of sleep disturbance and insomnia in patients with primary hyperparathyroidism (PHPT), and to evaluate the effect of parathyroidectomy.
Methods
A questionnaire was prospectively administered to adult patients with PHPT who underwent curative parathyroidectomy over an 11-month period. The questionnaire, administered pre- and 6-months post-operatively, included the Insomnia Severity Index (ISI) and eight additional questions regarding sleep pattern. Total ISI scores range from 0 to 28, with >7 signifying sleep difficulties and scores >14 indicating clinical insomnia.
Results
Of 197 eligible patients undergoing parathyroidectomy for PHPT, 115 (58.3%) completed the pre- and post-operative questionnaires. The mean age was 60.0±1.2 years and 80.0% were female. Pre-operatively, 72 patients (62.6%) had sleep difficulties, and 29 patients (25.2%) met criteria for clinical insomnia. Clinicopathologic variables were not predictive of clinical insomnia. There was a significant reduction in mean ISI score after parathyroidectomy (10.3±0.6 vs 6.2±0.5, p<0.0001). Post-operatively, 79 patients (68.7%) had an improved ISI score. Of the 29 patients with pre-operative clinical insomnia, 21 (72.4%) had resolution after parathyroidectomy. Pre-operative insomnia patients had an increase in total hours slept after parathyroidectomy (5.4±0.3 vs 6.1±0.3 hours, p=0.02), whereas both insomnia and non-insomnia patients had a decrease in the number of awakenings (3.7±0.4 vs 1.9±0.2 times, p=0.0001).
Conclusion
Sleep disturbances and insomnia are common in patients with PHPT, and the majority of patients will improve after curative parathyroidectomy.
Introduction
The clinical presentation of primary hyperparathyroidism (PHPT) has evolved over recent decades since the implementation of routine biochemical screening [1]. The classic presentation of overt manifestations of the disease, including kidney stones, bone disease, and neuromuscular dysfunction, is infrequently encountered today [2]. Currently, patients with PHPT typically present with mild hypercalcemia discovered on routine biochemical screening, in association with subtler, nonspecific symptomatology [3]. Nontraditional symptoms associated with PHPT include sleep disturbance, weakness, fatigue, depression, anxiety, irritability, and cognitive dysfunction [3–18]. Although these patients are often labeled as “asymptomatic” or mildly symptomatic due to the absence of overt symptoms, their disease burden can be quite substantial and negatively impact quality of life [7,9,19–22]. Given the increased incidence of patients with “asymptomatic” or mildly symptomatic PHPT, the National Institutes of Health (NIH) convened two consensus conferences in 1990 and 2002 to develop recommendations regarding which subset of patients would benefit from operative intervention [23,24]. While the criteria for parathyroidectomy included the classic symptoms and physiologic markers of PHPT, nonspecific physical and neuropsychological symptoms associated with PHPT were excluded. The panel concluded that the limited studies on the neuropsychological features of PHPT were inconsistent in confirming their association with the disease process and reversibility after parathyroidectomy, and recommended further study prior to guideline changes.
Sleep impairment is highly prevalent in patients with PHPT, with a reported incidence of 44% pre-parathyroidectomy [25]. Sleep impairment and insomnia are associated with a high symptom burden and significantly reduced quality of life [26]. Furthermore, nontraditional symptoms of PHPT, particularly neuropsychological dysfunction and fatigue, may be related to, or exacerbated by, sleep disturbance. Although these nontraditional symptoms have demonstrated improvement after parathyroidectomy, there are only a limited number of studies that have specifically investigated sleep disturbance in PHPT [25,27,28]. These studies had small sample sizes with ultimately inconclusive results, warranting further investigation into the association of PHPT, sleep disturbance, and parathyroidectomy.
Therefore, the objectives of this study were: 1) to determine the incidence of sleep disturbance and insomnia in patients with PHPT; 2) to assess the improvement in sleep measures with curative parathyroidectomy; and 3) to investigate associations between sleep disturbance and/or insomnia with biochemical profile.
Materials and Methods
Patients
Between November 2011 and October 2012, all patients with PHPT referred to our endocrine surgery clinic and scheduled to undergo a parathyroidectomy were asked to complete a questionnaire pertaining to their sleep. Identification of patients with PHPT was made by biochemical diagnosis, which was defined as hypercalcemia (serum calcium >10.2 mg/dL) with an elevated or inappropriately normal parathyroid hormone (PTH) level. Consent for study participation was obtained from all patients during the initial surgical consultation. Patients were excluded from the study if they: 1) were <18 years old, 2) could not read or understand English, 3) had undergone a previous parathyroidectomy, 4) required reoperation for persistent or recurrent PHPT, 5) declined to participate in the post-operative follow-up questionnaire, or 6) if the pre- or post-operative ISI questionnaires were incomplete. We collected information on patient age, gender, presence of obstructive sleep apnea, serum calcium and PTH levels, operative procedure, and histologic information from the electronic medical record.
Questionnaire
The questionnaire utilized in this study included the Insomnia Severity Index (ISI), as well as eight additional subjective questions regarding sleep pattern. The questionnaire was administered during the initial pre-operative surgical consultation, and again six months post-operatively during a follow-up clinic appointment or via telephone. The ISI is a clinically tested, reliable, and validated seven-item questionnaire that assesses patient perception of sleep quality over the prior two-week period (Figure 1) [29–31]. Patients rate each question on a five-point Likert scale ranging from 0 (‘not at all’ or ‘very dissatisfied’) to 4 (‘very much’ or ‘very satisfied’). Individual ISI scores are summed to give a composite score, and categorized as the following: 0–7 = no significant insomnia, 8–14 = subthreshold insomnia, 15–21 = clinical insomnia, moderately severe, and 22–28 = clinical insomnia, severe. An overall ISI score greater than seven is associated with sleep difficulties, and an ISI score greater than fourteen has been shown to distinguish patients with clinical insomnia from normal controls with a sensitivity of 94% and specificity of 94% [30,32]. The eight additional questions included with the ISI in the questionnaire evaluated overall sleep schedule, sleep latency, total hours slept, number of awakenings, and duration of awakenings.
Figure 1.
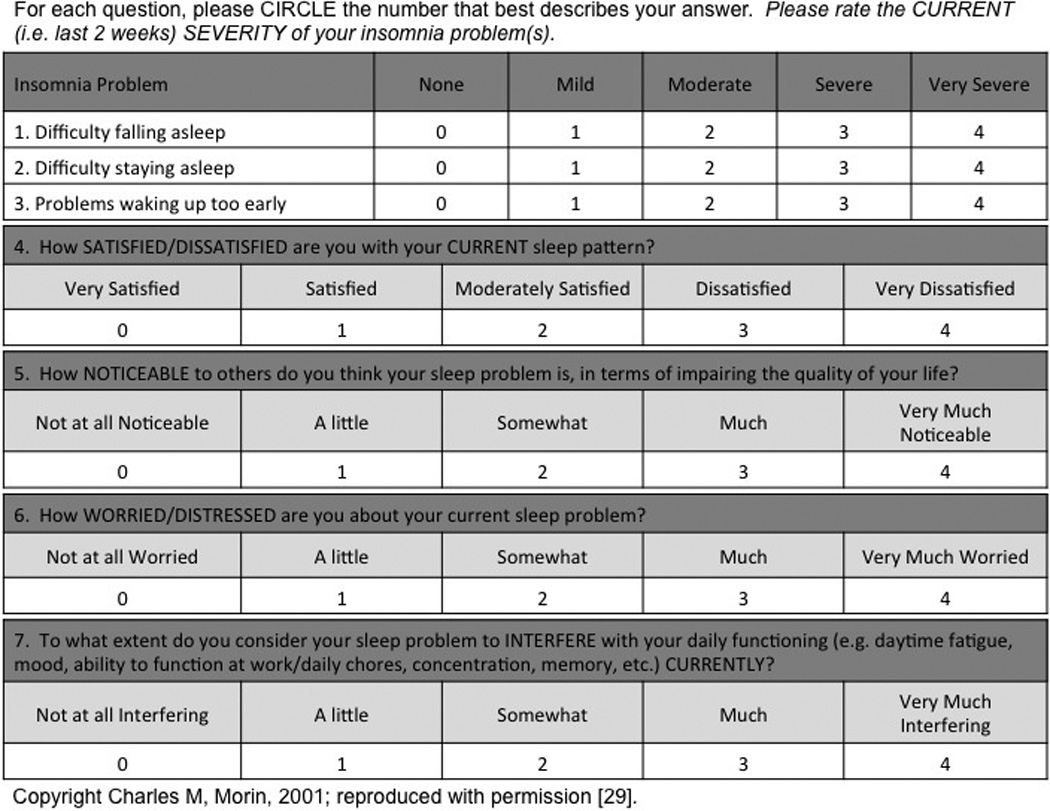
Insomnia Severity Index
Statistical Analysis
Data were analyzed using Stata version 12 software (StataCorp, College Station, TX), and are expressed as mean with standard error of the mean. A paired sample t-test was used to compare continuous variables, and a chi-squared test or Fisher’s exact test were utilized for categorical comparisons when appropriate. Values were reported to one significant decimal. P-values <0.05 were considered significant. For missing responses within the eight additional questions of the questionnaire, individual unreported values were excluded from the analyses. The institutional review board from the University of Wisconsin approved this study.
Results
During the study period, 197 patients with PHPT who underwent a parathyroidectomy were eligible to participate. One hundred and forty-two patients (72.1%) agreed to participate in the study and completed the pre-operative questionnaire. Overall, 115 patients (58.4%) successfully completed the pre- and post-operative questionnaires and were included in our analyses. There were no significant difference in demographic variables, biochemical profile, or parathyroid pathology between responders and non-responders.
The mean age of the cohort was 60.0±1.2 years and the majority of patients were female (n=92, 80.0%). Pre-operatively, 72 patients (62.6%) met criteria for sleep difficulties based on an ISI score >7, while 29 patients (25.2%) met criteria for clinical insomnia (ISI>14). Neither demographic variables, the magnitude of serum calcium and PTH elevations, nor the gland weight were predictive of clinical insomnia (Table 1). There was also no association between the diagnosis of obstructive sleep apnea and the incidence of clinical insomnia.
Table 1.
Clinicopathologic characteristics comparing patients with and without pre-operative clinical insomnia
| Variable | Without Insomnia | With Insomnia | P |
|---|---|---|---|
| n (%) | 86 (74.8) | 29 (25.2) | . |
| Age, mean ± SEM, yr | 61.0± 1.4 | 56.7 ± 2.2 | 0.1 |
| Females, n (%) | 67 (72.8) | 25 (27.2) | 0.4 |
| Serum calcium, mean ± SEM, mg/dL | 10.7 ± 0.08 | 10.9± 0.1 | 0.6 |
| Serum parathyroid hormone, mean ± SEM, pg/mL | 103 ± 6 | 106 ± 14 | 0.2 |
| Pathology, n (%) | |||
| Single adenoma | 70 (81.4) | 19 ( 65.5) | |
| Double adenoma | 6 (7.0) | 5 (17.2) | 0.1 |
| Hyperplasia | 10 (11.6) | 5 (17.2) | |
| Gland weight, mean ± SEM, mg | 604.3 ± 115.9 | 1045.8 ± 443.9 | 0.1 |
| Obstructive sleep apnea, n (%) | 11 (12.8) | 4 (13.8) | 1.0 |
SEM = Standard error of the mean
There was a significant reduction in the mean ISI score of all patients after parathyroidectomy, decreasing from 10.3±0.6 pre-operatively to 6.2±0.5 post-operatively (p<0.0001). This reduction was observed in patients with and without pre-operative clinical insomnia (Table 2). In total, 79 patients (68.7%) had an improved ISI score post-operatively. The frequency distribution of pre- versus post-operative ISI categories is displayed in Figure 2. A marked reduction in the ISI categories of ‘subthreshold insomnia’ and ‘clinical insomnia’, both moderate and severe, was observed, and the number of patients with ‘no clinically significant insomnia’ nearly doubled after parathyroidectomy. Figure 3 outlines in greater detail the movement between ISI categories pre- to post-operatively. As shown, 47 patients (40.9%) improved one or more ISI categories. There was no association between obstructive sleep apnea and improved ISI category (p=0.9).
Table 2.
Mean Insomnia Severity Index (ISI) scores and sleep patterns pre- and post-parathyroidectomy in patients with and without pre-operative clinical insomnia
| Variable | Pre-operatively | Post-operatively | P |
|---|---|---|---|
| Total ISI score | |||
| Insomnia | 19.1 ± 0.5 | 10.9 ± 1.3 | <0.0001 |
| No Insomnia | 7.3 ± 0.5 | 4.7 ± 0.4 | <0.0001 |
| Total hours slept per 24 hours | |||
| Insomnia | 5.4 ± 0.3 | 6.1 ± 0.3 | 0.02 |
| No Insomnia | 7.2 ± 0.1 | 7.1 ± 0.1 | 0.9 |
| Sleep latency | |||
| Insomnia | 35.5 ± 6.9 | 41.2 ± 8.9 | 0.6 |
| No Insomnia | 20.0 ± 1.9 | 22.3 ± 2.7 | 0.3 |
| Number of awakenings | |||
| Insomnia | 3.7 ± 0.4 | 1.9 ± 0.2 | 0.0009 |
| No Insomnia | 2.2 ± 0.2 | 1.7 ± 0.1 | 0.0004 |
| Duration of awakenings | |||
| Insomnia | 22.5 ± 4.7 | 22.8 ± 6.4 | 1.0 |
| No Insomnia | 13.7 ± 2.1 | 13.3 ± 2.4 | 0.9 |
Data displayed at mean ± standard error of the mean.
Figure 2.
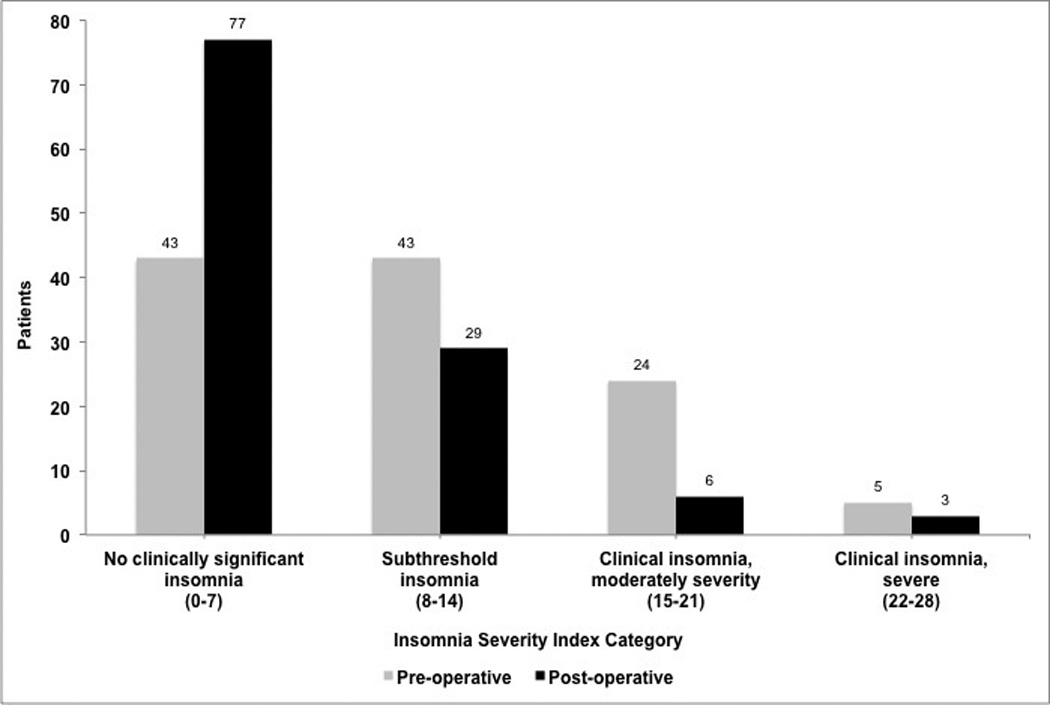
Frequency distribution of Insomnia Severity Index (ISI) categories pre- and post-parathyroidectomy
Figure 3.
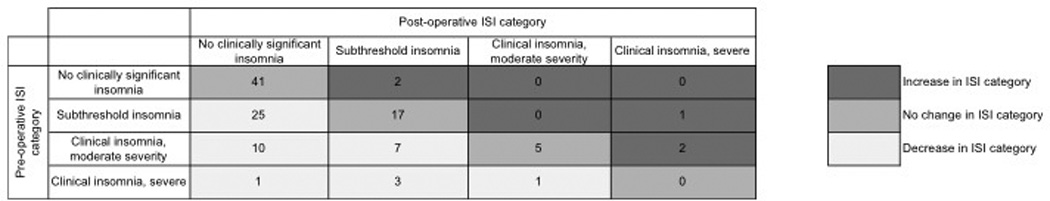
Changes in the Insomnia Severity Index (ISI) categories for patients undergoing parathyroidectomy
Patients with sleep difficulties (ISI>7) decreased from 72 (62.6%) pre-operatively to 38 (33.0%) post-operatively (p=0.04). Notably, of the 29 patients with pre-operative clinical insomnia, 21 (72.4%) had resolution after parathyroidectomy. In total, the number of patients with clinical insomnia decreased from 29 patients (25.2%) pre-operatively to 9 patients (7.8%) after parathyroidectomy (p<0.001). Five patients in the study had an increase in ISI category after surgery. No demographic, biochemical, or histologic differences were noted that distinguished these patients from the remainder of the cohort, nor did these five patients experience a higher rate of complications.
Regarding sleep pattern, there was a significant increase in mean total hours slept after surgery in patients with pre-operative clinical insomnia, whereas no difference was observed in patients without clinical insomnia (Table 2). The number of awakenings during sleep significantly decreased in both groups, though the duration of awakenings and sleep latency remained consistent.
Discussion
In this study, we evaluated the occurrence of sleep disturbance and insomnia in patients with PHPT, and the effect of curative parathyroidectomy. We found that clinical insomnia occurs in 25.2% of patients with PHPT, compared to an estimated 6% in the general population [33]. Nearly 70% of all patients in the series reported an improved ISI score, and there was a significant reduction in the mean ISI score post-parathyroidectomy. Importantly, over 70% of patients with pre-operative clinical insomnia had resolution after parathyroidectomy. Patients with and without insomnia both reported a significant decrease in the number of awakenings during sleep. In addition, patients with pre-operative insomnia experienced a significant increase in total hours slept post-operatively. We also demonstrated that neither patient demographics nor biochemical profile correlated with sleep disturbances or insomnia.
The majority of previous studies examining sleep disturbance in PHPT have incorporated a single item within a larger subjective questionnaire evaluating numerous non-traditional symptoms, and have conflicting conclusions regarding the effect of parathyroidectomy on sleep disturbance [11,14,15]. Our study is one of the few that focused specifically on sleep disturbance and insomnia in this patient population [25,27,28]. In addition, to our knowledge, no PHPT studies have utilized the ISI, which is a validated and reliable instrument used to identify patients with sleep difficulties and clinical insomnia [29–31].
The group from MD Anderson Cancer Center has similarly utilized objective tools to measure sleep impairment [25,27,28]. Using a subset of questions from the Brief Sleep Inventory, 44% of 55 patients with PHPT were identified as having pre-operative sleep impairment, which significantly decreased to 31% after parathyroidectomy [25]. These results reasonably correspond to the rate of sleep difficulties seen in our series – 62.6% pre-operatively decreasing to 33.0% post-operatively. In addition, similar to our findings, they found no association between sleep impairment and serum calcium or PTH. A subsequent pilot study with 18 patients estimated total sleep time using wrist actigraphy in patients with PHPT who did not meet NIH criteria for parathyroidectomy [28]. While there was a correlation between decreased serum PTH levels and improved sleep, no overall change in total sleep time was observed after parathyroidecotomy. This is in contrast to our findings of a significant increase in total hours slept after surgery in patients with pre-operative insomnia, although this was a subjective measurement. Our inclusion of all patients with PHPT, as opposed to only those who do not meet the NIH criteria, may account for this difference.
Although we did not address daytime sleepiness in this study, Perrier and colleagues reported that daytime sleepiness decreased after surgery in PHPT patients not meeting NIH criteria, as measured by the Epworth Sleepiness Scale [28,34]. This occurred without an increase in total sleep time, which suggests that sleep deprivation may not be the cause of sleepiness in PHPT patients. A possible explanation may be the decreased number of awakenings post-operatively demonstrated in our series, leading to improved sleep continuity. Further research utilizing polysomnography is necessary to determine the pattern of sleep disturbance in this population. Outlining a pattern of sleep impairment unique to PHPT would aid the clinician in properly counseling patients on post-operative expectations.
The mechanism of sleep disturbance in PHPT is likely multifactorial. The multitude of additional symptoms that frequently occur in PHPT may contribute to sleep impairment. These may include nocturia, gastroesophageal reflux disease, bone pain, myalgia, anxiety, or kidney stones. Many of these symptoms show improvement after parathyroidectomy, making difficult to ascertain cause and effect regarding sleep impairment in this population [4–9,18,35]. However, there is evidence that a biochemical component exists in relation to PHPT. Nilsson et al discovered altered cardiac autonomic nerve regulation in patients with hyperparathyroidism, which was shown to improve after parathyroidectomy and normalization of serum PTH [36]. This cardiac autonomic nerve dysfunction may alter circadian rhythm and impact sleep. Furthermore, evidence exists of a relationship between PTH and sleep. PTH has been shown to stimulate osteoblasts to produce the pro-inflammatory cytokine interleukin-6 (IL-6), which have been shown to enhance slow-wave non-rapid eye movement sleep. [37,38]. Further evidence is necessary to elucidate the complex interaction of hyperparathyroidism and sleep.
This study has several limitations. First, there is a potential non-responder bias as only 58.4% of eligible patients correctly completed the pre- and post-operative questionnaire. However, there were no significant differences in age, gender, biochemical profile, or parathyroid pathology between responders and non-responders, suggesting that our study sample is generalizable. Second, this study did not have a control sample, and therefore the observed improvement in sleep disturbance and insomnia post-parathyroidectomy may be partially due to placebo effect or other unmeasured factors unrelated to parathyroidectomy. A prospective study either comparing this population to patients undergoing an alternate elective operation (i.e. herniorraphy), or randomizing patients into surgical and non-surgical groups would provide stronger evidence of improvement.
In conclusion, sleep disturbance and insomnia are common problems in patients with PHPT. Although the severity of biochemical disease is not predictive of clinical insomnia, the majority of patients experience improvement or resolution after curative parathyroidectomy. Despite not being included in the NIH criteria for operative intervention, sleep disturbance has a significant impact on quality of life, and may contribute to many other non-specific symptoms of PHPT including fatigue and cognitive dysfunction. This study strongly suggests significant post-operative improvement in sleep disturbance and insomnia, although further investigation is necessary to determine if sleep impairment should be incorporated into the operative criteria for PHPT.
Acknowledgements
The authors would like to thank David F. Schneider, MD, and David S. Pontes for their contributions to this project. This work was supported by the University of Wisconsin, Physician Scientist Training in Career Medicine grant (National Institutes of Health T32 CA009614-22), and the Doris Duke Charitable Foundation Grant #2011119.
Footnotes
Abstract presented at the International Association of Endocrine Surgeons Annual Meeting, International Surgical Week in Helsinki, Finland, August 25–29, 2013.
Disclosures: None
References
- 1.Health H., III Clinical spectrum of primary hyperparathyroidism: evolution with changes in medical practices and technology. J Bone Miner Res. 1991;6:S63–S70. doi: 10.1002/jbmr.5650061415. [DOI] [PubMed] [Google Scholar]
- 2.Bilezikian JP, Silverberg SJ, Gartenberg F, et al. Clinical presentation of primary hyperparathyroidism. In: Bilezikian JP, editor. The parathyroids: basic and clinical concepts. New York: Raven Press; 1994. pp. 457–470. [Google Scholar]
- 3.Silverberg SJ, Lewiecki EM, Mosekilde L, et al. Presentation of asymptomatic primary hyperparathyroidism: Proceedings of the Third International Workshop. J Clin Endocrinol Metab. 2009;94:351–365. doi: 10.1210/jc.2008-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eigelberger MS, Cheah WK, Ituarte PHG, et al. The NIH criteria for parathyroidectomy in asymptomatic primary hyperparathyroidism. Are they too limited? Ann Surg. 2004;239:528–535. doi: 10.1097/01.sla.0000120072.85692.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasieka JL, Parsons LL. Prospective surgical outcome study of relief of symptoms following surgery in patients with primary hyperparathyroidism. World J Surg. 1998;22:513–518. doi: 10.1007/s002689900428. [DOI] [PubMed] [Google Scholar]
- 6.Pasieka JL, Parsons LL, Demeure MJ, et al. Patient-based surgical outcome tool demonstrating alleviation of symptoms following parathyroidectomy in patients with primary hyperparathyroidism. World J Surg. 2002;26:942–949. doi: 10.1007/s00268-002-6623-y. [DOI] [PubMed] [Google Scholar]
- 7.Caillard C, Sebag F, Mathonnet M, et al. Prospective evaluation of quality of life (SF-36v2) and nonspecific symptoms before and after cure of primary hyperparathyroidism (1-year follow-up) Surgery. 2007;141:153–160. doi: 10.1016/j.surg.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Sywak MS, Knowlton ST, Pasieka JL, et al. Do the National Institutes of Health consensus guidelines for parathyroidectomy predict symptom severity and surgical outcome in patients with primary hyperparathyroidism? Surgery. 2002;132:1013–1020. doi: 10.1067/msy.2002.128693. [DOI] [PubMed] [Google Scholar]
- 9.Gopinath P, Sadler GP, Mihai R. Persistent symptomatic improvement in the majority of patients undergoing parathyroidectomy for primary hyperparathyroidism. Langenbecks Arch Surgery. 2010;395:941–946. doi: 10.1007/s00423-010-0689-z. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto T, Kamo T, Obara T. Outcome study of psychological distress and nonspecific symptoms in patients with mild primary hyperparathyroidism. Arch Surg. 2002;137:779–783. doi: 10.1001/archsurg.137.7.779. [DOI] [PubMed] [Google Scholar]
- 11.Joborn C, Hetta J, Johansson H, et al. Psychiatric morbidity in primary hyperparathyroidism. World J Surg. 1988;12:476–481. doi: 10.1007/BF01655425. [DOI] [PubMed] [Google Scholar]
- 12.Joborn C, Hetta J, Lind L, et al. Self-rated psychiatric symptoms in patients operated on because of primary hyperparathyroidism and in patients with longstanding mild hypercalcemia. Surgery. 1989;105:72–78. [PubMed] [Google Scholar]
- 13.Numann PJ, Torppa AJ, Blumetti AE. Neuropsychologic deficits with primary hyperparathyroidism. Surgery. 1984;96:1119–1123. [PubMed] [Google Scholar]
- 14.McAllion SJ, Paterson CR. Psychiatric morbidity in primary hyperparathyroidism. Postgrad Med J. 1989;65:628–631. doi: 10.1136/pgmj.65.767.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsukahara K, Sugitani I, Fujimoto Y, et al. Surgery did not improve the subjective neuropsychological symptoms of patients with incidentally detected mild primary hyperparathyroidism. Eur Arch Otorhinolaryngol. 2008;265:565–569. doi: 10.1007/s00405-007-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Repplinger D, Schaefer S, Chen H, et al. Neurocognitive dysfunction: a predictor of parathyroid hyperplasia. Surgery. 2009;146:1138–1143. doi: 10.1016/j.surg.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bargen AE, Repplinger D, Chen H, et al. Can biochemical abnormalities predict symptomatology in patients with primary hyperparathyroidism? J Am Coll Surg. 2011;213:410–414. doi: 10.1016/j.jamcollsurg.2011.06.401. [DOI] [PubMed] [Google Scholar]
- 18.Murray SE, Pathak PR, Pontes DS, et al. Timing of symptom improvement after parathyroidectomy for primary hyperparathyroidism; Abstract presented at American Association of Endocrine Surgery Annual Meeting; Chicago, IL. April 2013; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adler JT, Sippel RS, Schaefer S, et al. Surgery improves quality of life in patients with"mild” hyperparathyroidism. Am J Surg. 2009;197:284–290. doi: 10.1016/j.amjsurg.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Burney RE, Jones KR, Coon JW, et al. Assessment of patient outcomes after operation for primary hyperparathyroidism. Surgery. 1996;120:1013–1019. doi: 10.1016/s0039-6060(96)80048-1. [DOI] [PubMed] [Google Scholar]
- 21.Burney RE, Jones KR, Peterson M, et al. Surgical correction of primary hyperparathyroidism improves quality of life. Surgery. 1998;124:987–999. [PubMed] [Google Scholar]
- 22.Talpos GB, Bone HG, Kleerekoper M, et al. Randomized trial of parathyroidectomy in mild asymptomatic primary hyperparathyroidism: patient description and effects on the SF-36 health survey. Surgery. 2000;128:1013–1020. doi: 10.1067/msy.2000.110844. [DOI] [PubMed] [Google Scholar]
- 23.NIH Conference. Diagnosis and management of asymptomatic primary hyperparathyroidism: consensus development conference statement. Ann Intern Med. 1991;114:593–597. doi: 10.7326/0003-4819-114-7-593. [DOI] [PubMed] [Google Scholar]
- 24.Bilezikian JP, Potts JT, Jr, Fuleihan Gel-H, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab. 2002;87:5353–5361. doi: 10.1210/jc.2002-021370. [DOI] [PubMed] [Google Scholar]
- 25.Mittendorf EA, Wefel JS, Meyers CA, et al. Improvement of sleep disturbance and neurocognitive function after parathyroidectomy in patients with primary hyperparathyroidism. Endocr Pract. 2007;13:338–344. doi: 10.4158/EP.13.4.338. [DOI] [PubMed] [Google Scholar]
- 26.Ishak ww, Bagot K, Thomas S, et al. Quality of life in patients suffering from insomnia. Innov Clin Neurosci. 2012;9:13–26. [PMC free article] [PubMed] [Google Scholar]
- 27.Perrier ND, Coker LH, Rorie KD, et al. Preliminary report: functional MRI of the brain may be the ideal tool for evaluating neuropsychologic and sleep complaints of patients with primary hyperparathyroidism. World J Surg. 2006;30:686–696. doi: 10.1007/s00268-005-0361-x. [DOI] [PubMed] [Google Scholar]
- 28.Perrier ND, Balachandran D, Wefel JS, et al. Prospective, randomized, controlled trial of parathyroidectomy versus observation in patients with"asymptomatic” primary hyperparathyroidism. Surgery. 2009;146:1116–1122. doi: 10.1016/j.surg.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 29.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 30.Savard MH, Savard J, Simard S, et al. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14:429–441. doi: 10.1002/pon.860. [DOI] [PubMed] [Google Scholar]
- 31.Morin CM, Belleville G, Belanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith S, Trinder J. Detecting insomnia: comparison of four self-report measures of sleep in a young adult population. J Sleep Res. 2001;10:229–235. doi: 10.1046/j.1365-2869.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 33.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 34.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 35.Reiher AE, Mazeh H, Schaefer S, et al. Symptoms of gastroesophageal reflux disease improve after parathyroidectomy. Surgery. 2012;152:1232–1237. doi: 10.1016/j.surg.2012.08.051. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson IL, Aberg J, Rastad J, et al. Circadian cardiac autonomic nerve dysfunction in primary hyperparathyroidism improves after parathyroidectomy. Surgery. 2003;134:1013–1019. doi: 10.1016/j.surg.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Safley SA, Villinger F, Jackson EH, et al. Interleukin-6 production and secretion by human parathyroids. Clin Exp Immunol. 2004;136:145–156. doi: 10.1111/j.1365-2249.2004.02419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9:355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]


