Abstract
Objectives: Microglia are considered as the primary immune effector cells in the brain and have a critical role in all brain lesions. We wanted to find out if there is any difference in the way that white and gray matter microglia react to the same type of lesion. Material and Method: We used 14-16 weeks old single transgenic CX3CR1-EGFP mice, whereon microglia were labeled by expression of the green fluorescent protein EGFP and the L1-L2 dorsal spinal columns were exposed. After 10 min of continuous base line image acquisition, we made a micro-lesion by focusing and raising the power of the laser and, than, we monitored it for an additional hour. Laser-lesion and image recording were also made in the right somato-sensory cortex. We quantified microglial response and compared white vs. grey matter. Results: 5-10 min after the lesion, microglia already showed signs of polarization by extending their processes both in white and gray matter. Processes were sent by the microglial bodies situated at a distance of 50 to 100 µm, depending on the lesion size. Microglial processes did not display a preferred target site from the lesion; in contrast, they formed a uniform “shielding” ring around the lesion. Conclusions: Microglia showed targeted responses to acute injuries in grey and white matter also; no major differences were observed besides the speed of the process, due probably to particular cortex and spine architecture.
Keywords: microglia, injury, gray matter, white matter
Introduction
High resolution imaging of the central nervous system (CNS) is now a real possibility, in vivo, by using two-photon laser scanning microscopy (2PLSM). The major limitation of this method is the presence of fluorescent proteins which could be exited using pulse laser [1].
This technique allows the scientists to further investigate the role of resident glial cells in the CNS. Transgenic animals express florescent proteins in different CNS cells; that permit scientists to analyze the labeled cells, for long periods of time, through windows to the brain [1, 2] or spinal cord [3, 3].
There are a number of injury models to investigate different levels of the CNS. These models can be used to evaluate different aspects of brain inflammation. Some of them disrupt the continuity of the dura like pial vessel disruption [5, 6], stab injury [7, 8] and so on. The inflammation in these models is too big for an accurate long term evaluation but they are perfect for acute cell reaction. Others keep the dura intact like middle cerebral artery occlusion using an intraluminal filament [1] or laser induced injuries [9] and these models are more suitable for long term imaging. From all of them only the laser induced injury allows investigators to evaluate an individual glia reaction towards inflammation, because in this model brain the process will be at the minimum level around the injured area.
In this study we wanted to see if inflammation of the brain has the same characteristics no matter the level at which it is produced. To see this, we tested if the immune response, initiated through microglial activity, is the same in white and gray matter.
Matherial and Method
For this purpose we used six adult transgenic TgH (CX3CR1-EGFP) mice (14-16 weeks of age at the beginning of the experiments) for in vivo imaging experiments. These mice expressed a green fluorescent protein in monocytes, macrophages and microglia, achieved by the placement of the EGFP reporter gene into the Cx3cr1 locus encoding the chemokine receptor CX3CR1 [10].
Mice were anaesthetized using Ketamine/Xylazine solution (120 mg/kg; 12 mg/kg), and supplemented when needed with half of the initial dose. Bepanthen gel (5% dexpanthenol) was applied to maintain eye moisture, as the cornea of mice got dry under anesthesia. The depth of anesthesia was verified by toe pinch reflex.
In order to have a good visualization of the cortex we removed the skin and subcutaneous tissue, and so we exposed the upper part of the skull. By using a driller we made a small craniotomy. After the bleeding has stopped we placed a small amount of low melting agar 1% on the intact durra. Over the agar we placed a glass cover slip and pressed it until we had a nice flat imaging plane. While the cover slip was hold in place so that no bubbles will get under it, we glued everything to the skull.
For the spine, around the surgical site, the hair was shaved and the skin was cleaned with iodine solution. In all mice we exposed L1-L2 spine by relieving the skin, tendons and muscle tissue at this level, so that the L1-L2 spinal processes and arcs appeared. The removal of spinal arcs needs a special attention in order to keep an intact durra mater and to have a clear view of the Goll and Burdach tracts.
For imaging we used a custom-made microscope able to record simultaneously up to four channels in vivo. ScanImage software version 3 release 1 [11], modified by ourselves, was used to drive the microscope. Photographic overviews of the spinal cord were acquired using a 5x objective (NA 0.15; Zeiss, Jena, Germany) and a CCD camera. High resolution in vivo imaging was obtained by stimulating the tissue with laser wavelength set to 920 nm. We used a 510±41 nm filter just before photomultiplier tube detection. We recorded, digitized and processed uniformly spaced planes to obtain z-stacks of images, around 40-60 images per stack.
The total acquisition time was approximately 3 min per stack. We recorded several stacks taken continuously to get time-lapse series of microglia action. After 30 min of continuous base line image acquisition, we made a micro-lesion. To do this laser damage, we focused the beam for 5-10 sec to a plane of 2-6 µm in diameter to destroy the tissue in a spheroid volume of 20-30 µm and 40-60 µm in depth in the cortex and 15-30 µm in the spine. When we applied the laser pulse we also saw, as others described before [9], auto florescent material caused by protein coagulation and photooxidation processes marking the lesion site. Microglia and blood vessels were not targeted when the laser lesion was done, but it is possible that in some cases, some microglia cells were destroyed.
Using ImageJ we analyzed individual cell movement and quantified the activation time for each cell and process speed. 15 cells were analyzed in the spine and 14 in the cortex.
One mouse died during the spine intervention, due to anesthetic complications.
Results
After a small baseline acquisition we have made a local tissue damage using high-power laser pulses in both, gray matter (cortex) or white matter (spine).
The microglia shown signs of polarization by extending their processes in cortex after an average time, called reaction time, of about 6.4 minutes with a SD=2.35. For the spine reaction time was found at 6.6 minutes in average with a SD=1.63 (Fig 1.1). There was no significant difference in the two types of microglia reaction (p>0.05).
Figure 1.
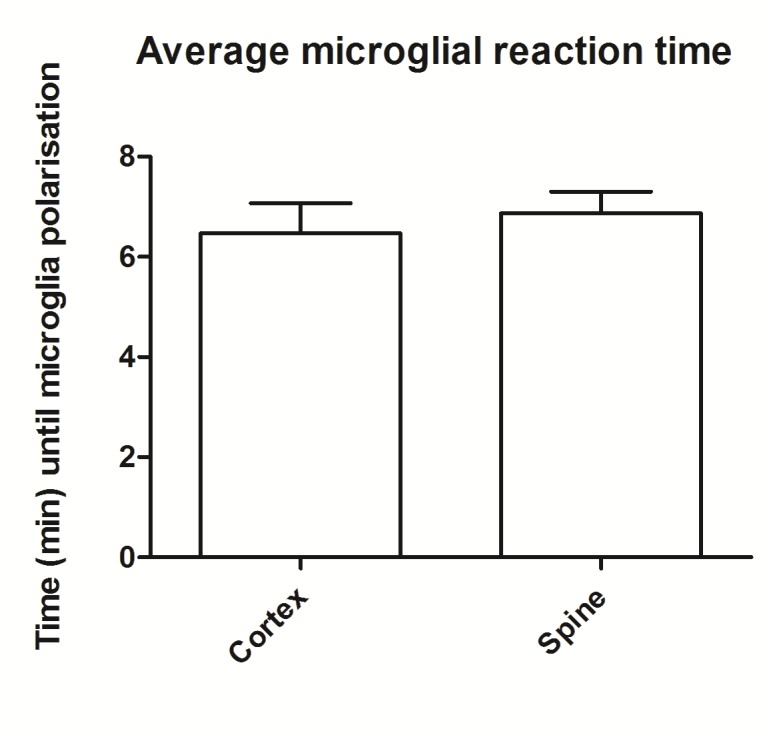
Microglia reaction time in cortex and spine generated by local injury. The data show no significant difference between the two groups.
Depending on the size of the lesion, the processes were sent from a distance of 50 up to 100 µm both in the spine or cortex. In the same time, the processes distal to the lesion were retracted.
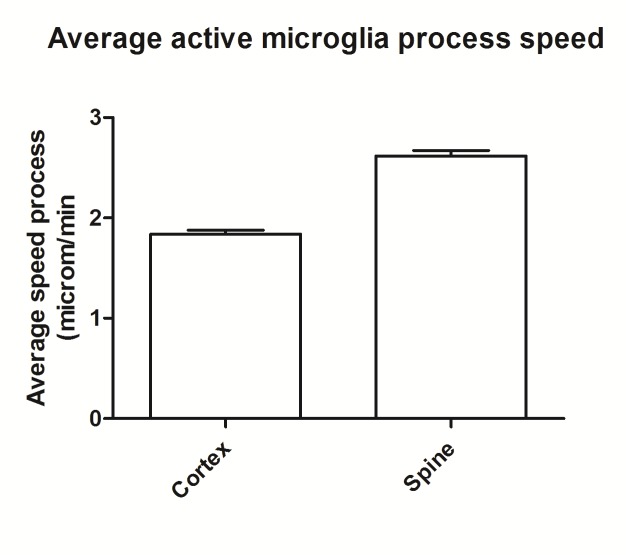
We also quantify the speed of microglia process sent towards the lesion site. We found that in the spine processes move faster (p<0.01) than in the cortex, with a average speed of 2.6 µm/min with a standard deviation of 0.6 compare to average speed in cortex of 1.87 µm/min with a standard deviation of 0.75 (Fig 2 2).
Figure 2.
Microglia process speed, in µm/min, sent as a resource to local injury.
No overall morphological difference was seen in the white and gray matter (Fig. 3).
Figure 3.
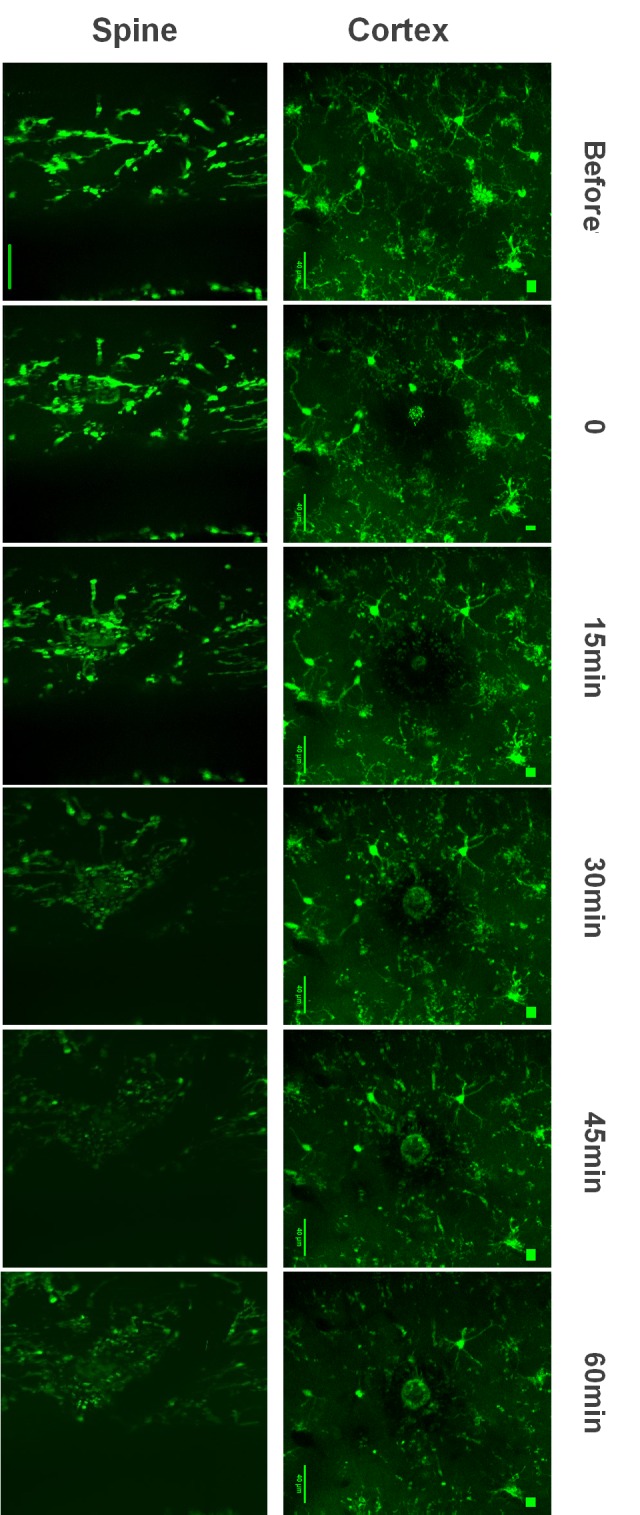
Microglia morphology reaction to local injury in spine and cortex. In both cases microglia reacted within the first 10 min by sending their processes towards the lesion. After 20 min, in the spine, microglia already formed a complete shielding ring around the lesion but in the cortex it was fully formed in about 30 min due to a slower process speed.
Conclusions
Microglia response to injury has been defined as a polarization of the cells with an extension of their processes, migration and, in the end, phagocytosis [12]. This reaction of microglia cells can be seen in gray [13] and white [9] matter with no real difference in between the two. Using a previous used method [9], we have also shown that, in contrast with the cell body that stays relatively in the same place, the processes of microglia cells are highly dynamic. Microglia processes will extend and retract all the time overseeing a neighboring microenvironment. Microglia will not survey the same microenvironment at the same time but border between them are highly dynamic, thus one cell can be overseen by more microglia over time. When ending processes of two neighboring microglia encounter one another, they will mutually repel each other [12].
It is the unique way that microglia exerts their functions permanently and “sees” the cells close to them: have a high motility due to the filamentous actin that the cells contain [14] and can extend processes by a rapid rearrangement of the actin cytoskeleton. By administering certain substances that inhibit actin polymerization (e.g. pertussis toxin) motility and migration of the cells can be also affected; instead complement 5a induces processes extension within few seconds [15].
Acknowledgments
The author thanks A. Scheller and A. Cupido for providing mice and laboratory equipment.
References
- 1.Sigler A, Murphy TH. In Vivo 2-Photon Imaging of Fine Structure in the Rodent Brain: Before, During, and After Stroke. Stroke. 2010;41(10 Suppl):S117–S123. doi: 10.1161/STROKEAHA.110.594648. [DOI] [PubMed] [Google Scholar]
- 2.Li P, Murphy TH. Two-photon imaging during prolonged middle cerebral artery occlusion in mice reveals recovery of dendritic structure after reperfusion. J Neurosci. 2008;28(46):11970–11979. doi: 10.1523/JNEUROSCI.3724-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenrich KK, Weber P, Hocine M, Zalc M, Rougon G, Debarbieux F. Long-term in vivo imaging of normal and pathological mouse spinal cord with sub-cellular resolution using implanted glass windows. J Physiol. 2012;590(16):3665–3675. doi: 10.1113/jphysiol.2012.230532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrar MJ, Bernstein IM, Schlafer DR, Cleland TA, Fetcho JR, Schaffer CB. Chronic in vivo imaging in the mouse spinal cord using an implanted chamber. Nature method. 2012;9(3):297–301. doi: 10.1038/nmeth.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayhan WG, Walz W. Pial vessel permeability to tracers using cranial windows. Meth Mol Med. 2003;89(1):121–131. doi: 10.1385/1-59259-419-0:121. [DOI] [PubMed] [Google Scholar]
- 6.Hua R, Walz W. Minocycline treatment prevents cavitation in rats after a cortical devascularizing lesion. Brain Res. 2006;1090(1):172–181. doi: 10.1016/j.brainres.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 7.Balasingam V, Tejada-Berges T, Wright E, Bouckova R, Yong VW. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosc. 1994;14:846–856. doi: 10.1523/JNEUROSCI.14-02-00846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eugenin EA, Eckardt D, Theis M, Willecke K, Bennett MV, Saez JC. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-g and tumor necrosis factor-a. PNAS. 2001;98:4190–4195. doi: 10.1073/pnas.051634298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dibaj P, Nadrigny F, Steffens H, Scheller A, Hirrlinger J, Schomburg ED, Neusch C, Kirchhoff F. NO mediates microglial response to acute spinal cord injury under ATP control in vivo. Glia. 2010;58:1133––1144. doi: 10.1002/glia.20993. [DOI] [PubMed] [Google Scholar]
- 10.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of Fractalkine Receptor CX3CR1 Function by Targeted Deletion and Green Fluorescent Protein Reporter Gene Insertion. Mol. Cell. Biol. 2000;20:4106-–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed Eng Online . 2003;2:13–13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker AJ, Ullian EM. New roles for astrocytes in developing synaptic circuits. Communicative & Integrative Biol. 2008;1:207–211. doi: 10.4161/cib.1.2.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capani F, Ellisman MH, Martone ME. Filamentous actin is concentrated in specific subpopulations of neuronal and glial structures in rat central nervous system. Brain Res. 2001;923(1-2):1–11. doi: 10.1016/s0006-8993(01)03189-4. [DOI] [PubMed] [Google Scholar]
- 14.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 15.Nolte C, Möller T, Walter T, Kettenmann H. Complement 5a controls motility of murine microglial cells in vitro via activation of an inhibitory G-protein and the rearrangement of the actin cytoskeleton. Neuroscience. 1996;73:1091–1107. doi: 10.1016/0306-4522(96)00106-6. [DOI] [PubMed] [Google Scholar]