Abstract
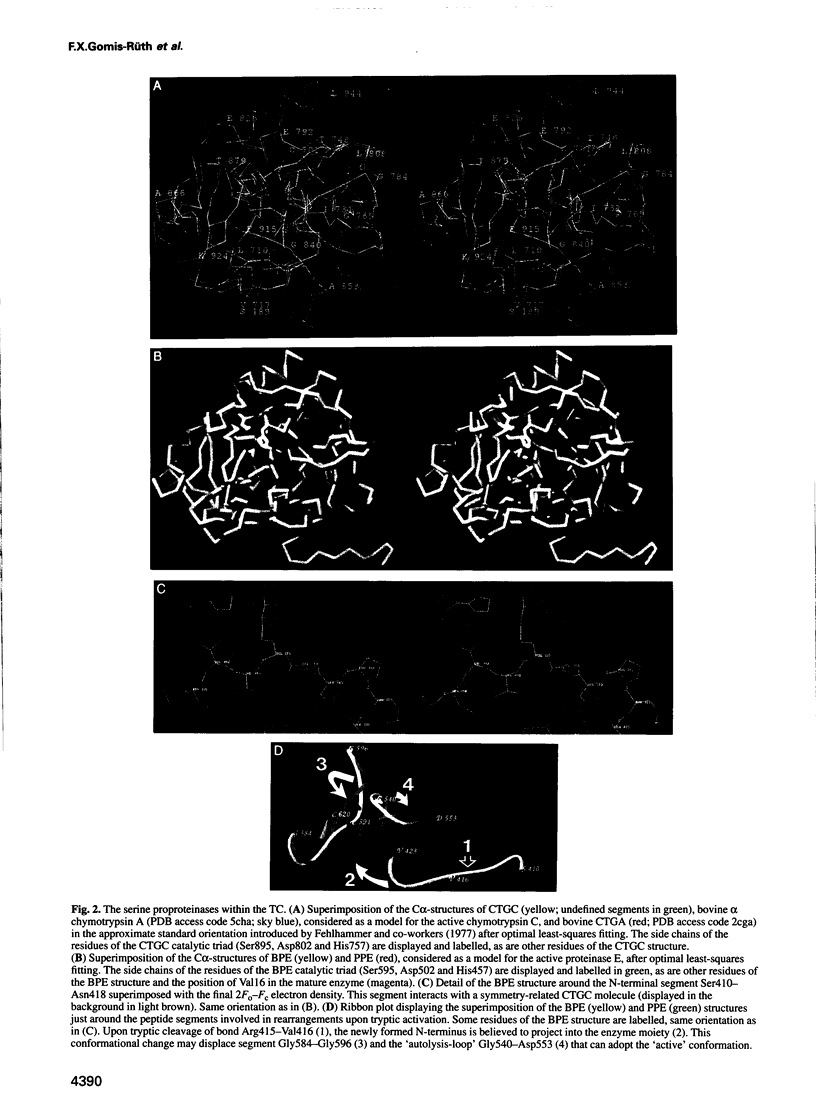
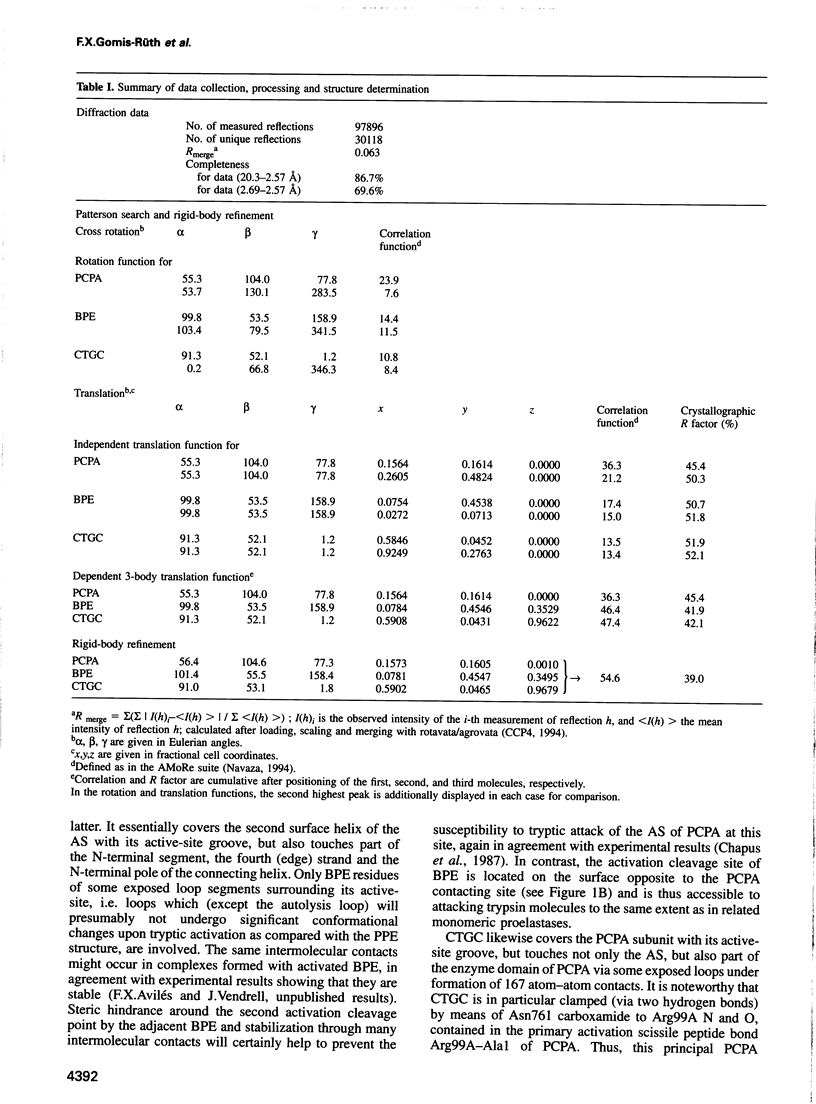
The metalloexozymogen procarboxypeptidase A is mainly secreted in ruminants as a ternary complex with zymogens of two serine endoproteinases, chymotrypsinogen C and proproteinase E. The bovine complex has been crystallized, and its molecular structure analysed and refined at 2.6 A resolution to an R factor of 0.198. In this heterotrimer, the activation segment of procarboxypeptidase A essentially clamps the other two subunits, which shield the activation sites of the former from tryptic attack. In contrast, the propeptides of both serine proproteinases are freely accessible to trypsin. This arrangement explains the sequential and delayed activation of the constituent zymogens. Procarboxypeptidase A is virtually identical to the homologous monomeric porcine form. Chymotrypsinogen C displays structural features characteristic for chymotrypsins as well as elastases, except for its activation domain; similar to bovine chymotrypsinogen A, its binding site is not properly formed, while its surface located activation segment is disordered. The proproteinase E structure is fully ordered and strikingly similar to active porcine elastase; its specificity pocket is occluded, while the activation segment is fixed to the molecular surface. This first structure of a native zymogen from the proteinase E/elastase family does not fundamentally differ from the serine proproteinases known so far.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avilés F. X., Vendrell J., Guasch A., Coll M., Huber R. Advances in metallo-procarboxypeptidases. Emerging details on the inhibition mechanism and on the activation process. Eur J Biochem. 1993 Feb 1;211(3):381–389. doi: 10.1111/j.1432-1033.1993.tb17561.x. [DOI] [PubMed] [Google Scholar]
- BROWN J. R., COX D. J., GREENSHIELDS R. N., WALSH K. A., YAMASAKI M., NEURATH H. The chemical structure and enzymatic functions of bovine procarboxypeptidase A. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1554–1560. doi: 10.1073/pnas.47.10.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Chapus C., Kerfelec B., Foglizzo E., Bonicel J. Further studies on the activation of bovine pancreatic procarboxypeptidase A by trypsin. Eur J Biochem. 1987 Jul 15;166(2):379–385. doi: 10.1111/j.1432-1033.1987.tb13526.x. [DOI] [PubMed] [Google Scholar]
- Coll M., Guasch A., Avilés F. X., Huber R. Three-dimensional structure of porcine procarboxypeptidase B: a structural basis of its inactivity. EMBO J. 1991 Jan;10(1):1–9. doi: 10.1002/j.1460-2075.1991.tb07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLK J. E., SCHIRMER E. W. CHYMOTRYPSIN C. I. ISOLATION OF THE ZYMOGEN AND THE ACTIVE ENZYME: PRELIMINARY STRUCTURE AND SPECIFICITY STUDIES. J Biol Chem. 1965 Jan;240:181–192. [PubMed] [Google Scholar]
- Fehlhammer H., Bode W., Huber R. Crystal structure of bovine trypsinogen at 1-8 A resolution. II. Crystallographic refinement, refined crystal structure and comparison with bovine trypsin. J Mol Biol. 1977 Apr 25;111(4):415–438. doi: 10.1016/s0022-2836(77)80062-4. [DOI] [PubMed] [Google Scholar]
- Freisheim J. H., Walsh K. A., Neurath H. The activation of bovine procarboxypeptidase A. II. Mechanism of activation of the succinylated enzyme precursor. Biochemistry. 1967 Oct;6(10):3020–3028. doi: 10.1021/bi00862a008. [DOI] [PubMed] [Google Scholar]
- Guasch A., Coll M., Avilés F. X., Huber R. Three-dimensional structure of porcine pancreatic procarboxypeptidase A. A comparison of the A and B zymogens and their determinants for inhibition and activation. J Mol Biol. 1992 Mar 5;224(1):141–157. doi: 10.1016/0022-2836(92)90581-4. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S. AMINO-ACID SEQUENCE OF BOVINE CHYMOTRYPSINOGEN-A. Nature. 1964 Mar 28;201:1284–1287. doi: 10.1038/2011284a0. [DOI] [PubMed] [Google Scholar]
- Kerfelec B., Cambillau C., Puigserver A., Chapus C. The inactive subunit of ruminant procarboxypeptidase A-S6 complexes. Structural basis of inactivity and physiological role. Eur J Biochem. 1986 Jun 16;157(3):531–538. doi: 10.1111/j.1432-1033.1986.tb09699.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi R., Kobayashi Y., Hirs C. H. The specificity of porcine pancreatic protease E. J Biol Chem. 1981 Mar 10;256(5):2460–2465. [PubMed] [Google Scholar]
- Kobayashi Y., Kobayashi R., Hirs C. H. Identification of zymogen E in a complex with bovine procarboxypeptidase A. J Biol Chem. 1981 Mar 10;256(5):2466–2470. [PubMed] [Google Scholar]
- Madison E. L., Kobe A., Gething M. J., Sambrook J. F., Goldsmith E. J. Converting tissue plasminogen activator to a zymogen: a regulatory triad of Asp-His-Ser. Science. 1993 Oct 15;262(5132):419–421. doi: 10.1126/science.8211162. [DOI] [PubMed] [Google Scholar]
- Meyer E., Cole G., Radhakrishnan R., Epp O. Structure of native porcine pancreatic elastase at 1.65 A resolutions. Acta Crystallogr B. 1988 Feb 1;44(Pt 1):26–38. doi: 10.1107/s0108768187007559. [DOI] [PubMed] [Google Scholar]
- Michon T., Granon S., Sauve P., Chapus C. The activation peptide of pancreatic procarboxypeptidase A is the keystone of the bovine procarboxypeptidase A-S6 ternary complex. Biochem Biophys Res Commun. 1991 Nov 27;181(1):449–455. doi: 10.1016/s0006-291x(05)81440-8. [DOI] [PubMed] [Google Scholar]
- Michon T., Sari J. C., Granon S., Kerfelec B., Chapus C. Microcalorimetric investigation of the interactions between the subunits of the bovine pancreatic procarboxypeptidase A-S6 complex. Eur J Biochem. 1991 Oct 1;201(1):217–222. doi: 10.1111/j.1432-1033.1991.tb16277.x. [DOI] [PubMed] [Google Scholar]
- Neurath H. Proteolytic processing and physiological regulation. Trends Biochem Sci. 1989 Jul;14(7):268–271. doi: 10.1016/0968-0004(89)90061-3. [DOI] [PubMed] [Google Scholar]
- Pascual R., Vendrell J., Avilés F. X., Bonicel J., Wicker C., Puigserver A. Autolysis of proproteinase E in bovine procarboxypeptidase A ternary complex gives rise to subunit III. FEBS Lett. 1990 Dec 17;277(1-2):37–41. doi: 10.1016/0014-5793(90)80804-r. [DOI] [PubMed] [Google Scholar]
- Pignol D., Gaboriaud C., Michon T., Kerfelec B., Chapus C., Fontecilla-Camps J. C. Crystal structure of bovine procarboxypeptidase A-S6 subunit III, a highly structured truncated zymogen E. EMBO J. 1994 Apr 15;13(8):1763–1771. doi: 10.1002/j.1460-2075.1994.tb06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver A., Desnuelle P. Reconstitution of bovine procarboxypeptidase A-S6 from the free subunits. Biochemistry. 1977 May 31;16(11):2497–2501. doi: 10.1021/bi00630a028. [DOI] [PubMed] [Google Scholar]
- Shen W. F., Fletcher T. S., Largman C. Primary structure of human pancreatic protease E determined by sequence analysis of the cloned mRNA. Biochemistry. 1987 Jun 16;26(12):3447–3452. doi: 10.1021/bi00386a030. [DOI] [PubMed] [Google Scholar]
- Uren J. R., Neurath H. Mechanism of activation of bovine procarboxypeptidase A S 5 . Alterations in primary and quaternary structure. Biochemistry. 1972 Nov 21;11(24):4483–4492. doi: 10.1021/bi00774a010. [DOI] [PubMed] [Google Scholar]
- Vendrell J., Cuchillo C. M., Avilés F. X. The tryptic activation pathway of monomeric procarboxypeptidase A. J Biol Chem. 1990 Apr 25;265(12):6949–6953. [PubMed] [Google Scholar]
- Venot N., Sciaky M., Puigserver A., Desnuelle P., Laurent G. Amino acid sequence and disulfide bridges of subunit III, a defective endopeptidase present in the bovine pancreatic 6 S procarboxypeptidase A complex. Eur J Biochem. 1986 May 15;157(1):91–99. doi: 10.1111/j.1432-1033.1986.tb09642.x. [DOI] [PubMed] [Google Scholar]
- Wang D., Bode W., Huber R. Bovine chymotrypsinogen A X-ray crystal structure analysis and refinement of a new crystal form at 1.8 A resolution. J Mol Biol. 1985 Oct 5;185(3):595–624. doi: 10.1016/0022-2836(85)90074-9. [DOI] [PubMed] [Google Scholar]
- Wicker C., Puigserver A. Further studies on subunit III of bovine procarboxypeptidase A. Structure and reactivity of the weakly functional active site. FEBS Lett. 1981 Jun 1;128(1):13–16. doi: 10.1016/0014-5793(81)81067-8. [DOI] [PubMed] [Google Scholar]