Abstract
Background
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) combined with solid-organ transplantation is a feasible method to achieve long-lasting organ allograft tolerance through the induction of hematopoietic chimerism in recipients. However, the allo-HSCT engraftment puts recipients at risk of life-threatening graft-versus-host disease (GVHD). Novel immunomodulatory approaches are required to effectively control GVHD while preserving the status of hematopoietic chimerism. We have reported that histone methylation inhibitor 3-deazaneplanocin A (DZNep) can control ongoing GVHD in mice by selectively inducing apoptosis of alloreactive effector T cells.
Methods
Using donor-derived CD8+ T cell-mediated mouse GVHD model, we further investigated the effect of in vivo administration of DZNep on allogeneic CD8+ T cell response and the hematopoietic chimerism in recipients.
Results
We found that DZNep delayed the in vivo proliferation of donor-derived alloreactive CD8+ T cells and also reduced the interleukin-2 production by these T cells. Moreover, DZNep treatment resulted in a significant decrease of interferon-γ, tumor necrosis factor-α, granzyme B, TRAIL, and Fas ligand expressing donor-derived CD8+ T cells, suggesting a multilevel modulation role on T-cell survival and effect in vivo. Notably, DZNep treatment did not hamper the generation of hematopoietic chimerism in recipients.
Conclusions
These findings suggest that modulation of histone methylation through DZNep may be a potential strategy for the induction of hematopoietic chimerism to achieve donor-specific organ allograft tolerance through donor allo-HSCT combined with solid-organ transplantation.
Keywords: Immune response, DZNep, GVHD, Hematopoietic chimerism
With the help of new and superactive immunosuppressants, short-term survival rates of organ allograft have increased progressively, but long-term success remains less satisfactory (1). Lifelong immunosuppression is associated with severe side effects, such as diabetes, cardiovascular diseases, cancer, infection, and allograft toxicity (2). Besides these, current immunosuppressants cannot prevent chronic allograft rejection, thereby limiting long-term allograft survival (3–5). The development of immune tolerance to organ allografts has long been considered the ideal for eliminating the side effects of immunosuppressants and for preventing long-term allograft loss due to chronic rejection and/or allograft toxicity of these drugs.
One strategy for inducing organ allograft tolerance is combining organ transplantation and donor-derived allogeneic hematopoietic stem cell transplantation (allo-HSCT). Some clinical trials have also shown that the infusion of donor hematopoietic stem cells is efficient to induce organ allograft tolerance (6–13). The hematopoietic chimeric recipient deletes both self-reactive and alloreactive T cells and thereby becomes tolerant to donor organ allograft. In allo-HSCT, transfusion of donor T cells in recipients are critical for engraftment of donor HSC and reconstitution of T-cell immunity in the host (14). However, donor T cells infusion often leads to donor T-cell reactivity to alloantigens in normal host tissue and induces broadly attack of recipients’ tissues in a process called graft-versus-host disease (GVHD), which is a major cause of morbidity and mortality after allo-HSCT (15–17). Some degree of GVHD is considered acceptable in patients with malignancies because of the associated beneficial graft-versus-leukemia effects (18), but this complication is unacceptable in patients without malignant disease, who receive HSCT expressly to induce organ allograft tolerance through mixed chimerism. Achievement of mixed chimerism in recipients without GVHD is going to make HSCT more safe and effective in inducing clinical organ allograft tolerance (11, 13).
3-Deazaneplanocin A (DZNep) is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation (19). Importantly, DZNep seems to be a unique chromatin remodeling compound that can deplete the expression level of PRC2 and inhibit the associated histone methylation through selectively inhibiting H3K27me3 and H4K20me3 (20). Target gene changes induced by DZNep were not heritable and that the cell can return to its “ground” state within 24 hr after drug removal (19). Our previous research has demonstrated that DZNep caused selective apoptosis in alloantigen-activated T cells and arrest of ongoing GVHD. This effect was associated with the ability of DZNep to selectively reduce trimethylation of histone H3K27me3, deplete the histone methyltransferase Ezh2 specific to trimethylation of histone H3K27me3, and activate proapoptotic gene Bim repressed by Ezh2 in antigenic-activated T cells. Importantly, inhibition of histone methylation by DZNep in vivo preserved the antileukemia activity of donor T cells leading to significantly improved survival of recipients after allo-HSCT (21).
In this present study, we demonstrated that DZNep can inhibit the proliferation of alloreactive donor-derived CD8+ T cells and decrease the expression of tumor necrosis factor (TNF)-α and interleukin (IL)-2 on early alloreactive donor-derived CD8+ T cells. DZNep treatment also significantly decreased the absolute number but not the frequency of donor-derived CD8+ T cells producing interferon (IFN)-γ, granzyme B, TNF-related apoptosis-inducing ligand (TRAIL), and Fas ligand (FasL). Notably, DZNep treatment did not hamper the achievement of hematopoietic chimerism and hematopoietic reconstitution in recipients. These findings indicate that modulation of histone methylation through DZNep may take us to an important research direction and develop a new strategy for HSCT to induce organ allograft tolerance through mixed chimerism.
RESULTS
DZNep Inhibits CD8+ T-Cell–Mediated GVHD
We tested the impact of in vivo DZNep administration on donor CD8+ T-cell–dependent major histocompatibility complex–identical but minor histocompatibility antigens–mismatched GVHD model (Fig. 1). Male C57BL/6 (B6) mice were lethally irradiated at least 6 hr before allo-HSCT followed by the infusion of C3H.SW (C3H) T-cell–depleted bone marrow (TCD-BM) alone or C3H TCD-BM plus CD44lowCD8+ T cells. As we expected, compared with phosphate-buffered saline (PBS)–treated control mice, DZNep-treated mice showed significantly attenuated GVHD development with improved survival rate (Fig. 1A), body weight loss (Fig. 1B), and also the clinical score of GVHD signs (Fig. 1C). Histologic examination further showed that DZNep treatment reduced the inflammatory infiltration in liver, intestine, and skin of recipients and also relieved tissue damage for those typical GVHD target organs (Fig. 1D). These data indicate that in vivo DZNep treatment can inhibit the development of CD8+ T-cell–mediated acute GVHD.
FIGURE 1.
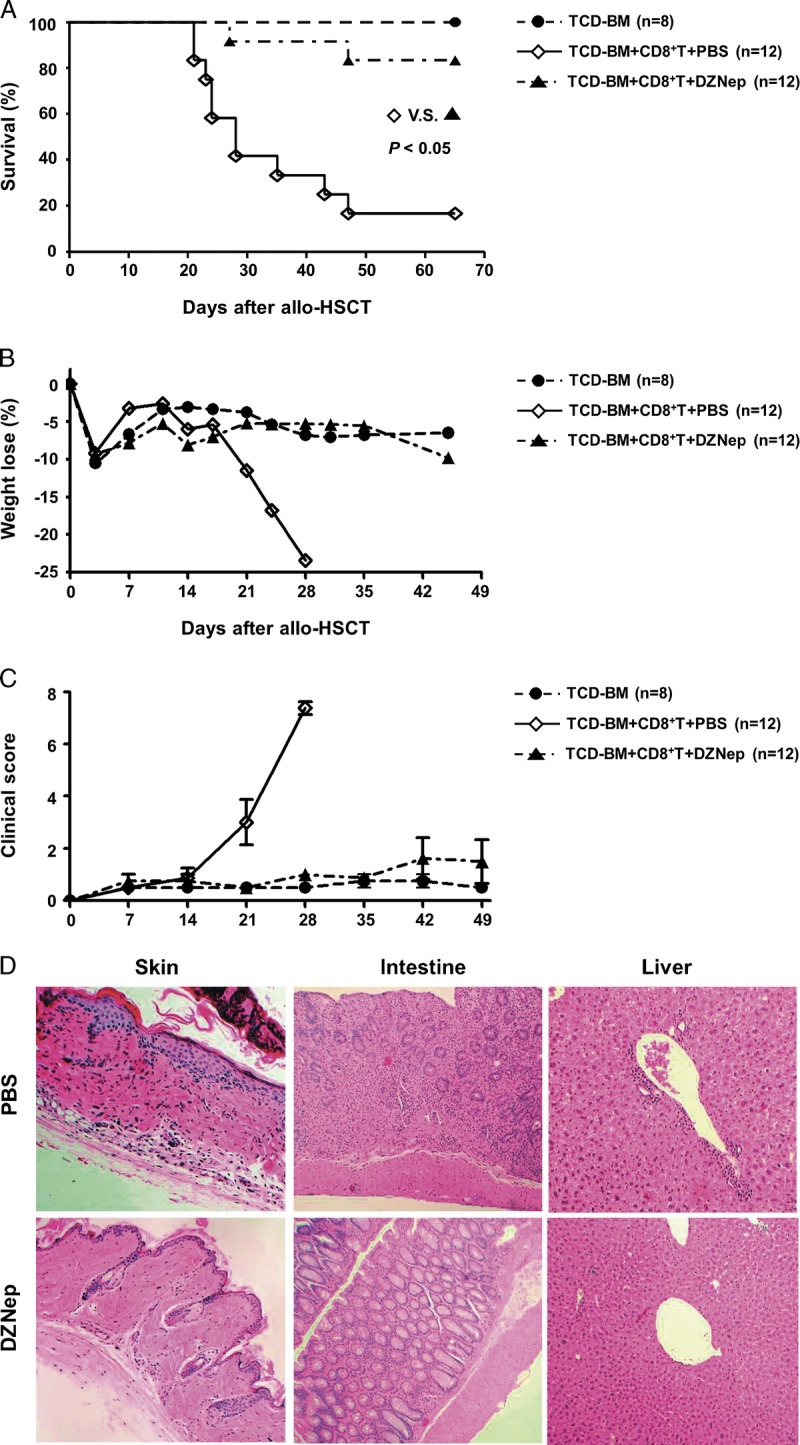
DZNep controls donor CD8+ T-cell–mediated GVHD. Lethally irradiated B6 recipients (1000 rads) were transplanted intravenously with TCD-BM (6.0×106) derived from C3H mice or with TCD-BM+CD44lowCD8+ T cells (1.0×106). Eight doses of DZNep (1 mg/kg; D3, D5, D7, D9, D11, D13, D15, and D17) were injected subcutaneously to recipients (DZNep; n=12; ▲) with PBS treatment as positive control (PBS; n=12; ◊) and TCD-BM transplanted mice as negative control (TCD-BM; n=8; •). Survival data (A), body weight loss (B), and clinical score (C) were monitored over time. Tissues were collected on day 14 (before DZNep treatment) after allo-HSCT for histologic GVHD examination (×100) (D).
DZNep Hampers In vivo Survival of Pathogenic Cytotoxic T Lymphocyte
We then assessed the effects of DZNep on distribution of donor-derived CD8+ T cells in recipients. Thy1.2 was used as the cell marker of donor-derived CD8+ T cells. Compared with control group, DZNep treatment markedly decreased the absolute number of donor-derived CD8+ T cells in the spleen and lymph node on days 8 and 14 after allo-HSCT (Fig. 2A).
FIGURE 2.
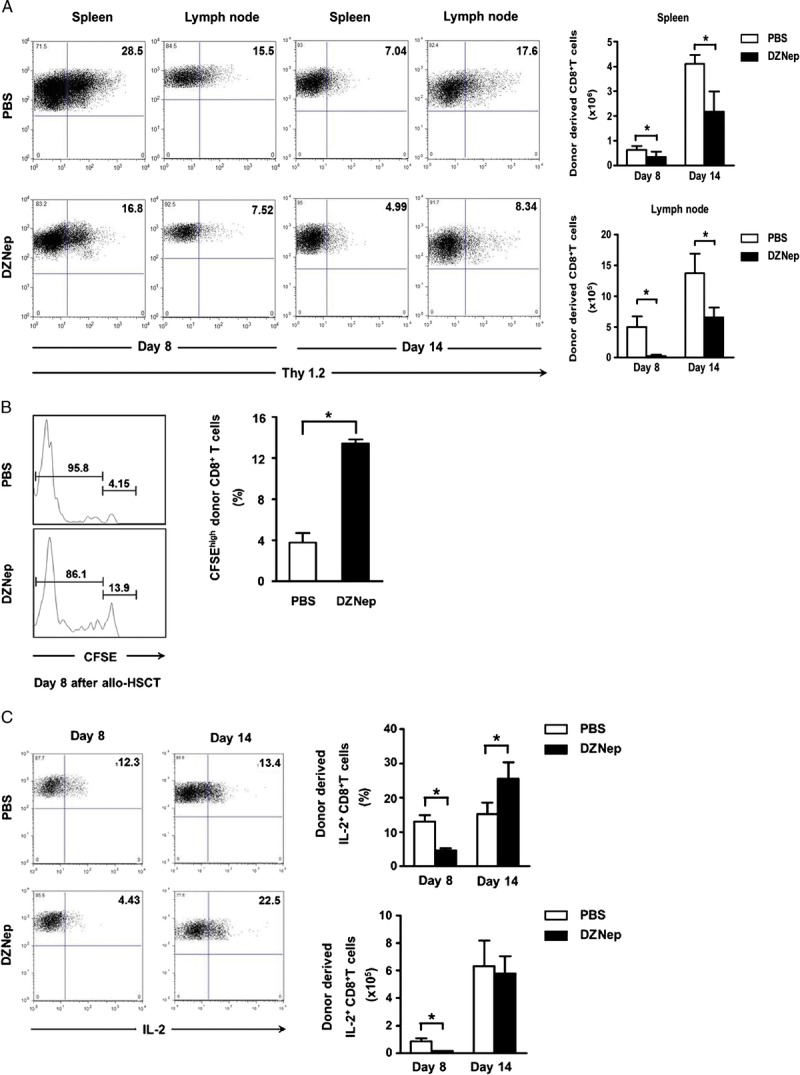
DZNep hampers the in vivo survival of pathogenic CTL. TCD-BM (6.0×106) and CD44lowCD8+ T cells (1.0×106) derived from C3H mice (H2-2b, Thy1.2) were transplanted intravenously into lethally irradiated B6 recipients (H2-2b, Thy1.1). Spleen and lymph node were collected on days 8 and 14 after allo-HSCT. Donor-derived CD8+ T cells were analyzed and calculated based on cell marker Thy1.2. A, mean±SD representative FACS analysis and absolute number of donor-derived CD8+ T cells in spleen and lymph node on day 8 (n=4 for each group) and day 14 (n=4 for each group) after transplantation. Donor-divided and nondivided CD8+ T cells were analyzed based on CFSE and cell marker Thy1.2. B, mean±SD percentage of nondivided donor-derived CD8+ T cells day 8 (n=4 for each group) in spleen after transplantation. Flow cytometry was preformed after intracellular staining of IL-2. C, mean±SD representative FACS analysis, frequency, and absolute cell number of alloreactive IL-2+ CD8+ T cells on days 8 and 14 (n=4 for each group) in spleen after transplantation. PBS (TCD-BM+CD8+ T+PBS; n=4; □); DZNep (TCD-BM+CD8+ T+DZNep; n=4; ▪). *P<0.05.
To investigate the effect of DZNep on proliferation of donor-derived CD8+ T cells after transplantation, carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled donor CD44lowCD8+ T cells were infused into recipient mice. As proliferation of these labeled donor-derived CD8+ T cells, the CFSE intensity in these proliferated cells will decrease gradually. The CFSElowCD8+ T cells and CFSEhighCD8+ T cells indicate the proliferated and nonproliferated donor-derived CD8+ T cells, respectively. Compared with PBS control group, DZNep treatment markedly inhibited the proliferation of donor-derived CD8+ T cells in spleen on day 8 after transplantation (Fig. 2B). Besides the promotion of apoptosis, this may be another reason why DZNep can reduce the absolute number of donor-derived CD8+ T cells in spleen and lymph node.
In our previous reports, we have shown that DZNep can promote the apoptosis of donor alloreactive CD8+ T cells (21), but the effects of DZNep on the proliferation of donor alloreactive CD8+ T cells was still unknown. Here, using CFSE-labeled donor CD8+ T cells, we found that DZNep also delayed the in vivo proliferation of donor-derived CD8+ T cells in response to allogeneic antigen.
IL-2 is one of the most important extrinsic survival factors for T cells. We found that DZNep treatment decreased IL-2 production on the early stage (Fig. 2C), which supported the in vivo T-cell survival inhibition effect by DZNep. It is interesting that no differences in IL-2 production were observed on day 14 after transplantation, which may represent that the effect of DZNep on T-cell survival was occurred mainly on the early stage, but not the whole disease course of GVHD, which may be beneficial to graft-versus-leukemia effect and anti-infection after transplantation. Taken together, all these data suggest that DZNep can hamper the survival of pathogenic cytotoxic T lymphocytes (CTL) during the early stages of GVHD after allo-HSCT.
DZNep Treatment Ameliorates Inflammation in Recipients
Inflammatory environment represents in vivo CTL function and tissue damage, and inflammation controlling is crucial for tolerance maintaining after transplantation. There are two major mechanisms for the proinflammatory effect of CTL, one is direct cytotoxic effect to target cells. Fas/FasL, TRAIL, and granzyme B signal pathway are involved in this mechanism (22, 23). The other way is indirect proinflammatory cytokine production; IFN-γ and TNF-α are two of the most important cytokines for this mechanism (24, 25). In this study, DZNep treatment significantly reduced the numbers of FasL, TRAIL, or granzyme B–expressing CTL, suggesting decreased cytotoxic status in recipients (Fig. 3A). Moreover, both IFN-γ– and TNF-α–expressing CTL were also down-regulated by DZNep (Fig. 3B). All these data indicate ameliorated inflammatory environment in recipients after DZNep treatment, which would benefit the maintaining of immune tolerance after allo-HSCT.
FIGURE 3.
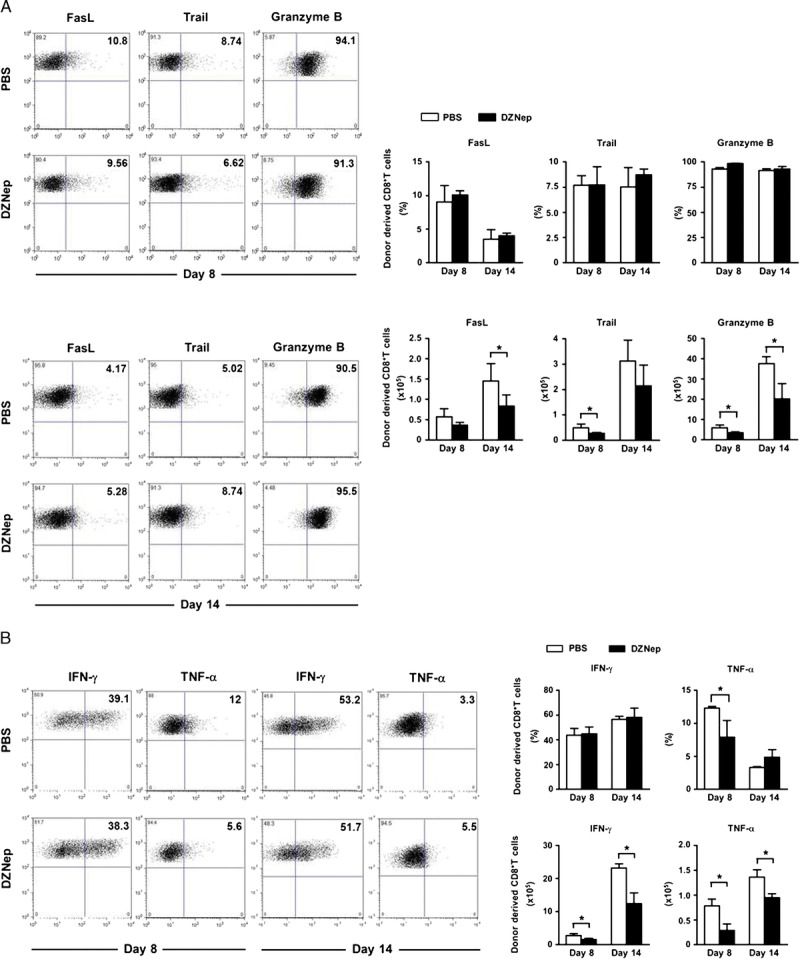
DZNep treatment ameliorates inflammation in recipients. Lethally irradiated B6 recipients were transplanted intravenously with TCD-BM (6.0×106) and CD44lowCD8+ T cells (1.0×106) derived from C3H mice. Spleen was collected on days 8 and 14 after allo-HSCT. Flow cytometry was preformed after intracellular staining of FasL, TRAIL, granzyme B, IFN-γ, and TNF-α. Mean±SD frequency and absolute cell number of alloreactive CD8+ T cells on days 8 and 14 (n=4 for each group) after transplantation. A, representative FACS analysis, frequency, and absolute number of donor-derived CD8+ T cells producing FasL, TRAIL, and granzyme B. B, representative FACS analysis, frequency, and absolute number of donor-derived CD8+ T cells producing IFN-γ and TNF-α. PBS (TCD-BM+CD8+ T+PBS; n=4; □); DZNep (TCD-BM+CD8+ T+DZNep; n=4; ▪). *P<0.05.
Effect of DZNep on Generation of Hematopoietic Chimerism
The existence of hematopoietic chimerism is crucial for the induction of specific tolerance of organ allograft from donor. Because chimerism in thymus is important for the tolerance induction (11, 13), we checked donor-derived CD4+CD8+ T cells in recipient’s thymus on day 14 after transplantation. Thy1.2 and Thy1.1 was used as the phenotype marker of donor and recipient mice, respectively. We found that both donor- and recipient-derived CD4+CD8+ T cells coexist in recipient’s thymus (P<0.05) (Fig. 4A) on day 14. Compared with PBS group, DZNep treatment did not hamper the ratio of donor-derived CD4+CD8+ T cells in the thymus (Fig. 4B, C). These data indicate that DZNep treatment can preserve normal generation of hematopoietic chimerism in recipients.
FIGURE 4.
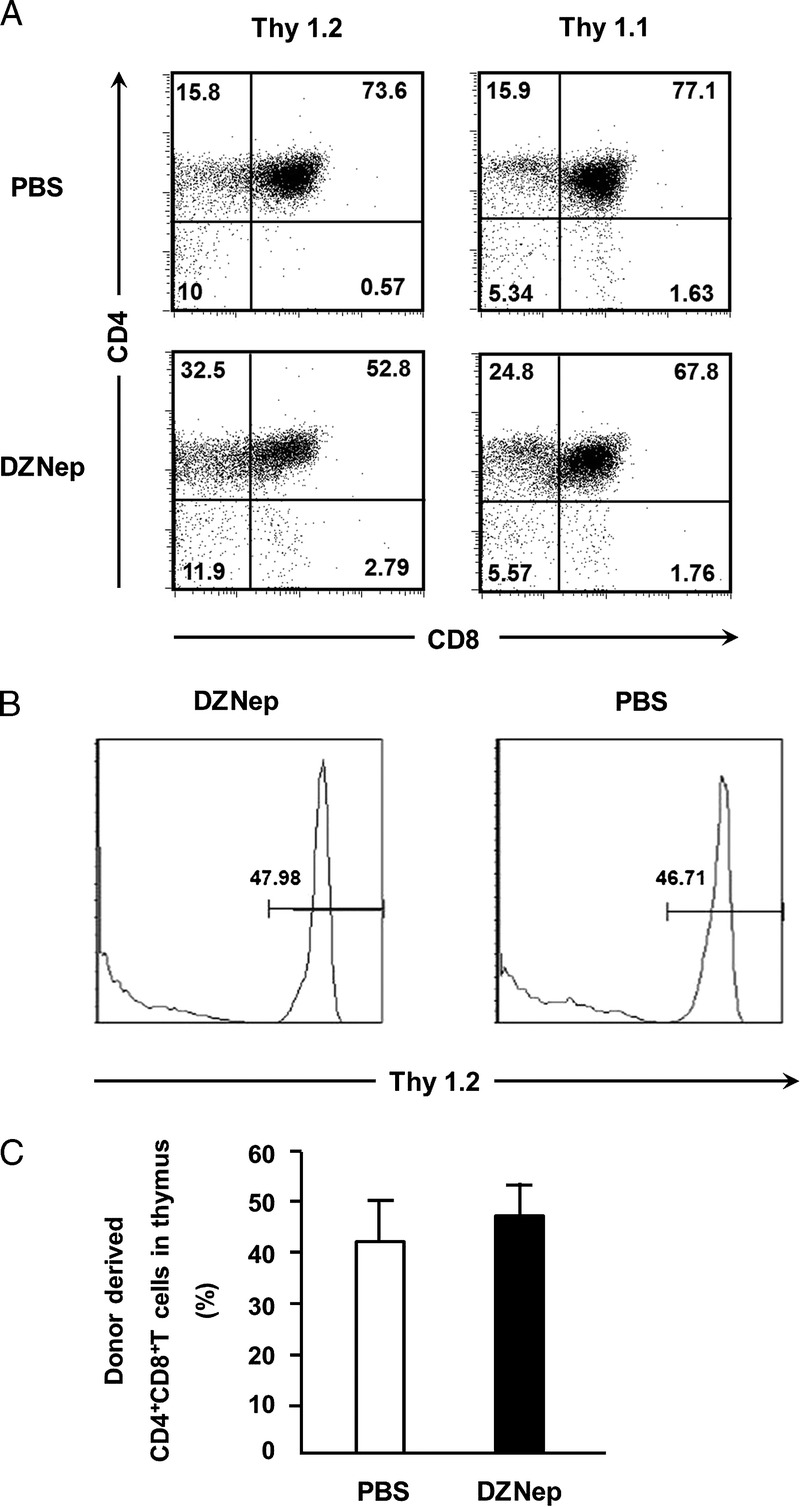
Chimerism status in the thymus of recipient mice. Irradiated B6 recipients (H-2b, Thy1.1) were transplanted intravenously with TCD-BM (6.0×106) and CD44lowCD8+ T cells (1.0×106) derived from C3H mice (H-2b, Thy1.2). Recipient’s thymus was collected on day 14 after allo-HSCT. Donor- and recipient-derived CD4+CD8+ T cells were analyzed based on Thy1.2 and Thy1.1, respectively. Mean±SD ratio of donor-derived CD4+CD8+ T cells in thymus on day 14 (n=4 for each group) after transplantation. A, representative FACS analysis demonstrating coexist of donor (Thy1.2)–derived and recipient (Thy1.1)–derived CD4+CD8+ T cells in thymus. B and C, ratio of donor-derived CD4+CD8+ T cells in thymus. PBS (TCD-BM+CD8+ T+PBS; n=4; □), DZNep (TCD-BM+CD8+ T+DZNep; n=4; ▪). *P<0.05.
DISCUSSION
The concept of mixed chimerism—a state wherein hematopoietic cells of both host and donor coexist in the host for the rest of the live—can be established after HSCT (11, 13). Induction of organ allograft tolerance through mixed hematopoietic chimerism also has a long history that is presented in detail elsewhere (26). GVHD, which is frequently a severe complication of allo-HSCT, would likewise be unacceptable for ordinary clinical organ allograft tolerance induction. Therefore, we have attempted to design preparative regimens that can avoid GVHD while preserving mixed chimerism.
To date, strategies to prevent and control GVHD have been limited (24). Many studies have reported that continual generation of pathogenic host-reactive effector T cells from donors is associated with the persistence and progression of GVHD (27). In our previous study, we demonstrated that inhibition of histone methylation by DZNep could control ongoing GVHD in mice models of allo-HSCT through promoting apoptosis in alloreactive effector T cells in vivo (21). Based on our previous study, we focused on the effect of DZNep on alloreactive CD8+ T cells response in this study, especially on the function of alloreactive CD8+ T cells. We found that DZNep inhibition of histone methylation can also drastically reduce the proliferation of alloreactive CD8+ T cells and infiltration of inflammatory cells in GVHD target organs of mice after HSCT. Due to the inhibited apoptosis and proliferation of alloreactive CD8+ T cells, the absolute number of alloreactive CD8+ T cells was reduced in spleen and lymph nodes. Besides these, DZNep treatment can reduce the expression of TNF-α and IL-2 on single-cell level of alloreactive CD8+ T cells. Although DZNep did not inhibit the expression of FasL, TRAIL, granzyme B, and IFN-γ on alloreactive CD8+ T cells in spleen, the reduced absolute number of alloreactive CD8+ T cells in spleen also led to attenuated pathogenic function of these T cells. This may be one of the reasons why DZNep treatment can control GVHD after allo-HSCT.
As soon as hematopoietic chimerism is established, transplanted organs derived from the HSC donor usually survive with less or no immunosuppressive medication of the host (11, 13). The basic principle of tolerance through mixed chimerism relies on educating the hematopoietic repertoire in central organs to become tolerant toward donor-antigen like it is tolerant to self-antigen. In the experimental setting, the key mechanism of this tolerance strategy is intrathymic deletion of newly developing donor-reactive T cells, one of the major mechanisms by which also self-tolerance is maintained (28). Coexistence of donor and recipient cells in the recipient thymus environment is needed to assure the lifelong maintain of central, deletional T-cell tolerance (28, 29). We checked the mixed chimerism in recipients mice and found that both donor- and recipient-derived CD4+CD8+ T cells exist in DZNep-treated recipient’s thymus. DZNep treatment preserved the normal achievement of mixed hematopoietic chimerism in recipients, which makes it possible to tolerate the organ allograft form donor without immunosuppressants.
DZNep may be not a simple traditional immunosuppressive agent. First, we found that in vivo administration of DZNep did not impair the induction of effector T cells during graft-versus-leukemia reaction and the survival of T cells stimulated by cytokines (21). The expression of genes in DZNep-treated cells could return to the original levels within 24 hr after removal of the inhibitor (19). Second, DZNep has broad and potent antiviral activity, including against vesicular stomatitis virus, rotavirus, and vaccinia virus (30, 31). In particular, this antiviral spectrum of DZNep extends to human cytomegalovirus (31), which causes serious infection in patients after organ transplantation (32). Third, DZNep has been investigated as an effective therapy in mice model target on PRC2 to treat the breast cancer (20, 33), acute myeloid leukemia (34), glioblastoma (35), and so on. These observations suggest that DZNep should not be an traditional immunosuppressive agent, and modulation of histone methylation by DZNep could have translational potential for treating some pathogenic T-cell–mediated inflammatory diseases.
In summary, we have demonstrated that histone methylation inhibitor DZNep can preserve mixed hematopoietic chimerism in recipients without CD8+ T-cell–mediated GVHD. Besides the selective apoptosis of alloreactive effector T cells (21), this effect was also associated with inhibited proliferation and attenuated pathogenic function of alloreactive CD8+ T cells. Our findings may provide a new experimental approach for inducing solid-organ allograft tolerance through allo-HSCT by modulating histone methylation. Given its powerful effects on reducing allogeneic T-cell responses, DZNep may also be used for controlling other T-cell–mediated inflammatory conditions, such as allograft rejection after solid-organ transplantation.
MATERIALS AND METHODS
Antibodies and Reagents
The following antibodies were used: mouse anti-CD3, CD8, CD4, IFN-γ, IL-2, TNF-α, TRAIL, FasL, granzyme B, Thy1.1, and Thy1.2. All antibodies were obtained from eBiosciences (San Diego, CA), BioLegend (San Diego, CA), or BD Biosciences (San Jose, CA). Fluorescent conjugates were fluorescein isothiocyanate, phycoerythrin (PE), PE-cyanin7, allophycocyanin, allophycocyanin-Cy7, peridinin-chlorophyll-cyanin5.5, or PE-Texas red. Biotinylated antibodies were revealed with streptavidin conjugated to PE-cyanin7, peridinin-chlorophyll-cyanin5.5, or PE-Texas red. For ex vivo restimulation, we used plate-bound anti-CD3 (clone 145-2C11) and anti-CD28 (clone 37.51) (Biolegend, San Diego, CA; 2.5 μg/mL each). CFSE was obtained from Molecular Probes (Eugene, OR). Cells were labeled with CFSE at a final concentration of 7.5 μmol/L in 2.5% PBS–fetal bovine serum at 37°C for 13 min. DZNep was bought from the Cayman Chemical (Ann Arbor, MI).
Mice and Induction of GVHD
Female C3H (H-2b, Thy1.2) and male B6 (H-2b, Thy1.1) mice were obtained from Shanghai Laboratory Animal Commission (Shanghai, China). Mice were used between 8 and 12 weeks old. Age- and sex-matched littermate mice were used as controls for all experiments. GVHD model was induced as described previously (36). The severity of GVHD was assessed with a clinical GVHD scoring system, as described previously (37). Experimental protocols were approved by Committee of Zhongshan Hospital, Fudan University on Use and Care of Animals.
Flow Cytometric Analysis
After blocking unspecific binding with unlabeled rat and mouse IgG (Sigma, St. Louis, MO), cells were stained in fluorescence-activated cell sorter (FACS) buffer (PBS with 2.5% fetal bovine serum and 0.05% sodium azide) and incubated for 30 min at 4°C with antibodies and washed twice with FACS buffer. BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit with BD GolgiPlug (BD Pharmingen, East Rutherford, NJ) was used for intracellular staining according to the manufacturer’s instructions. Stained cells were resuspended in FACS buffer and analyzed on a FACSCalibur flow cytometer (BD Biosciences) with CellQuest. Files were analyzed with FlowJo software (Tree Star, San Carlos, CA).
Statistical Analysis
Survival data in different groups were analyzed by Kaplan–Meier estimator with log-rank test. All results shown in histograms represent the mean±SD and were analyzed using a two-tailed Student’s t test. Normal distribution analysis was performed before the analysis of Student’s t test. Differences were considered statistically significant if P<0.05.
ACKNOWLEDGMENTS
The authors thank Yi Zhang, Shuaiying Cui, Koji Kato, and Shan He (University of Michigan) for technical guidance in this study and Fang Zhao (Northwest University) for thoughtful discussion and critical reading of the article.
Footnotes
This study was supported by grant from the National Nature Science Foundation of China (81100533, 81100534, and 81070595), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20110071120057), the Science and Technology Commission of Shanghai Municipality (09411952000), and the Shanghai Health Bureau (XBR2011019 and 11JC1402300).
The authors declare no conflicts of interest.
Address correspondence to: Tongyu Zhu, M.D., Ph.D., 180 FengLin Road Shanghai, 200032, China.
E-mail: tyzhu_dr@163.com
J.W. and L.L. contributed equally to this article. J.W. designed and performed the research, analyzed the data, and wrote the article. L.L. performed the research, contributed ideas, and helped write the article. M.X. and R.R. provided guidance and revised the article. T.Z. designed the experiments, provided overall guidance, and helped write the article.
Received 3 April 2013. Revision requested 21 April 2013.
Accepted 20 June 2013.
REFERENCES
- 1. Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004; 4: 378. [DOI] [PubMed] [Google Scholar]
- 2. Pascual M, Theruvath T, Kawai T, et al. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med 2002; 346: 580. [DOI] [PubMed] [Google Scholar]
- 3. Yang H. Maintenance immunosuppression regimens: conversion, minimization, withdrawal, and avoidance. Am J Kidney Dis 2006; 47: S37. [DOI] [PubMed] [Google Scholar]
- 4. Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 2003; 3: 178. [DOI] [PubMed] [Google Scholar]
- 5. Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349: 931. [DOI] [PubMed] [Google Scholar]
- 6. Fudaba Y, Spitzer TR, Shaffer J, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant 2006; 6: 2121. [DOI] [PubMed] [Google Scholar]
- 7. Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008; 358: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med 2008; 358: 362. [DOI] [PubMed] [Google Scholar]
- 9. Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant 2012; 12: 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dave SD, Vanikar A, Trivedi HL, et al. Stem cells versus donor specific transfusions for tolerance induction in living donor renal transplantation: a single-center experience. Transplantation 2013; 95: 155. [DOI] [PubMed] [Google Scholar]
- 11. Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med 2012; 4: 124ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leventhal J, Miller J, Abecassis M, et al. Evolving approaches of hematopoietic stem cell-based therapies to induce tolerance to organ transplants: the long road to tolerance. Clin Pharmacol Ther 2013; 93: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leventhal J, Abecassis M, Miller J, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation 2013; 95: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol Rev 1997; 157: 61. [DOI] [PubMed] [Google Scholar]
- 15. Reddy P, Maeda Y, Liu C, et al. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med 2005; 11: 1244. [DOI] [PubMed] [Google Scholar]
- 16. Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol 2007; 7: 340. [DOI] [PubMed] [Google Scholar]
- 17. Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol 2007; 25: 139. [DOI] [PubMed] [Google Scholar]
- 18. Weiden PL, Sullivan KM, Flournoy N, et al. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med 1981; 304: 1529. [DOI] [PubMed] [Google Scholar]
- 19. Miranda TB, Cortez CC, Yoo CB, et al. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol Cancer Ther 2009; 8: 1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan J, Yang X, Zhuang L, et al. Pharmacologic disruption of polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev 2007; 21: 1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He S, Wang J, Kato K, et al. Inhibition of histone methylation arrests ongoing graft-versus-host disease in mice by selectively inducing apoptosis of alloreactive effector T cells. Blood 2012; 119: 1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chawla-Sarkar M, Lindner DJ, Liu YF, et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003; 8: 237. [DOI] [PubMed] [Google Scholar]
- 23. Kornacker M, Moldenhauer G, Herbst M, et al. Cytokine-induced killer cells against autologous CLL: direct cytotoxic effects and induction of immune accessory molecules by interferon-gamma. Int J Cancer 2006; 119: 1377. [DOI] [PubMed] [Google Scholar]
- 24. Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet 2009. 373: 1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Francoeur C, Escaffit F, Vachon PH, et al. Proinflammatory cytokines TNF-alpha and IFN-gamma alter laminin expression under an apoptosis-independent mechanism in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2004; 287: G592. [DOI] [PubMed] [Google Scholar]
- 26. Sykes M. Mixed chimerism and transplant tolerance. Immunity 2001; 14: 417. [DOI] [PubMed] [Google Scholar]
- 27. Yamashita K, Choi U, Woltz PC, et al. Severe chronic graft-versus-host disease is characterized by a preponderance of CD4(+) effector memory cells relative to central memory cells. Blood 2004; 103: 3986. [DOI] [PubMed] [Google Scholar]
- 28. Manilay JO, Pearson DA, Sergio JJ, et al. Intrathymic deletion of alloreactive T cells in mixed bone marrow chimeras prepared with a nonmyeloablative conditioning regimen. Transplantation 1998; 66: 96. [DOI] [PubMed] [Google Scholar]
- 29. Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Peripheral mechanisms do not contribute to maintenance of tolerance. Transplantation 1996; 62: 380. [DOI] [PubMed] [Google Scholar]
- 30. De Clercq E, Cools M, Balzarini J, et al. Broad-spectrum antiviral activities of neplanocin A, 3-deazaneplanocin A, and their 5′-nor derivatives. Antimicrob Agents Chemother 1989; 33: 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Snoeck R, Andrei G, Neyts J, et al. Inhibitory activity of S-adenosylhomocysteine hydrolase inhibitors against human cytomegalovirus replication. Antiviral Res 1993; 21: 197. [DOI] [PubMed] [Google Scholar]
- 32. Wu CJ, Ritz J. Induction of tumor immunity following allogeneic stem cell transplantation. Adv Immunol 2006; 90: 133. [DOI] [PubMed] [Google Scholar]
- 33. Puppe J, Drost R, Liu X, et al. BRCA1-deficient mammary tumor cells are dependent on EZH2 expression and sensitive to polycomb repressive complex 2-inhibitor 3-deazaneplanocin A. Breast Cancer Res 2009; 11: R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fiskus W, Wang Y, Sreekumar A, et al. Combined epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor panobinostat against human AML cells. Blood 2009; 114: 2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suva ML, Riggi N, Janiszewska M, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res 2009; 69: 9211. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Joe G, Hexner E, et al. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med 2005; 11: 1299. [DOI] [PubMed] [Google Scholar]
- 37. Zhang Y, Louboutin JP, Zhu J, et al. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell-mediated acute graft-versus-host disease. J Clin Invest 2002; 109: 1335. [DOI] [PMC free article] [PubMed] [Google Scholar]


