Background: We studied the role of the mineralocorticoid receptor (MR) for atrial fibrotic remodeling.
Results: Increased 11β-hydroxysteroid dehydrogenase type 2 in atrial fibrillation enhances mineralocorticoid receptor pro-fibrotic signaling through connective tissue growth factor, lysyl oxidase, and microRNA-21.
Conclusion: The MR regulates fibrogenesis in atrial fibrillation.
Significance: The MR may represent a target for the prevention of atrial fibrosis.
Keywords: Fibrosis, Heart, Lysyl Oxidase, MicroRNA, Mineralocorticoid Receptor, CTGF
Abstract
We studied the role of the mineralocorticoid receptor (MR) in the signaling that promotes atrial fibrosis. Left atrial myocardium of patients with atrial fibrillation (AF) exhibited 4-fold increased hydroxyproline content compared with patients in sinus rhythm. Expression of MR was similar, as was 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which also increased. 11β-HSD2 converts cortisol to receptor-inactive metabolites allowing aldosterone occupancy of MR. 11β-HSD2 was up-regulated by arrhythmic pacing in cultured cardiomyocytes and in a mouse model of spontaneous AF (RacET). In cardiomyocytes, aldosterone induced connective tissue growth factor (CTGF) in the absence but not in the presence of cortisol. Hydroxyproline expression was increased in cardiac fibroblasts exposed to conditioned medium from aldosterone-treated cardiomyocytes but not from cardiomyocytes treated with both cortisol and aldosterone. Aldosterone increased connective tissue growth factor and hydroxyproline expression in cardiac fibroblasts, which were prevented by BR-4628, a dihydropyridine-derived selective MR antagonist, and by spironolactone. Aldosterone activated RhoA GTPase. Rho kinase inhibition by Y-27632 prevented CTGF and hydroxyproline, whereas the RhoA activator CN03 increased CTGF expression. Aldosterone and CTGF increased lysyl oxidase, and aldosterone enhanced miR-21 expression. MR antagonists reduced the aldosterone but not the CTGF effect. In conclusion, MR signaling promoted fibrotic remodeling. Increased expression of 11β-HSD2 during AF leads to up-regulation of collagen and pro-fibrotic mediators by aldosterone, specifically RhoA activity as well as CTGF, lysyl oxidase, and microRNA-21 expression. The MR antagonists BR-4628 and spironolactone prevent these alterations. MR inhibition may, therefore, represent a potential pharmacologic target for the prevention of fibrotic remodeling of the atrial myocardium.
Introduction
Atrial fibrillation (AF)2 is the most common arrhythmia. The prevalence is increasing, especially in the elderly (1). AF is a major cause of thromboembolic stroke and cardiovascular morbidity (1). Atrial enlargement and fibrosis are predisposing factors for the development of AF. On the other hand, AF contributes to structural changes and fibrosis in the left atrium (2). Fibrosis is a hallmark of structural remodeling in left atria of AF patients. Interstitial fibrosis perturbs cardiac electrophysiological properties and promotes the formation micro-reentry circles leading to AF (2). Despite substantial progress in the field of structural and electrical remodeling, additional understanding of the underlying molecular pathways is needed to identify pharmacologic targets to prevent AF (3, 4).
The activation of the mineralocorticoid aldosterone leads to left ventricular remodeling (5–9). Clinical trials show beneficial effects of mineralocorticoid receptor (MR) antagonist in patients with systolic heart failure (10–12). There is evidence that MR antagonism may reduce the incidence of AF in heart failure patients (13). However it is currently unknown whether improvements in the hemodynamic situation or direct atrial effects of MR antagonist treatment are responsible for the beneficial effects. Recently, aldosterone was found to be able to induce AF and fibrosis (14). Aldosterone passes the cell membrane and binds at and activates the intracellular localized MR (6). Activation of the MR is regulated by the 11-β-hydroxysteroid dehydrogenase 2 (11β-HSD2), which oxidizes the glucocorticoid cortisol to the inactive metabolite cortisone, thereby preventing cortisol occupancy at the MR. The concentration of cortisol in the human serum is 100–1000-fold higher compared with aldosterone, but its binding affinity to the mineralocorticoid receptor is similar. Therefore, cortisol mainly occupies the MR binding site in cells that do not express 11β-HSD2, and 11β-HSD2 expression prevents illicit MR binding by cortisol (15).
We have recently observed that atrial fibrillation is associated with structural remodeling, including overexpression of connective tissue growth factor (CTGF), lysyl oxidase (LOX), and micro-RNA-21 (16–18), but the role of the MR in this context is unknown. Therefore, the aim of this study was to investigate the role of the MR in the pathways that promote structural remodeling and fibrosis in the left atrial myocardium during atrial fibrillation.
EXPERIMENTAL PROCEDURES
Human Left Atrial Tissue
Tissue samples of the left atrial appendage of patients undergoing mitral valve surgery were analyzed in patients with sinus rhythm (SR) and with permanent AF (documented by ECG for >3 months). The samples were matched for atrial diameter (see Table 1). The patients did not receive drugs at least 12 h before surgery. The analysis was approved by the ethics committee of the Ärztekammer des Saarlandes (no. 131/00).
TABLE 1.
Characteristics of patients who underwent mitral valve cardiac surgery
ACE, angiotensin converting enzyme; AT1, angiotensin II receptor type 1; CAD, coronary artery disease; FS, fractional shortening; IVSd, interventricular septum diameter; LVEDd, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESd, left ventricular end-systolic diameter; LVPWd, left ventricular posterior wall diameter; receptor; S.E. = standard error of the mean; SR = sinus rhythm.
| Parameter | SR | S.E. | AF | S.E. | p Value |
|---|---|---|---|---|---|
| n | 9 | 21 | |||
| Male | 5 | 14 | ns | ||
| Age, years | 63.0 | 4.7 | 70.0 | 2.0 | ns |
| Height, cm | 166.9 | 3.1 | 176.1 | 1.45 | <0.05 |
| Weight, kg | 79.4 | 3.7 | 84.6 | 2.74 | ns |
| LA, mm | 51.8 | 1.8 | 52.9 | 1.98 | ns |
| LVEF, % | 58.7 | 5.1 | 63.7 | 2.82 | ns |
| LVEDd, mm | 55.0 | 1.8 | 54.6 | 1.76 | ns |
| LVESd, mm | 39.0 | 1.9 | 38.6 | 1.41 | ns |
| FS, % | 30.7 | 1.5 | 29.8 | 1.07 | ns |
| IVSd, mm | 11.7 | 0.4 | 12.6 | 0.28 | ns |
| LVPWd, mm | 11.4 | 0.5 | 11.7 | 0.39 | ns |
| Beta blocker | 6 (67) | 16 (76) | ns | ||
| ACE-/AT1-antagonist | 9 (100) | 14 (67) | ns | ||
| MR antagonist | 2 (22) | 3 (14) | ns | ||
| Statin | 1 (11) | 4 (19) | ns | ||
| Diuretic | 7 (78) | 16 (76) | ns | ||
| CAD | 3 (33) | 10 (48) | ns | ||
| Hypertension | 6 (67) | 18 (86) | ns | ||
| Smoking | 1 (11) | 7 (33) | ns | ||
| Diabetes | 0 (0) | 2 (10) | ns |
Cell Isolation and Culture
Neonatal rat cardiomyocytes and cardiac fibroblasts were isolated from 5-day-old neonatal Sprague-Dawley rat hearts (Charles River, Germany) of mixed sex as described (19, 20). After 48 h in culture, cardiomyocytes exhibited regular spontaneous contractions and were used for experiments after 3–5 days of culture. Fibroblasts were separated by adhesion, and purity of fibroblasts was confirmed by vimentin staining. Neonatal cardiac fibroblasts were grown in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal calf serum, gentamicin (0.08 mg/ml), and penicillin (100 IU/ml). When subconfluent in passage three, cells were kept in serum-free medium for 24 h before treatment. Cellular protein content was quantified by a modified Lowry assay.
Electric Stimulation of Neonatal Rat Cardiomyocytes
Cellular viability of cardiomyocytes was determined by cell counting, morphology, and trypan blue exclusion. Cardiomyocytes were plated in 6-well culture trays (BD Falcon 6-well plate 353846, BD Biosciences) at 0.5–0.6 × 106 per well (∼9 cm2) containing F-10 medium (Invitrogen) supplemented with 10% horse serum, 5% fetal calf serum, 1% penicillin, and streptomycin and maintained in 95% air and 5% CO2 at 37 °C. After 48 h of culture, ∼70% of the cells adhered to the culture dishes and began to oscillate (beat spontaneously). Thereafter, the culture medium was changed daily. On day 4, most cardiomyocytes beat synchronously and at a constant frequency. Thereafter cardiomyocytes were stimulated with an electronic multichannel cell stimulator (Cell Culture EP Stimulator, IonOptix Ltd) via carbon electrodes (C-Dish CLD6WBFC, IonOptix) at a frequency of 3 Hz, either regular or irregular. The irregular pacing was produced by a pseudo-random variation of the specified frequency within a 50% window. For example, if a frequency of 1 Hz with 50% variability is selected, 100 pulses will have occurred after 100 s, but the time period between any two pulses can be anything from 500 to 1500 ms. An equal number of electrical stimulations per min were applied either rhythmically or arrhythmically. Therefore, this model specifically addresses arrhythmia- rather than tachycardia-mediated cellular alterations. The duration of each pulse was 2–3 ms, and the strength of the electrical field was <7 V/cm, which is well below that reported to cause cell injury (21). Effective contraction by electric stimulation was checked by optical microscopy at 20× magnification at the beginning and the end of each stimulation. To minimize electrolysis at the electrodes and a possible generation of oxidative species, the polarity of the electrodes was altered with each electrical pulse. After use, the electrodes were cleaned in distilled water with changing the water every 6–12 h for at least 4 times and till the water was absolutely clear. Directly before use electrodes were soaked into 70% ethanol for 1 h for disinfection. After electric stimulation cells were washed with 2 ml of PBS, lysed with 200 μl of ice-cold lysis buffer, scraped, and homogenized with 5 min of ultrasound. After homogenization, debris was removed by centrifugation at 14,000 × g for 15 min at 4 °C, and the supernatant was transferred to Eppendorf cups for storage at −20 °C. Total protein concentration was quantified by a modified Lowry assay for Western blot analysis.
Animal Studies
Mice with cardiac overexpression of constitutively active (V12) Rac1 under the control of the α-myosin heavy chain promoter (RacET; provided by Prof. Mark A. Sussman, San Diego State University Heart Institute and Department of Biology, San Diego State University, San Diego, CA) and their wild-type (WT) controls (22) were fed with normal chow (ssniff, Germany). RacET mice show spontaneous or inducible atrial tachyarrhythmia in 45% as well as atrial conduction abnormalities at the age of 6 months (23). At a high age (16 months) they develop permanent AF spontaneously (24). The study was approved by the Animal Ethics Committee of the Universität des Saarlandes and is in accord with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (no. 85–23, revised 1996).
Specification of Drugs
Aldosterone and TGF-β1 (both Sigma) were used as endogenous mediators of fibrosis. Aldosterone is a physiological mineralocorticoid that mediates its cellular effects via binding at the MR but also possesses non-MR effects. Cortisol (synonym, hydrocortisone; Rotexmedica GmbH) is an endogenous glucocorticoid that binds at the glucocorticoid and the mineralocorticoid receptor. To investigate the competitive binding between aldosterone and cortisol at the MR, we used either a physiological concentration of cortisol (127 ng/ml) (25) or a 100-fold higher concentration (12.7 μg/ml) for mechanistic studies, as indicated in the figure legends. TGF-β was used at the concentration of 5 ng/ml that was shown to stimulate the expression of CTGF and cardiac fibrosis (26). The MR antagonist spironolactone (Sigma) is a widely used therapeutic drug with well characterized clinical and biochemical properties. BR-4628 (Bayer Pharma AG) is a new MR antagonist derived from the chemical structure of dihydropyridines with a advanced specificity for the MR (27). BR-4628 and spironolactone exhibit a similar IC50 at the MR and were, therefore, used in the same concentrations (500 nm). This concentration is based on pharmacological studies to inhibit the MR activation by aldosterone binding (27). CN03 (Cytoskeleton) is a RhoA activator with an active site based on the catalytic domain of the bacterial cytotoxic necrotizing factor toxins. CN03 activates Rho GTPase isoforms by deamidating glutamine-63. This modification converts glutamine 63 to glutamate that blocks GTPase activity, resulting in constitutively active Rho (28). We used concentrations following the manufacturer's datasheet (1 μg/ml) to achieve a constitutive active RhoA. The Rho kinase inhibitor Y-27632 was used at a concentration of 10 μm that has been shown to down-regulate the Rho-dependent protein kinase (29). The concentration of CTGF was established in previous studies to induce pro-fibrotic changes in cardiac fibroblasts (16, 18).
Western Blot Analysis
Total protein lysates were prepared as described (19). Immunoblotting was performed using anti-MR (sc-11412, Santa Cruz), SPARC (sc-25574, Santa Cruz), CTGF (sc-14939, Santa Cruz), LOX (ab31238, Abcam), Sprouty-1 (ab75492, Abcam), 11β-HSD2 (sc-365529, Santa Cruz), RhoA (sc-179, Santa Cruz), tubulin (sc-9104, Santa Cruz), and GAPDH (sc-32233, Santa Cruz). Immunodetection was accomplished using goat anti-rabbit, goat anti-mouse, or rabbit anti-goat secondary antibody (1:4000 dilution, Sigma), and an enhanced chemiluminescence kit (Amersham Biosciences) followed by densitometry.
Rho (Rhotekin) Pulldown Assay
Cells were harvested in magnesium-containing lysis buffer (125 mmol/liter HEPES, pH 7.5, 750 mmol/liter sodium chloride; 5% Igepal; 10% glycerol; 25 mmol/liter sodium fluoride; 50 mmol/liter magnesium chloride; 5 mmol/liter EDTA; 1 mmol/liter sodium orthonvanadate; 10 μg/ml leupeptin; 10 μg/ml aprotinin) and centrifuged at 1 rpm for 5 min at room temperature. Equal amounts of supernatant protein were incubated with 20 μl of agarose labeled Rho Assay Reagent Rhotekin RGB (#14-383, Upstate Biotechnology) at 4 °C overnight. Beads were washed 3 times with magnesium-containing lysis buffer, eluted in Laemmli buffer (125 mmol/liter Tris, pH 6.8, 4% sodium dodecyl sulfate, 10% β-mercaptoethanol, 20% glycerol, 0.002% bromphenol blue), and analyzed for active Rho in relation to total protein content by Lowry assay.
Micro-RNA Isolation and TaqMan-PCR
MicroRNAs were isolated by a miRNA isolation kit (mirVana, Ambion). For TaqMan-PCR, a target-specific stem loop structure and reverse transcription primers were used. After reverse transcription miR-21 expression was quantified with specific TaqMan hybridization probes (TaqMan miR-21 microRNA assay, Applied Biosystems). The small RNA molecule U6 small nuclear (Rnu6-2) was amplified as a control. The 11β-HSD2 mRNA expression was measured using the TaqMan probe Rn-00492539-m1 from Applied Biosystems (30). The 18 S rRNA was amplified as the control.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA isolation, reverse transcription, and competitive PCR were performed according to standard techniques. The following primers were used: human MR forward, 5′-AGAACCCTCGGTCAACACAG-3′; human MR reverse, 5′-CAGCTCAAGGCAAATGATGA-3′; rat MR forward, 5′-GGATTGGTGCTCAAGGTACAA-3′; rat MR reverse, 5′-GATAGTTGTGTTGCCCTTCCA-3′; rat CTGF forward, 5′-AGAGTGGAGATGCCAGGAGA-3′; rat CTGF reverse, 5′-CACACACCCAGCTCTTGCTA-3′; rat LOX forward, 5′-TGGATATGGCACCGGTTACT-3′; rat LOX reverse, 5′-CCATGCTGTGGTAATGTTGG-3′; rat GAPDH forward, 5′-AGACAGCCGCATCTTCTTGT-3′; rat GAPDH, reverse 5′-CTTGCCGTGGGTAGAGTCAT-3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as the external standard. Each PCR cycle consisted of denaturing at 95 °C for 45 s, annealing at 53 °C, 53 °C, 55 °C, 53 °C, and 63 °C for 30 s for human MR, rat MR, rat CTGF, rat LOX, and GAPDH, respectively, and elongation at 72 °C for 60 s. Equal amounts of corresponding target gene and GAPDH RT-PCR products were loaded on 1.5% agarose gels, and optical densities of ethidium bromide-stained DNA bands were quantified.
Measurement of Hydroxyproline
Hydroxyproline content in left atrial myocardium (LA) and supernatants of cultured cardiac fibroblasts were determined by enzyme-linked immunosorbent assay using a hydroxyproline assay kit (Quickzyme, The Netherlands) according to the manufacturer's instruction.
Histological and Immunofluorescence Analyses
Cryosections (10 μm) were stained with 0.1% Sirius Red F3BA (Polysciences) as described (24). Lucia Measurement Version 4.6 software was used for quantification of interstitial fibrosis (Nikon, Germany). Indirect immunofluorescence was performed on paraffin-embedded sections of the human LA applying monoclonal antibodies against α-sarcomeric actin (cardiomyocytes (Sigma)), SPARC (Santa Cruz), and vimentin (fibroblasts (Dako Cytomation)). Fluorescein isothiocyanate- or tetramethylrhodamine isothiocyanate-conjugated (Dianova) anti-mouse IgM, anti-mouse IgG, or anti-goat IgG were used as secondary antibodies. Sections were counter-stained with 4′,6 diamidino-2-phenylindole (Calbiochem). Immunostaining with fluorescein isothiocyanate- or tetramethylrhodamine isothiocyanate-conjugated anti-mouse IgM, anti-mouse IgG, or anti-goat IgG as a negative control for nonspecific staining was performed in parallel sections.
Statistical Analysis
Band intensities were analyzed by densitometry. Data were calculated from at least three independent experiments and are presented as the mean S.E. Statistical analyses were calculated using Sigma Stat software, Version 2.0. Unpaired Student's t test and in the case of failing normality test, the Mann-Whitney Rank Sum test for single comparison was performed. For multiple comparisons, an analysis of variance followed by Newman-Keuls post-hoc analysis was applied. Differences were considered significant at p < 0.05.
RESULTS
Mineralocorticoid Receptor and 11β-Hydroxysteroid Dehydrogenase Type 2 in Human LA
We investigated samples of LA from patients undergoing mitral valve cardiac surgery who had either permanent atrial fibrillation or sinus rhythm. The characteristics of the patients are displayed in Table 1. Groups did not differ significantly with regard to cardiovascular medications. The samples were matched for atrial diameter. Patients with AF are characterized by increased left atrial fibrosis compared with patients with SR (Sirius red staining; Fig. 1, A and B), and total collagen content was quantitated by a hydroxyproline assay (425 ± 103%, p < 0.05; Fig. 1C).
FIGURE 1.
Fibrosis and the mineralocorticoid receptor in human left atrium. Samples from patients with AF or SR are matched for atrial size. A, representative Sirius red staining of SR and AF patients; 10- and 100-fold magnification. B, quantification of fibrosis by Sirius red staining. C, content of hydroxyproline assessed by hydroxyproline assay in LA. Shown are mRNA expression (RT-PCR) (D) and protein expression (Western blot) (E) of mineralocorticoid receptor in LA of SR and AF patients. F, protein expression of 11β-HSD2 in LA. *, p < 0.05.
The expression of MR mRNA and protein was similar in AF and SR patients (107 ± 13% (p = ns) and 87 ± 9% (p = ns), respectively; Fig. 1, D and E). MR expression did not differ between very large LA (63 ± 2 mm) and large LA (48 ± 3 mm) (p = 0.42, data not shown). Because the MR has similar affinities to bind aldosterone and glucocorticoids, the expression of the cortisol-inactivating enzyme 11β-HSD2 was investigated. LA of AF patients exhibited increased expression of 11β-HSD2 compared with SR patients (416 ± 124%, p < 0.05; Fig. 1F).
Mediators of Fibrosis in Human LA from Patients with AF and SR
SPARC is a collagen-binding matricellular protein that mediates collagen maturation and assembly of the extracellular matrix (31). SPARC expression in human LA was elevated in AF patients (165 ± 23%, p < 0.05; Fig. 2B). There are conflicting data regarding the cell types that express SPARC (32, 33). Immunohistochemical staining revealed that SPARC was localized in cardiac myocytes (α-sarcomeric actin staining) as well as in connective tissue, i.e. extracellular collagen and fibroblasts (vimentin stained) (Fig. 2A). Association studies in human LA revealed that classical mediators and enzymes of fibrotic remodeling correlate with hydroxyproline. Hydroxyproline concentration correlated positively with 11β-HSD2 (r = 0.874, p = 0.0002; Fig. 2C), CTGF (r = 0.800, p = 0.014; Fig. 2D), SPARC (r = 0.75, p = 0.026; Fig. 2E), miR-21 (r = 0.8485, p = 0.0327; Fig. 2F), Sprouty-1 (r = −0.700, p = 0.0364, Fig. 2G), and RhoA (r = 0.7818, p = 0.0105, Fig. 2H).
FIGURE 2.
SPARC expression in human left atria and correlations with mediators of fibrosis. A, immunohistochemical staining for SPARC (TRITC, red) and vimentin (lower panel, green, FITC) or α-sarcomeric actin (upper panel, green, FITC) in LA (10-fold magnification). Both cardiac myocytes (α-sarcomeric actin-positive) and fibroblasts (vimentin-positive) express SPARC. B, quantification of SPARC protein expression in LA. C–H, correlations between hydroxyproline expression in human LA and 11β-HSD2 (C), CTGF (D), SPARC (E), miR-21 (F), Sprouty-1 (G), and RhoA (H). *, p < 0.05.
MR, Hydroxyproline and CTGF Expression in Cultured Cardiac Fibroblasts
MR expression and its role in fibrotic remodeling were studied in cultured neonatal rat cardiac fibroblasts. Cardiac fibroblasts express MR mRNA and protein. Neither aldosterone nor TGF-β treatment altered the basal MR expression (Fig. 3A). We found no protein expression of 11β-HSD2 in cultured cardiac fibroblasts (data not shown).
FIGURE 3.
Expression of hydroxyproline, MR and CTGF in cultured rat cardiac fibroblasts. A, MR mRNA expression in cells treated with aldosterone (Aldo, 10−8 m, 24 h), TGF-β (5 ng/ml, 24 h), or vehicle as control. B, hydroxyproline concentration in supernatants of fibroblasts using hydroxyproline assay. Spirono, spironolactone. C, aldosterone concentration curve (1, 10, and 100 nm, all 24 h) regarding CTGF expression. D, CTGF expression in fibroblasts treated with either with BR-4628, spironolactone, or vehicle as control. Shown are CTGF protein expression in fibroblasts treated with aldosterone (10−8 m, 24 h) and preincubation with BR-4628 (500 nm, 25 h) (E) or spironolactone (500 nm, 25 h) (F). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Aldosterone treatment of cardiac fibroblasts increased hydroxyproline concentration in cellular supernatants (244 ± 46%, p < 0.001; Fig. 3B). This increase was prevented by pretreatment with the MR antagonists BR-4628 and spironolactone (123 ± 16% (p < 0.01) versus aldosterone and 125 ± 12% (p < 0.01) versus aldosterone, respectively; Fig. 3B).
The mineralocorticoid aldosterone increased CTGF protein expression in a concentration-dependent manner (144 ± 11% (p < 0.05), 165 ± 23% (p < 0.01), 183 ± 10% (p < 0.001) for aldosterone 1, 10, and 100, respectively; Fig. 3C). The subsequent experiments were performed with 10 nm aldosterone because this concentration is close to the IC50 of aldosterone (2.0 nm) (34). In contrast, the glucocorticoid cortisol did not significantly alter CTGF expression (120 ± 18%, p = ns; data not shown). The MR antagonists BR-4628 and spironolactone reduced CTGF expression to a similar extent (59 ± 17% (p < 0.05) and 51 ± 15% (p < 0.05) and respectively; Fig. 3D). Aldosterone treatment of cardiac fibroblasts elevated CTGF protein expression (207 ± 34%, p < 0.01; Fig. 3E), and preincubation with BR-4628 or spironolactone completely prevented these aldosterone-induced alterations (95 ± 12% (p < 0.05) versus aldosterone and 99 ± 17% (p < 0.05) versus aldosterone, respectively; Fig. 3, E and F).
The RhoA/Rho Kinase Pathway Regulates Fibrotic Changes Downstream of MR in Cardiac Fibroblasts
Exposure of cardiac fibroblasts to transforming growth factor β (TGF-β) increased CTGF protein expression (180 ± 13%, p < 0.001; Fig. 4A). Preincubation with BR-4628 completely prevented this change (86 ± 9%, p < 0.001 versus aldosterone; Fig. 4A). TGF-β is an activator of the RhoA/Rho kinase pathway (35). Indeed, the specific Rho kinase inhibitor Y-27632 completely prevented the TGF-β induced CTGF overexpression (95 ± 8%, p < 0.05 versus TGF-β; Fig. 4B). Furthermore, treatment of cardiac fibroblasts with Y-27632 decreased both basal and aldosterone-induced CTGF protein expression (64 ± 6% (p < 0.01) versus aldosterone and 52 ± 15% (p < 0.01) versus aldosterone, respectively; Fig. 4C). The RhoA activator CN03 increased CTGF protein expression (226 ± 28%, p < 0.001; Fig. 4D), which persisted despite BR-4628 or spironolactone preincubation (Fig. 4D). Aldosterone increased RhoA activity in cardiac fibroblasts (165 ± 4%, p < 0.05, Fig. 4E), whereas total RhoA protein expression remained unaffected (93 ± 10%, p = ns; Fig. 4F). The aldosterone-induced up-regulation of hydroxyproline was prevented by Y-27632 (91 ± 9%, p < 0.01 versus aldosterone; Fig. 4G), demonstrating the functional importance of RhoA for the MR signaling. Aldosterone increased CTGF mRNA expression (132 ± 12%, p < 0.01, Fig. 4H), which was prevented by BR-4628, spironolactone, and Y-27632 preincubation similar to the regulation of the protein expression (95 ± 8%, 97 ± 9%, and 96 ± 5%, respectively; all p < 0.05, Fig. 4H).
FIGURE 4.
RhoA/Rho kinase pathway mediates fibrotic remodeling downstream of MR in cardiac fibroblasts. A, CTGF protein expression in fibroblasts treated with TGF-β (5 ng/ml, 24 h) with and without preincubation with BR-4628. B, CTGF protein expression in fibroblasts treated with TGF-β (5 ng/ml, 24 h) with and without preincubation with Y-27632 (10 μm, 25 h), a Rho kinase inhibitor. C, CTGF protein expression after aldosterone (Aldo) treatment with and without preincubation of Y-27632. D, CTGF protein expression in fibroblasts treated with RhoA activator CN03 (1 μg/ml, 3 h) only and with preincubation with BR-4628 or spironolactone (Spirono). RhoA activity (pulldown assay) (E) and RhoA total protein expression (Western blot) (F) in aldosterone treatment in cardiac fibroblasts. G, hydroxyproline concentration in aldosterone-treated cardiac fibroblasts with and without preincubation with Y-27632. H, CTGF mRNA expression (RT-PCR) in cardiac fibroblasts incubated with aldosterone and BR-4628, spironolactone, or Y-27632 pretreatment, respectively. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
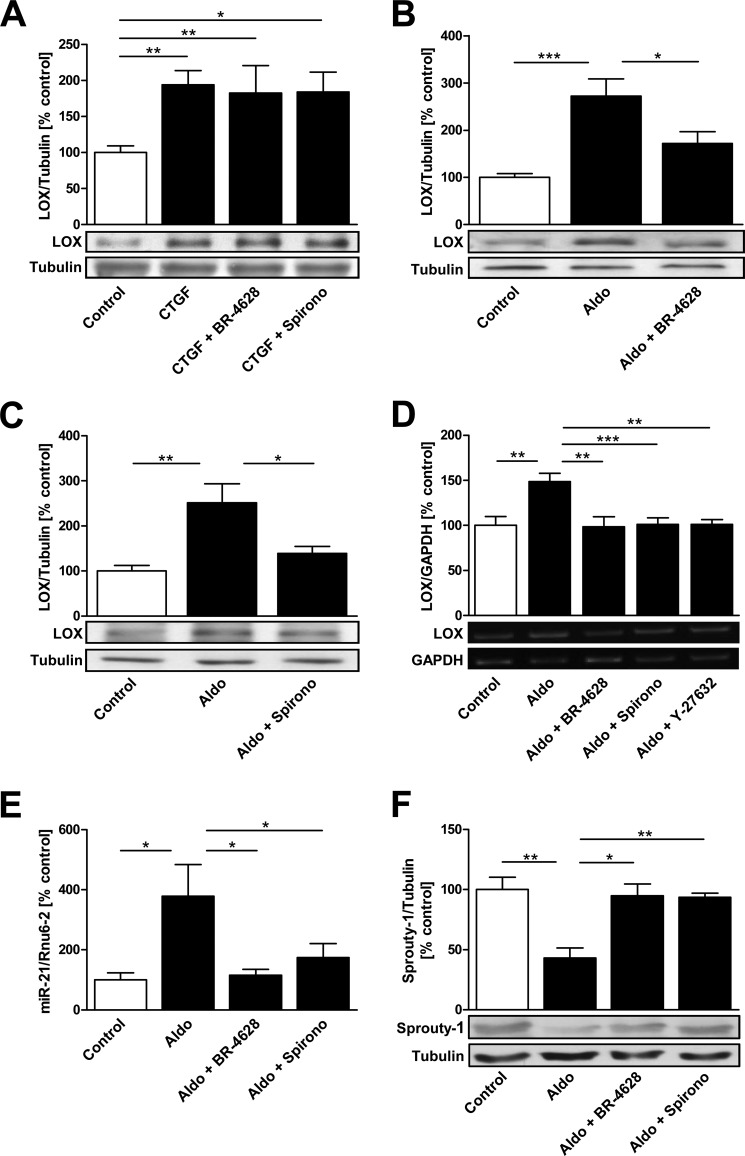
LOX Is a Downstream Mediator of CTGF in Cardiac Fibroblasts
LOX is an important enzyme for collagen cross-linking (46). Previously we found that LOX is more abundant in the LA of AF patients and contributes to atrial fibrosis (18). CTGF increased LOX protein expression (194 ± 19%, p < 0.01; Fig. 5A) in cardiac fibroblasts, and neither pretreatment with BR-4628 nor with spironolactone affected CTGF-induced up-regulation (Fig. 5A). Aldosterone also increased LOX expression (272 ± 37%, p < 0.001; Fig. 5B), but pretreatment with BR-4628 or spironolactone reduced the aldosterone effect on LOX expression (172 ± 25% (p < 0.05) versus aldosterone and 139 ± 16% (p < 0.05) versus aldosterone, respectively; Fig. 5, B and 5C). Additionally, aldosterone increased LOX mRNA expression (149 ± 9%, p < 0.01, Fig. 5D). This change was completely prevented by BR-4628, spironolactone, and Y-27632 preincubation (98 ± 11%, 101 ± 8%, and 101 ± 5%, respectively; all p < 0.01; Fig. 5D).
FIGURE 5.
Regulation of LOX and miR-21 via MR in cardiac fibroblasts. A, LOX expression in CTGF-treated (1 ng/ml, 1 h) fibroblasts with or without pretreatment with BR-4628 or spironolactone (Spirono). LOX expression in cardiac fibroblasts incubated with aldosterone (Aldo) and pretreated with either BR-4628 (B) or spironolactone (C) is shown. D, LOX mRNA expression (RT-PCR) in cardiac fibroblasts incubated with aldosterone and BR-4628, spironolactone, or Y-27632 pretreatment. E, microRNA-21 (miR-21) expression quantified by TaqMan-PCR in fibroblasts treated with aldosterone, aldosterone and BR-4628 or aldosterone and spironolactone. F, protein expression of the miR-21 downstream target Sprouty-1 in cells treated with aldosterone, aldosterone and BR-4628, or aldosterone and spironolactone. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Micro-RNA-21 and Sprouty-1 Expression in Cardiac Fibroblasts
MicroRNA-21 (miR-21) contributes to myocardial remodeling and fibrosis (36). Human left atria of patients with AF exhibit increased miR-21 expression compared with SR patients (17). miR-21 expression was increased in aldosterone-treated cardiac fibroblasts (379 ± 106%, p < 0.05; Fig. 5E). BR-4628 as well as spironolactone reduced miR-21 up-regulation (115 ± 20% (p < 0.05) versus aldosterone and 174 ± 46% (p < 0.05) versus aldosterone, respectively; Fig. 5E). Sprouty-1, a downstream target of miR-21, was conclusively decreased in aldosterone-treated cardiac fibroblasts (43 ± 8%, p < 0.01; Fig. 5F). Treatment with BR-4628 or spironolactone normalized Sprouty-1 protein expression (95 ± 10% (p < 0.05) versus aldosterone and 93 ± 4% (p < 0.01) versus aldosterone, respectively; Fig. 5F). Treatment of cardiac fibroblasts with Rho activator CN03 did not alter miR-21 expression (97 ± 10%, p = ns; data not shown).
Regulation of 11β-HSD2 in Cardiomyocytes and Transgenic RacET Mice
11β-HSD2 expression was not found in cardiac fibroblasts (data not shown), which is in concordance with the literature (40). Cardiomyocytes expressed 11β-HSD2, and neither aldosterone nor TGF-β treatment altered 11β-HSD2 expression (data not shown). Aldosterone increased CTGF expression in cardiomyocytes (141 ± 12%, p < 0.05; Fig. 6A), but a physiological concentration of cortisol in the cell culture medium completely abolished the aldosterone effect (72 ± 8%, p < 0.01 versus aldosterone; Fig. 6A), indicating MR occupancy by cortisol. Cortisol itself did not alter CTGF expression (Fig. 6A). To investigate the functional interaction between cardiomyocytes and cardiac fibroblasts, the latter were incubated with cardiomyocyte-conditioned medium. We observed an increase of collagen production by cardiac fibroblasts exposed to cell culture medium from cardiomyocytes that were incubated with aldosterone (154 ± 12%, p < 0.001; Fig. 6B). Co-incubation with cortisol prevented this aldosterone-induced increase of collagen production (114 ± 12%, p < 0.05 versus aldosterone; Fig. 6B).
FIGURE 6.
Role and regulation of 11β-HSD2 in neonatal cardiomyocytes and transgenic RacET mice. A, CTGF protein expression in myocytes treated with aldosterone (Aldo, 10−8 m, 24 h), hydrocortisone (127 ng/ml, 24 h), or both. B, hydroxyproline concentration in cardiac fibroblasts 24 h after the addition of conditioned medium from cardiomyocytes, which were cultured with aldosterone (10−8 m, 24 h), hydrocortisone (12.7 μg/ml, 24 h), or both. MR (C) and 11β-HSD2 (D) protein expression in neonatal cardiac myocytes paced rhythmic or arrhythmic at 3 Hz for 24 h. MR (RT-PCR) (E) and 11β-HSD2 (F) mRNA expression (TaqMan-PCR) in neonatal cardiac myocytes paced rhythmic or arrhythmic (3 Hz, 24 h). Protein expression of MR (G) and protein expression of 11β-HSD2 (H) in atria of 12-month-old transgenic RacET compared with wild type control mice is shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
In cardiomyocytes that were paced rhythmically or arrhythmically with 3 Hz over 24 h, 11β-HSD2 protein expression was up-regulated (175 ± 25%, p < 0.05; Fig. 6D), whereas MR protein and mRNA expression remained unaltered (123 ± 14% (p = ns) and 78 ± 9% (p = ns), respectively; Fig. 6, C and E). The 11β-HSD2 mRNA expression was slightly increased but not statistically significant (122 ± 17%, p = ns; Fig. 6F).
To characterize the expression of 11β-HSD2 in vivo we studied transgenic mice with cardiac overexpression of constitutively active (V12) Rac1 under the control of the α-myosin heavy chain promoter (RacET). These mice show a marked 30-fold up-regulation of Rac1 activity compared with WT mice and developed AF spontaneously at old age (24). 12-Month-old RacET mice exhibited a >6-fold up-regulation of atrial 11β-HSD2 expression compared with wild type control mice (FVBN) (665 ± 189%, p < 0.05; Fig. 6H). MR expression was similar in RacET and wild type control mice (120 ± 5%, p = ns; Fig. 6G).
DISCUSSION
The data identify the mineralocorticoid receptor and its regulation by the 11β-hydroxysteroid dehydrogenase 2 as important contributors for atrial fibrotic remodeling. The 11β-HSD2 converts cortisol to receptor-inactive metabolites and thereby allows aldosterone to bind at the MR. Importantly, 11β-HSD2 is up-regulated in human LA from patients with AF compared with SR, in a mouse model of spontaneous AF (RacET mice) and in irregularly compared with regularly paced cardiomyocytes. This finding explains the increased pro-fibrotic MR-dependent signaling despite equal expression of MR. The subsequent mechanistic studies identified the RhoA/Rho kinase pathway, CTGF, LOX, miR-21, and Sprouty-1 as components of the MR-controlled pro-fibrotic atrial remodeling.
Atrial fibrillation is a disease of the left atrial myocardium. Therefore, the use of left atrial samples for the analysis is appropriate. The expression of MR in moderately enlarged LA was similar in AF and SR patients, whereas other studies have reported increased expression of MR in AF patients (38, 39). Potential explanations for these differences include the use of left atrial tissue compared with the right atrial appendage in other studies (38) and the study of more severely diseased hearts and atria (39).
The MR has similar affinities for aldosterone and cortisol and, hence, MR is occupied by cortisol under physiological conditions because of its 1000 time higher serum concentrations. The enzyme 11β-HSD2 inactivates cortisol to cortisone. High expression levels of 11β-HSD2 protect MR from glucocorticoid binding and enables aldosterone to bind to the MR (15, 40). Our data demonstrate that 11β-HSD2 is up-regulated in LA of AF compared with SR patients despite similar MR expression and that 11β-HSD2 correlates with fibrosis in human LA. We, therefore, studied the role of 11β-HSD2 regarding MR-mediated pro-fibrotic signaling in cardiomyocytes. Aldosterone increased the expression of CTGF but in the presence of cortisol the aldosterone effect was abolished. Cardiac fibroblasts do not express 11β-HSD2 (40). Conditioned medium from cardiomyocytes treated with aldosterone induced a pro-fibrotic response in fibroblasts; however, this effect was absent when the cardiomyocytes were treated with aldosterone in the presence of cortisol. These findings suggest that under physiological conditions aldosterone has little pro-fibrotic activity because the MR is occupied by cortisol. In paced cardiomyocytes we observed an arrhythmia-associated increase in the 11β-HSD2 protein expression. Because 11β-HSD2 mRNA expression was not increased significantly, the regulation of 11β-HSD2 appears to be, at least in part, posttranscriptional, as described (42, 43). Transgenic mice with cardiac overexpression of constitutive active Rac1 (RacET) that develop spontaneous AF (23, 24) showed increased atrial 11β-HSD2 expression compared with wild type mice. These data point to an arrhythmia and Rac1-mediated regulation of 11β-HSD2. In summary, these results provide evidence that 11β-HSD2 up-regulation in AF facilitates a pro-fibrotic signaling via the MR.
Aldosterone increased collagen production in cardiac fibroblasts, thus validating this cell culture model for investigating MR-dependent fibrotic remodeling in vitro. CTGF is a mediator of fibrosis in AF (16, 18) and of aldosterone-induced cellular alterations (41, 44). Our data demonstrate that aldosterone induces CTGF expression via the MR, whereas cortisol did not. Recent data identified RhoA/Rho kinase as a potential mediator of aldosterone-induced renal injury including up-regulation of CTGF (45). We recently showed that RacET mice have an increased RhoA activity in the myocardium (16). RhoA correlates with fibrosis in human left atrial myocardium. Our in vitro data reveal that aldosterone increases RhoA activity and that the RhoA/Rho kinase pathway regulates CTGF expression and collagen production downstream of the MR. RhoA/Rho kinase was confirmed as a common pathway where TGF-β and MR signaling converge.
Fibroblasts exposed to CTGF exhibited increased expression of LOX, the key enzyme for collagen cross-linking (18, 46). Aldosterone increased LOX expression in a MR- and Rho kinase-dependent manner. These results confirm a dependence of LOX on CTGF as a subsequent pathway, and both CTGF and LOX are induced by aldosterone via the MR.
MicroRNAs are small non-coding RNAs that control expression of complementary target messenger RNAs (47). MicroRNA-21 increases fibroblast survival that leads to cardiac fibrosis and dysfunction (36). We and others have observed that miR-21 promotes atrial structural remodeling (17, 48, 49). LA of patients with AF exhibit increased miR-21 expression (17), and miR-21 as well as Sprouty-1 expression in human LA correlates positively with fibrosis. Here, these data are extended showing that the MR-dependent pro-fibrotic signaling involves miR-21 and Sprouty-1.
BR-4628 is a dihydropyridine-derived non-steroidal and selective MR antagonist (27). BR-4628 and spironolactone exhibit similar potency against MR but differ in their affinity to other steroid receptors; BR-4628 possesses the highest specificity for MR (27). The prevention of aldosterone induced alterations, e.g. collagen production, CTGF, LOX, miR-21, and Sprouty-1 expression, by the MR antagonists BR-4628 and spironolactone suggests that 1) aldosterone-induced fibrotic changes are strongly MR-dependent, and 2) MR blockade is a potential target to prevent structural remodeling and fibrosis.
Important limitations of this study include that isolated human atrial myocytes and fibroblasts in culture were not available for the mechanistic studies. We are aware of the limitations of neonatal rat cardiac fibroblasts and myocytes prepared from left ventricles. To prevent differentiation, these cells were used until passage 3 or less only. As all animal models and cell culture have potential limitations with regard to the human disease, the human left atrial myocardium is the primary readout of the study, and our cell culture and animal studies are performed to dynamically study the signal transduction.
CONCLUSION
In summary, MR signaling promotes fibrotic atrial remodeling. Increased expression of 11β-HSD2 leads to up-regulation of pro-fibrotic mediators by aldosterone, specifically RhoA activity as well as CTGF, LOX, miR-21 expression, and collagen synthesis. The MR antagonists BR-4628 and spironolactone prevented these alterations. MR inhibition may, therefore, represent a potential pharmacologic target for the prevention of fibrotic remodeling of the atrial myocardium.
Acknowledgments
We thank Catrin Pittke, Simone Jäger, and Ellen Becker for excellent technical assistance.
This work was supported by the Universität des Saarlandes, an unrestricted grant from Bayer Pharma AG to the Universität des Saarlandes and by the Deutsche Forschungsgemeinschaft DFG (KFO 196, AD 455/1-1).
- AF
- atrial fibrillation
- 11β-HSD2
- 11β hydroxysteroid dehydrogenase type 2
- CTGF
- connective tissue growth factor
- LA
- left atrial myocardium
- LOX
- lysyl oxidase
- MR
- mineralocorticoid receptor
- miR-21
- microRNA-21
- SPARC
- secreted protein acidic and rich in cysteine
- SR
- sinus rhythm
- TRITC
- tetramethylrhodamine isothiocyanate
- ns
- not significant
- FITC
- fluorescein isothiocyanate.
REFERENCES
- 1. Lip G. Y., Tse H. F., Lane D. A. (2012) Atrial fibrillation. Lancet 379, 648–661 [DOI] [PubMed] [Google Scholar]
- 2. Burstein B., Nattel S. (2008) Atrial fibrosis. Mechanisms and clinical relevance in atrial fibrillation. J. Am. Coll. Cardiol. 51, 802–809 [DOI] [PubMed] [Google Scholar]
- 3. Nattel S., Burstein B., Dobrev D. (2008) Atrial remodeling and atrial fibrillation. Mechanisms and implications. Circ. Arrhythm Electrophysiol. 1, 62–73 [DOI] [PubMed] [Google Scholar]
- 4. Dobrev D., Carlsson L., Nattel S. (2012) Novel molecular targets for atrial fibrillation therapy. Nat. Rev. Drug. Discov. 11, 275–291 [DOI] [PubMed] [Google Scholar]
- 5. Zhou G., Kandala J. C., Tyagi S. C., Katwa L. C., Weber K. T. (1996) Effects of angiotensin II and aldosterone on collagen gene expression and protein turnover in cardiac fibroblasts. Mol. Cell. Biochem. 154, 171–178 [DOI] [PubMed] [Google Scholar]
- 6. Briet M., Schiffrin E. L. (2010) Aldosterone. Effects on the kidney and cardiovascular system. Nat. Rev. Nephrol. 6, 261–273 [DOI] [PubMed] [Google Scholar]
- 7. Young M., Fullerton M., Dilley R., Funder J. (1994) Mineralocorticoids, hypertension, and cardiac fibrosis. J. Clin. Invest. 93, 2578–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lother A., Berger S., Gilsbach R., Rösner S., Ecke A., Barreto F., Bauersachs J., Schütz G., Hein L. (2011) Ablation of mineralocorticoid receptors in myocytes but not in fibroblasts preserves cardiac function. Hypertension 57, 746–754 [DOI] [PubMed] [Google Scholar]
- 9. Weber K. T. (2001) Aldosterone in congestive heart failure. N. Engl. J. Med. 345, 1689–1697 [DOI] [PubMed] [Google Scholar]
- 10. Pitt B., Remme W., Zannad F., Neaton J., Martinez F., Roniker B., Bittman R., Hurley S., Kleiman J., Gatlin M. (2003) Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 348, 1309–1321 [DOI] [PubMed] [Google Scholar]
- 11. Pitt B., Zannad F., Remme W. J., Cody R., Castaigne A., Perez A., Palensky J., Wittes J. (1999) The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N. Engl. J. Med. 341, 709–717 [DOI] [PubMed] [Google Scholar]
- 12. Zannad F., McMurray J. J., Krum H., van Veldhuisen D. J., Swedberg K., Shi H., Vincent J., Pocock S. J., Pitt B. (2011) Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 364, 11–21 [DOI] [PubMed] [Google Scholar]
- 13. Swedberg K., Zannad F., McMurray J. J., Krum H., van Veldhuisen D. J., Shi H., Vincent J., Pitt B. (2012) Eplerenone and atrial fibrillation in mild systolic heart failure. J. Am. Coll. Cardiol. 59, 1598–1603 [DOI] [PubMed] [Google Scholar]
- 14. Reil J. C., Hohl M., Selejan S., Lipp P., Drautz F., Kazakow A., Münz B. M., Müller P., Steendijk P., Reil G. H., Allessie M. A., Böhm M., Neuberger H. R. (2012) Aldosterone promotes atrial fibrillation. Eur. Heart J. 33, 2098–2108 [DOI] [PubMed] [Google Scholar]
- 15. Odermatt A., Kratschmar D. V. (2012) Tissue-specific modulation of mineralocorticoid receptor function by 11β-hydroxysteroid dehydrogenases. An overview. Mol. Cell. Endocrinol. 350, 168–186 [DOI] [PubMed] [Google Scholar]
- 16. Adam O., Lavall D., Theobald K., Hohl M., Grube M., Ameling S., Sussman M. A., Rosenkranz S., Kroemer H. K., Schäfers H. J., Böhm M., Laufs U. (2010) Rac1-induced connective tissue growth factor regulates connexin 43 and N-cadherin expression in atrial fibrillation. J. Am. Coll Cardiol. 55, 469–480 [DOI] [PubMed] [Google Scholar]
- 17. Adam O., Löhfelm B., Thum T., Gupta S. K., Puhl S. L., Schäfers H. J., Böhm M., Laufs U. (2012) Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res. Cardiol. 107, 278. [DOI] [PubMed] [Google Scholar]
- 18. Adam O., Theobald K., Lavall D., Grube M., Kroemer H. K., Ameling S., Schäfers H. J., Böhm M., Laufs U. (2011) Increased lysyl oxidase expression and collagen cross-linking during atrial fibrillation. J. Mol. Cell. Cardiol. 50, 678–685 [DOI] [PubMed] [Google Scholar]
- 19. Custodis F., Eberl M., Kilter H., Böhm M., Laufs U. (2006) Association of RhoGDIα with Rac1 GTPase mediates free radical production during myocardial hypertrophy. Cardiovasc. Res. 71, 342–351 [DOI] [PubMed] [Google Scholar]
- 20. Laufs U., Kilter H., Konkol C., Wassmann S., Böhm M., Nickenig G. (2002) Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc. Res. 53, 911–920 [DOI] [PubMed] [Google Scholar]
- 21. Tovar O., Tung L. (1992) Electroporation and recovery of cardiac cell membrane with rectangular voltage pulses. Am. J. Physiol. 263, H1128–H1136 [DOI] [PubMed] [Google Scholar]
- 22. Sussman M. A., Welch S., Walker A., Klevitsky R., Hewett T. E., Price R. L., Schaefer E., Yager K. (2000) Altered focal adhesion regulation correlates with cardiomyopathy in mice expressing constitutively active rac1. J. Clin. Invest. 105, 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reil J. C., Hohl M., Oberhofer M., Kazakov A., Kaestner L., Müller P., Adam O., Maack C., Lipp P., Mewis C., Allessie M., Laufs U., Böhm M., Neuberger H. R. (2010) Cardiac Rac1 overexpression in mice creates a substrate for atrial arrhythmias characterized by structural remodelling. Cardiovasc. Res. 87, 485–493 [DOI] [PubMed] [Google Scholar]
- 24. Adam O., Frost G., Custodis F., Sussman M. A., Schäfers H. J., Böhm M., Laufs U. (2007) Role of Rac1 GTPase activation in atrial fibrillation. J. Am. Coll Cardiol. 50, 359–367 [DOI] [PubMed] [Google Scholar]
- 25. He Y. H., Zhang H. N., Zhang G. P., Hou N., Xiao Q., Huang Y., Wu J. H., Luo M. S., Zhang G. S., Yi Q., Chen M. S., Luo J. D. (2011) A physiological concentration of glucocorticoid inhibits the proinflammatory cytokine-induced proliferation of adult rat cardiac fibroblasts. Roles of extracellular signal-regulated kinase 1/2 and nuclear factor-κB. Clin. Exp. Pharmacol. Physiol. 38, 739–746 [DOI] [PubMed] [Google Scholar]
- 26. Jiang D., Jiang Z., Han F., Zhang Y., Li Z. (2008) HGF suppresses the production of collagen type III and α-SMA induced by TGF-β1 in healing fibroblasts. Eur. J. Appl. Physiol. 103, 489–493 [DOI] [PubMed] [Google Scholar]
- 27. Fagart J., Hillisch A., Huyet J., Bärfacker L., Fay M., Pleiss U., Pook E., Schäfer S., Rafestin-Oblin M. E., Kolkhof P. (2010) A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J. Biol. Chem. 285, 29932–29940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flatau G., Lemichez E., Gauthier M., Chardin P., Paris S., Fiorentini C., Boquet P. (1997) Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387, 729–733 [DOI] [PubMed] [Google Scholar]
- 29. Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klusonová P., Reháková L., Borchert G., Vagnerová K., Neckár J., Ergang P., Miksík I., Kolár F., Pácha J. (2009) Chronic intermittent hypoxia induces 11β-hydroxysteroid dehydrogenase in rat heart. Endocrinology 150, 4270–4277 [DOI] [PubMed] [Google Scholar]
- 31. McCurdy S., Baicu C. F., Heymans S., Bradshaw A. D. (2010) Cardiac extracellular matrix remodeling. Fibrillar collagens and secreted protein acidic and rich in cysteine (SPARC). J. Mol. Cell. Cardiol. 48, 544–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harris B. S., Zhang Y., Card L., Rivera L. B., Brekken R. A., Bradshaw A. D. (2011) SPARC regulates collagen interaction with cardiac fibroblast cell surfaces. Am. J. Physiol. Heart Circ. Physiol. 301, H841–H847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen H., Huang X. N., Stewart A. F., Sepulveda J. L. (2004) Gene expression changes associated with fibronectin-induced cardiac myocyte hypertrophy. Physiol. Genomics 18, 273–283 [DOI] [PubMed] [Google Scholar]
- 34. Grillo C., Vallee S., McEwen B. S., De Nicola A. F. (1990) Properties and distribution of binding sites for the mineralocorticoid receptor antagonist [3H]ZK 91587 in brain. J. Steroid Biochem. 35, 11–15 [DOI] [PubMed] [Google Scholar]
- 35. Mu Y., Gudey S. K., Landström M. (2012) Non-Smad signaling pathways. Cell Tissue Res. 347, 11–20 [DOI] [PubMed] [Google Scholar]
- 36. Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M., Galuppo P., Just S., Rottbauer W., Frantz S., Castoldi M., Soutschek J., Koteliansky V., Rosenwald A., Basson M. A., Licht J. D., Pena J. T., Rouhanifard S. H., Muckenthaler M. U., Tuschl T., Martin G. R., Bauersachs J., Engelhardt S. (2008) MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456, 980–984 [DOI] [PubMed] [Google Scholar]
- 37. Funder J. W. (2009) Reconsidering the roles of the mineralocorticoid receptor. Hypertension 53, 286–290 [DOI] [PubMed] [Google Scholar]
- 38. Tsai C. T., Chiang F. T., Tseng C. D., Hwang J. J., Kuo K. T., Wu C. K., Yu C. C., Wang Y. C., Lai L. P., Lin J. L. (2010) Increased expression of mineralocorticoid receptor in human atrial fibrillation and a cellular model of atrial fibrillation. J. Am. Coll. Cardiol. 55, 758–770 [DOI] [PubMed] [Google Scholar]
- 39. De-An P., Li L., Zhi-Yun X., Jin-Yu H., Zheng-Ming X., Min W., Qiang Y., Shi-Eng H. (2010) Increased expression of mineralocorticoid receptor and 11β-hydroxysteroid dehydrogenase type 2 in human atria during atrial fibrillation. Clin. Cardiol. 33, 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Odermatt A., Atanasov A. G. (2009) Mineralocorticoid receptors. Emerging complexity and functional diversity. Steroids 74, 163–171 [DOI] [PubMed] [Google Scholar]
- 41. Koitabashi N., Arai M., Kogure S., Niwano K., Watanabe A., Aoki Y., Maeno T., Nishida T., Kubota S., Takigawa M., Kurabayashi M. (2007) Increased connective tissue growth factor relative to brain natriuretic peptide as a determinant of myocardial fibrosis. Hypertension 49, 1120–1127 [DOI] [PubMed] [Google Scholar]
- 42. Kadereit B., Fustier P., Shojaati K., Frey B. M., Frey F. J., Mohaupt M. G. (2005) Extracellular ATP determines 11β-hydroxysteroid dehydrogenase type 2 activity via purinergic receptors. J. Am. Soc. Nephrol. 16, 3507–3516 [DOI] [PubMed] [Google Scholar]
- 43. van Beek J. P., Guan H., Julan L., Yang K. (2004) Glucocorticoids stimulate the expression of 11β-hydroxysteroid dehydrogenase type 2 in cultured human placental trophoblast cells. J. Clin. Endocrinol. Metab. 89, 5614–5621 [DOI] [PubMed] [Google Scholar]
- 44. Azibani F., Benard L., Schlossarek S., Merval R., Tournoux F., Fazal L., Polidano E., Launay J. M., Carrier L., Chatziantoniou C., Samuel J. L., Delcayre C. (2012) Aldosterone inhibits antifibrotic factors in mouse hypertensive heart. Hypertension 59, 1179–1187 [DOI] [PubMed] [Google Scholar]
- 45. Sun G. P., Kohno M., Guo P., Nagai Y., Miyata K., Fan Y. Y., Kimura S., Kiyomoto H., Ohmori K., Li D. T., Abe Y., Nishiyama A. (2006) Involvements of Rho-kinase and TGF-β pathways in aldosterone-induced renal injury. J. Am. Soc. Nephrol. 17, 2193–2201 [DOI] [PubMed] [Google Scholar]
- 46. Kagan H. M., Trackman P. C. (1991) Properties and function of lysyl oxidase. Am. J. Respir. Cell Mol. Biol. 5, 206–210 [DOI] [PubMed] [Google Scholar]
- 47. Bartel D. P. (2004) MicroRNAs. Genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 48. Wang Z., Lu Y., Yang B. (2011) MicroRNAs and atrial fibrillation. New fundamentals. Cardiovasc. Res. 89, 710–721 [DOI] [PubMed] [Google Scholar]
- 49. Cardin S., Guasch E., Luo X., Naud P., Le Quang K., Shi Y., Tardif J. C., Comtois P., Nattel S. (2012) Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ. Arrhythm. Electrophysiol. 5, 1027–1035 [DOI] [PubMed] [Google Scholar]