Background: Heterochromatin is enriched for di- and tri-methylated lysine 9 of histone H3 (H3K9Me2/3) and heterochromatin protein 1 (HP1Hsα .).
Results: The association of HP1Hsα with H3K9Me3-containing nucleosome arrays facilitated array compaction and cross-array interactions.
Conclusion: HP1Hsα association caused intra- and inter-array associations, leading to chromatin condensation and looping.
Significance: An understanding of HP1Hsα-nucleosome interactions provides insights on the structure and functions of heterochromatin.
Keywords: Chromatin Structure, Chromosomes, DNA Binding Protein, Heterochromatin, Histone Modification
Abstract
HP1Hsα-containing heterochromatin is located near centric regions of chromosomes and regulates DNA-mediated processes such as DNA repair and transcription. The higher-order structure of heterochromatin contributes to this regulation, yet the structure of heterochromatin is not well understood. We took a multidisciplinary approach to determine how HP1Hsα-nucleosome interactions contribute to the structure of heterochromatin. We show that HP1Hsα preferentially binds histone H3K9Me3-containing nucleosomal arrays in favor of non-methylated nucleosomal arrays and that nonspecific DNA interactions and pre-existing chromatin compaction promote binding. The chromo and chromo shadow domains of HP1Hsα play an essential role in HP1Hsα-nucleosome interactions, whereas the hinge region appears to have a less significant role. Electron microscopy of HP1Hsα-associated nucleosomal arrays showed that HP1Hsα caused nucleosome associations within an array, facilitating chromatin condensation. Differential sedimentation of HP1Hsα-associated nucleosomal arrays showed that HP1Hsα promotes interactions between arrays. These strand-to-strand interactions are supported by in vivo studies where tethering the Drosophila homologue HP1a to specific sites promotes interactions with distant chromosomal sites. Our findings demonstrate that HP1Hsα-nucleosome interactions cause chromatin condensation, a process that regulates many chromosome events.
Introduction
Heterochromatin is located in centric regions of chromosomes and regulates a wide range of DNA-mediated processes, including DNA recombination, transcriptional regulation, DNA replication/repair, and the maintenance of genomic stability (1). Centric heterochromatin adopts a condensed state that persists through the cell cycle and contributes to its biological functions (2, 3). However, relatively little is known about the structure of heterochromatin.
Two key components of heterochromatin are histone H3 lysine 9 di- and tri-methylation (H3K9Me2/3), and heterochromatin protein 1Hsα (HP1Hsα), a highly conserved non-histone chromosomal protein. HP1 family members are relatively small (∼200 amino acids) and possess two highly conserved domains: an amino-terminal chromo domain (CD)5 and a carboxyl chromo shadow domain (CSD), separated by a hinge domain (4). The CD binds H3K9Me2/3 (5–7), and the CSD mediates homodimerization (8, 9). Dimerization generates an interaction platform for a wide range of nuclear proteins containing a PXVXL consensus sequence (10) and forms a linkage between two separate CDs.
In vivo, association of specific HP1 family members with heterochromatin limits accessibility of the underlying DNA sequences to nuclease digestion and transcription factor binding, consistent with a condensed chromatin state (11–13). Protection of the DNA template is thought to occur by HP1 homodimers binding to H3K9me2/3 on adjacent nucleosomes and compacting chromatin (14).
To better understand HP1-nucleosome interactions, studies have examined HP1 proteins bound to nucleosome arrays that are predominantly methylated on H3K9 or contain H3K9Me2/3 mimics (15, 16). These studies showed that HP1 binds H3K9Me2/3-containing arrays; however, the requirements for binding are not well understood. In the case of Drosophila HP1a, in vitro binding required the addition of other chromatin proteins (15).
Binding of HP1 proteins to nucleosomes in vitro has also been observed in the absence of histone methylation (16, 17). Corroborating these findings, genome-wide analyses showed that HP1 proteins associate with both sites that possess and lack H3K9Me2/3 (18, 19). Moreover, the structure of HP1 proteins bound to methylated nucleosomes and the consequences of this binding within the context of a nucleosome array are unknown.
To determine the factors that affect binding of HP1 proteins to nucleosomes and the structural ramifications of this binding, we used in vitro reconstitution, in silico modeling, and in vivo functional analyses. Taking this approach, we demonstrate that human HP1Hsα promotes nucleosome associations within and between nucleosome arrays that drive chromatin condensation.
EXPERIMENTAL PROCEDURES
Preparation of HP1Hsα
Wild type His6-HP1Hsα fusion protein, a gift from Professor Christian Muchardt (Pasteur Institute, Paris, France) (20), and HP1Hsα point mutants were expressed and then lysed into HP1 buffer (50 mm NaH2PO4, pH 8, 300 mm NaCl, 10 mm imidazole, 1 mm 2-mercaptoethanol, 10% glycerol, 1 μm PMSF, 10 μg/ml leupeptin, 10 μg/μl aprotinin, and 50 μg/ml DNase I). HP1Hsα proteins were affinity purified using nickel-nitrilotriacetic acid resin and then separated from HP1Hsα aggregates by size exclusion using Sephacryl S-300 resin.
The extent of dimerization was determined by sedimentation equilibrium analysis. The protein was dialyzed in a buffer containing 100 mm NaCl, 50 mm NaH2PO4, pH 8, 0.2 mm EDTA, and 0.05 mm Tris(2-carboxyethyl)phosphine. 120 ml of protein samples were prepared with A280 of 0.3, 0.5 and 0.7, centrifuged at 30,000 rpm in a Beckman XL-A ProteomeLab ultracentrifuge, and the data were collected in triplicate. Using Ultrascan software (version 7.0), these data were subjected to non-linear least-squares global fits to several models (monomer, dimer, monomer-dimer equilibrium, tetramer), where molecular weight was allowed to float. The molecular weight associated with the model with the smallest root mean square deviation is reported.
The binding of HP1Hsα to H3K9me3 peptide (fragments 2–23) was analyzed using fluorescence anisotropy. H3 peptide (residues 1–23) with K9 trimethylation and an amino-terminal fluorescene label was generated by standard Fmoc-based solid-phase peptide synthesis (University of Wisconsin Biotechnology Center). For fluorescence anisotropy experiments, HP1 was dialyzed in a buffer containing 50 mm K2PO4, pH 8, 25 mm NaCl, and 2 mm DTT as reported (21). 100 nm fluorescein peptide were titrated with increasing concentrations of HP1 at room temperature for 30 min. The anisotropy vales determined using a Varian fluorescence spectrophotometer fitted with a polarizer. KD values were fit according to a simple saturation model of binding using Kaleidagraph (version 3.6). Data for the WT and CSD mutant form of HP1α were normalized to give a maximum fit amplitude of one. The CD mutant data were normalized according to the WT fit.
Preparation of Nucleosomal Systems
Recombinant, unmodified, Xenopus laevis H2A, H2B, H3, and H4 histones were prepared according to standard protocols (22). Uniformly trimethylated histone H3 at lysine 9 was generated by native chemical ligation using a synthesized H3 1–23 peptide (University of Wisconsin Biotechnology Center) according to methods described previously (23). Methylated and unmethylated histone octamers were assembled according to standard techniques (22). 601-177-12 and 601-177-1 DNA templates were liberated from a 601-177-12-containing plasmid using EcoRV and ScaI, respectively (24). Carrier DNA was prepared by PCR amplification as described previously (25). 601-177-1 mononucleosomes were prepared by rapid dilution deposition with molar ratios of octamer to DNA between 0.8 and 1.2 (26). 601-177-12 arrays were assembled by salt step dialysis as described previously (24), with ratios of template to carrier to octamer of 1.0:0.3:1.1–1.3.
Mononucleosome composition/saturation was characterized by native PAGE analysis as described previously (26). Nucleosomal array composition/saturation was characterized using four different techniques. 1) Array composition was determined by resolution of ScaI digestion products by native PAGE analysis as described previously (27). 2) The distribution of array sedimentation coefficients was determined at 12,000 rpm in a Beckman XL-A ProteomeLab ultracentrifuge using the enhanced van Holde-Weischet method in Ultrascan (version 8.0) as described previously (28). 3) The absolute weight-averaged molecular weights of the DNA and histone components of the nucleosomal arrays were determined by protein conjugate analysis. In this approach, ∼6 μg of array in 50 μl of buffer (10 mm HEPES, 2.5 mm NaCl, 0.1 mm EDTA, pH 8.0) was resolved by size exclusion HPLC chromatography (7.8 × 300 mm, 5 μm, 2000 Å, Wyatt Technology) with an isocratic flow of 0.5 ml/min HPLC buffer (10 mm Tris, 2.5 mm NaCl, 0.1 mm EDTA, pH 8.0). The eluted peak was subjected to protein conjugate analysis (Astra version 6 software, Wyatt Technology), where the peak was detected by UV-visible absorption (SPD-20A UV detector, Shimadzu Scientific Instruments), refractive index change (Optilab T-rEX, Wyatt Technology), and multi-angle static light scattering intensity (Dawn Helios II, Wyatt Technology). For protein conjugate analysis, component concentrations were determined using both molar extinction coefficients (23.8 ml·mg−1·cm−1 for DNA and 0 ml·mg−1·cm−1 for histone octamer) and differential refractive indexes (0.178 ml/g for DNA and 0.185 ml/g for histone octamer). 4) To directly count the number of nucleosomes per array, electron microscopy images of arrays under low-compacting conditions (8 mm NaCl) were obtained as described in the main text. In these images, only nucleosomal arrays that were clearly individual arrays were counted.
EMSA Analysis
HP1Hsα was dialyzed in 50 mm HEPES, pH 8, 150 mm NaCl, 1 mm EDTA, and 10% glycerol. For our standard EMSA analysis, nucleosome arrays or mononucleosomes (8 nm nucleosome final) were mixed with HP1Hsα (500 nm final) in a total volume of 20 μl (50 mm NaCl, 23.33 mm HEPES, pH 8, 0.067% Tris(2-carboxyethyl)phosphine, and 0.33 mm EDTA, final buffer concentrations). The HP1Hsα-containing nucleosomal arrays or mononucleosome mixture was mixed gently for 30 min at 4 °C and then applied on 0.5% agarose (type IV) gel made in 50 mm HEPES, pH 8 and 150 mm NaCl buffer, and electrophoresed in the same buffer at 70 volts and 0.4 amp for 2 h at 4 °C. For experiments in which the ionic strength was varied, the NaCl concentration was adjusted to the appropriate concentration both in the binding mixture and gel. Gels were visualized with Sybr Gold (Invitrogen).
The following model was used to calculate how the EMSA half-shift of an HP1-bound 12-mer nucleosomal array might relate to the HP1 binding of a mononucleosome with dissociation constant of KD. If the initial concentration of HP1 is much greater than the initial concentration of mononucleosome, then the probability, P, that a particular mononucleosome is bound by HP1 is as follows.
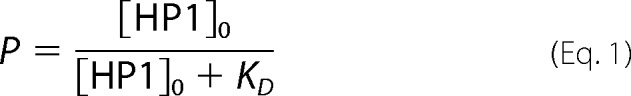 |
For nucleosomal arrays with n nucleosomes, where each nucleosome can bind an HP1 molecule, if 1) each nucleosome binds HP1 independently, 2) each nucleosome binds HP1 with the same affinity as the mononucleosome, P, and 3) the initial concentration of HP1 is much greater than nucleosome (i.e. HP1 is not limiting), then the probability of binding exactly k nucleosomes, Pk, is the sum of all the ways that an array can have k nucleosomes bound and n−k nucleosomes unbound.
 |
If k or more HP1 molecules need to be bound to observe an EMSA gel shift, for the most limiting case of requiring one HP1 bound to an array to produce a gel shift, i.e. gel shift occurs when k is one or more, then the probability of k is equal to one or more is simply unity minus the probability that k equals zero. Thus, for a 12-mer array, see Equation 3.
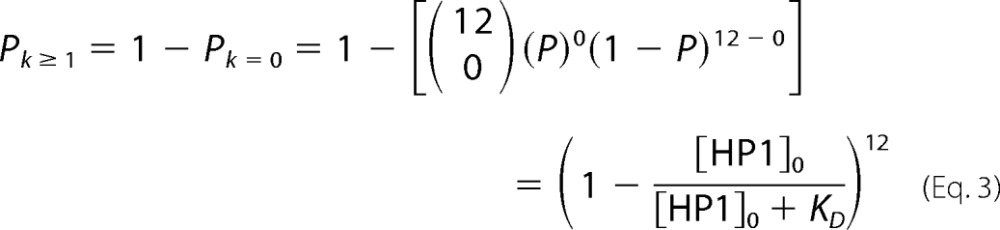 |
For the case of half-array shift, i.e. 50% of the arrays have one or more HP1 molecular bound
 |
Then,
That is, if only one HP1 needs to bind to an array to give a gel shift, an initial concentration of HP1 that is only ∼6% of the KD for and individual nucleosome is required for half-shift or the apparent half-shift concentration is 16.8-fold less for the array than for mononucleosomes.
Although some of the assumptions made may not hold in practice, reasonable deviations from these assumptions would tend to support the idea that arrays bind HP1 in a way that is fundamentally different from individual mononucleosomes. For example, if more than one HP1 is required to bind an array and generate an EMSA shift (a very reasonable possibility), the apparent half-shift concentration will be <16.8-fold. As another example, if binding to nucleosomes in arrays is not independent (i.e. binding of one nucleosome affects the binding of others) or is not identical between nucleosomes, then by definition, the act of stringing together individual nucleosomes into an array changes how nucleosomes interact with HP1. Finally, if HP1 is limiting (which is likely to be the case in the array HP1 titrations), the half-saturation concentration is likely to be higher than would be observed if HP1 was not limiting.
Preparation of Electron Microscopy Samples
HP1Hsα (500 nm) was incubated with arrays (68–86 nm nucleosome) in 50 μl of binding solution (8 or 50 mm NaCl, 23.33 mm HEPES, pH 8.0, and 0.33 mm EDTA) for 30 min at 4 °C. Samples were fixed by dialyzing against 160 ml of binding buffer containing 0.1% glutaraldehyde and 3.24% glycerol for 4 h at 4 °C. Samples were then dialyzed overnight into 2.5 mm NaCl, 10 mm HEPES, pH 8.0, at 4 °C. Fixed arrays were diluted 5- to 10-fold with 50 mm NaCl, applied to glow-discharged thin carbon films for 5 min, washed with 5 mm magnesium acetate, and stained for 10 s with 0.04% aqueous uranyl acetate and then washed with H2O and air dried. Grids were examined with a Tecnai 12 TEM (FEI Co., Hillsboro, OR) operated at 100 kV in tilted dark field mode, and images were recorded using a 2048 × 2048 CCD camera (TVIPS, Gauting, Germany) with 2 × 2 binning. The final pixel size was 0.908 nm. Salt concentrations above 50 mm NaCl were not analyzed because significant aggregation of the arrays occurred. Statistical analysis was performed on multiple particles; only arrays that were well separated from their neighbors were used in the analysis.
Differential Sedimentation Analysis
Reversible self-association of nucleosomal arrays in the presence of HP1Hsα was characterized using a differential sedimentation assay described previously (29) Briefly, HP1Hsα in buffer (150 mm NaCl, 50 mm HEPES, 0.2 mm EDTA, pH 8.0) was diluted to a stock concentration of 13.2 μm and final buffer concentrations of 150 mm NaCl, 12.5 mm HEPES, and 0.05 mm EDTA. Prior to mixing with arrays, HP1Hsα was added to varying concentrations of MgCl2 in array buffer (2.5 mm NaCl, 10 mm Tris, pH 8.0, 0.25 mm EDTA, 0.1 mm Tris(2-carboxyethyl)phosphine) to create 2× MgCl2 and HP1Hsα solutions, which were then mixed in equal volume with nucleosomal arrays (9.1 ng/μl final concentration template DNA). After incubation for 15 min at room temperature, samples were centrifuged at 14,000 × g for 10 min. The percentage of arrays remaining in the supernatant was determined by comparing the A260 of each sample with the A260 of a sample with no MgCl2 added.
Molecular Dynamics Simulations of HP1Hsα Conformational Dynamics
Molecular dynamics simulations of human HP1Hsα dimer were performed to assess the range of motion available to the CDs. Eight different explicit-solvent molecular dynamics simulations were performed with Gromacs software (version 4) (30, 46): four independent simulations of human wild type HP1Hsα dimers and four independent simulations of the hinge deletion ΔHP1Hsα dimer (residues Pro-81–Gly-116 were deleted). Each simulation was performed with a different starting geometry of the CDs relative to the dimerized CSDs, and each simulation was conducted for 100 ns with coordinates saved every 1 ps for analysis. To assess the distance between the CDs within a dimer, we measured the distances between the centers of mass of the aromatic rings of the three residues (Tyr-19, Trp-40, and Phe-43) that form each H3K9Me3 binding site.
In Silico Modeling of HP1Hsα-chromatin Interactions
All model building was done using the molecular modeling software VMD (31), with steric clashes assessed visually. To model HP1Hsα, we manually positioned the high resolution structures of the CD (32) and CSDs (10) such that the CDs were close to where the H3 N-terminal tails exit from the nucleosome core and the CSDs were close enough to the CDs that the linker domains could be connected using the molecular modeling program Loopy (33). We then used Loopy to complete the histone H3 tails. The regions modeled by Loopy did not violate atomic bond constraints. A complete atomic model of a mononucleosome and a dinucleosome was constructed using a high-resolution mononucleosome crystal structure as a starting point (46). Given the high degree of conformational flexibility in HP1, the resulting model represents only one of a possible multitude of structures.
Drosophila Cultures
Drosophila stocks were maintained at room temperature on standard sucrose/cornmeal medium. The generation of the LacI-HP1a and lacO repeat transgenic stocks were described previously (34). For the genetic complementation studies, genetic crosses were set up to introduce a single copy of a given transgene into a trans-heterozygous HP1a mutant background, and the resulting larvae were heat shocked daily at 37 °C for 1 h throughout development.
Polytene Chromosome Staining
Third instar larvae were subjected to a 1 h heat-shock at 37 °C. After a 1-h recovery period at room temperature, salivary glands were dissected, fixed, squashed, and stained with mouse antibodies to LacI (Upstate Biotechnology no. 05-501, clone 9A5, 1:300 dilution) followed by donkey anti-mouse Alexa Fluor 488 (Invitrogen, 1:300). Images were obtained using a Bio-Rad confocal microscope.
RESULTS
Interactions between HP1Hsα and Nucleosomal Arrays
To better understand the determinants and structural ramifications of HP1Hsα binding to nucleosomal arrays, we utilized a minimal system reconstituted from pure, homogenous components.
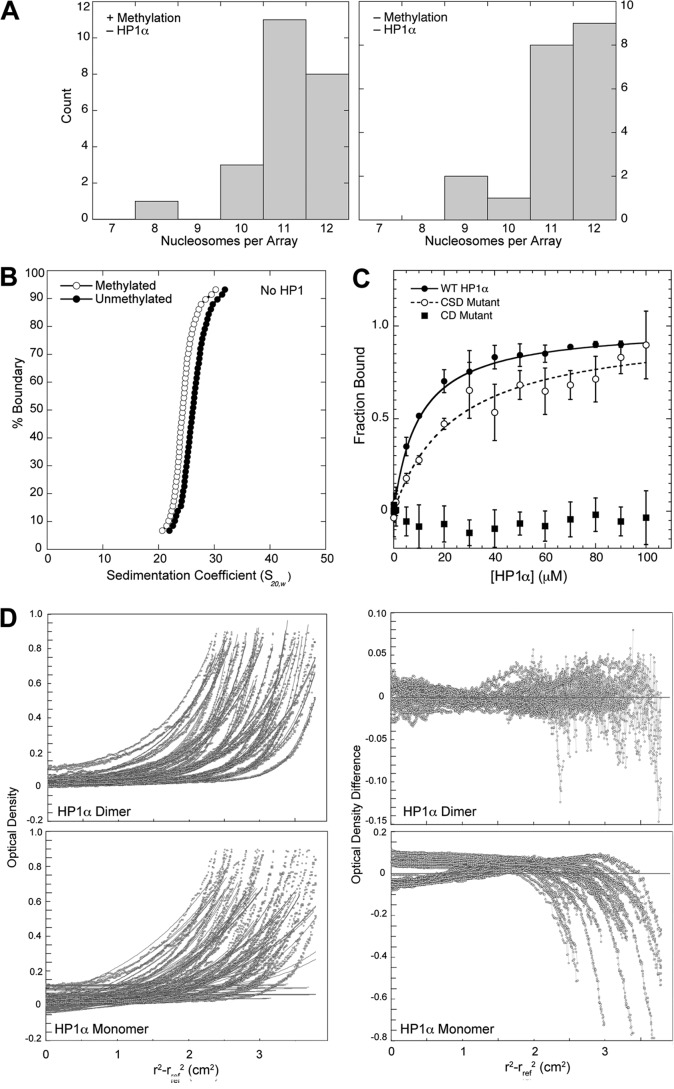
Nucleosomal arrays were assembled from recombinant purified histones deposited on a DNA template consisting of 12 tandem head-to-tail repeats of a 177-bp 601 positioning sequence (22, 24). Homogeneous histone H3K9Me3 was prepared by native chemical ligation (23). Nucleosomal arrays containing histone H3K9Me3 and unmethylated H3 were prepared with the same initial ratio of histone octamer to DNA and showed similar distributions of nucleosome occupancy (Fig. 1A), with an average number of nucleosomes of 11.10 ± 0.20 and 11.20 ± 0.21, respectively. Size exclusion chromatography coupled with static light scattering analysis of the wild type arrays showed molecular weights consistent with an average array saturation of 11.15-histone octamers per array (data not shown). In the absence of HP1Hsα, these arrays exhibited similar biophysical properties, although sedimentation velocity analysis indicated that the H3K9Me3 arrays adopt a slightly more extended conformation than the unmethylated arrays (Fig. 1B). This might be due to the increased negative charge character of the histone H3 tail that results from the partial deprotonation of the A25C substitution used to install H3K9 methylation by native chemical ligation.
FIGURE 1.
Characterization of 601-177-12 nucleosomal arrays and recombinant HP1Hsα. A, distribution of nucleosomes per array for histone H3K9Me3-containing and unmethylated arrays at 8 mm NaCl. Numbers of nucleosomes/per array were made from electron micrographs similar to those shown in Fig. 4, A and B. B, integrated sedimentation coefficient distribution of arrays possessing histone H3K9Me3 and unmethylated H3. Midpoint S values for the methylated and unmethylated arrays are 24.8S and 26.0S, respectively. Sedimentation values were calculated using the enhanced van Holde-Weischet method and normalized to standard conditions of pure water at 20 °C. C, HP1Hsα binding to a histone H3K9me3 peptide was determined by fluorescence anisotropy for wild type, CD mutant, and CSD mutant proteins. KD values for the wild type, CSD mutant, and CD mutant were 9 ± 2 μm, 24 ± 1 μm, and >500 μm, respectively. S.D. for each data point is shown. D, characterization of HP1Hsα dimerization by equilibrium analytical ultracentrifugation. Wild type and mutant forms of HP1Hsα were analyzed at nine different concentrations in triplicate. Shown are the fits and residuals for wild type HP1Hsα, where the fits are forced to conform to either a dimeric species (top panels) or a monomeric species (bottom panels). Fits to the data are shown on the left; fit residuals are shown on the right. The expected molecular weight for the HP1Hsα monomers is 25.4 kDa, and the best-fit molecular weight values of wild type, CD mutant, and CSD mutant HP1Hsα proteins were 48.6, 45.2, and 22.6 KDa, respectively.
Full-length recombinant human HP1Hsα (20) was expressed, purified, and functionally characterized. This HP1Hsα showed a binding affinity of 9 ± 2 μm for a histone H3K9Me3 peptide (Fig. 1C). This is consistent with a value of 4 ± 1 μm for Drosophila HP1a tested under similar conditions (21). Additionally, HP1Hsα showed expected dimer formation (Fig. 1D).
To study the binding of HP1Hsα to nucleosomal arrays, EMSA titration experiments were performed. In the absence of HP1Hsα, histone H3K9Me3-containing and unmethylated nucleosome arrays exhibited a single major electrophoretic species (Fig. 2A, left lane, top and bottom panel). Addition of HP1Hsα to the histone H3K9Me3-containing arrays generated a reduced mobility with a half-shift concentration of 16 nm (Fig. 2A, top panel). In contrast, arrays lacking H3K9Me3 showed no shift in mobility at comparable concentrations and required HP1Hsα concentrations of >2 μm to generate a shifted complex (Fig. 2A, bottom panel). To test for specificity, arrays containing histone H3K4Me3 were incubated with HP1Hsα. These arrays showed a half-shift concentration between 128–256 nm (Fig. 2B), which was 8-fold greater than that observed for arrays containing histone H3K9Me3. Taken together, these data demonstrate that histone H3K9Me3 promotes binding of HP1Hsα to nucleosomal arrays and HP1Hsα preferentially binds histone H3K9Me3, consistent with published studies (16, 21).
FIGURE 2.

Properties of nucleosomal arrays that affect HP1Hsα binding. A, EMSA analysis of HP1Hsα and methylated and unmethylated nucleosomal arrays. HP1Hsα was incubated with 12-mer nucleosomal arrays containing histone H3K9Me3-containing arrays (top) or unmodified H3 (bottom) at 50 mm NaCl. All titrations were performed using 8.0 nm of 177 bp of DNA repeat. Bound products were resolved on an agarose gel with the running buffer containing 150 mm NaCl. Arrays were visualized by staining with SYBR Gold. B, EMSA analysis of HP1Hsα and histone H3K4Me3-containing arrays under identical conditions as described in A. C, EMSA analysis of HP1Hsα and H3K9Me3-containing monocucleosomes (monos) under conditions identical to A. D, EMSA analysis of HP1Hsα and unmethylated nucleosomal arrays and template DNA under different ionic strengths. The salt conditions are indicated at the left and were used for both HP1Hsα binding and gel electrophoresis.
We investigated how neighboring nucleosomes affect HP1Hsα binding. Titrations were performed using histone H3K9Me3-containing mononucleosomes assembled on a single repeat unit of the 601-177-12 DNA template (Fig. 2C). Similar to the nucleosomal arrays, a reduced mobility species was observed at higher HP1Hsα concentrations; however, the half-saturation concentration for binding was ∼1.0 μm, greater than that observed for the arrays. These data demonstrate that neighboring nucleosomes are not essential for HP1Hsα, yet they appear to foster binding.
Compared with published studies (16, 17), our data showed relatively weak binding of HP1Hsα to nucleosome arrays lacking histone methylation. This difference might be due in part to their use of relatively low ionic strengths in the HP1 binding or EMSA running buffers, where nonspecific electrostatic DNA interactions are favored. Indeed, we found that reducing the ionic strength of the EMSA binding and electrophoresis buffer resulted in increased binding of HP1Hsα to unmethylated arrays (Fig. 2D, left panel). To determine whether these interactions could be DNA-mediated, titrations were performed with the DNA template used for array assembly (Fig. 2D, right panel). The results showed similar, if not enhanced, HP1Hsα-mediated shifts in mobility, suggesting that the methylation-independent nucleosomal array interactions have a nonspecific DNA-binding component.
The HP1Hsα CD and CSD Play Essential Roles in Nucleosome Interactions in Vitro and in Vivo
To determine the role of HP1Hsα domains in directing nucleosome binding, titration studies with wild type and mutant versions of HP1Hsα were performed (Fig. 3A). An HP1Hsα mutant possessing amino acid substitution W71A (CD mutant) that eliminates binding to a histone H3K9Me3 peptide (Fig. 1C) (36) did not show significant binding to methylated arrays and methylated (and unmethylated) mononucleosomes (Fig. 3, A and B). An HP1Hsα mutant possessing amino acid substitution I195E (CSD mutant), which abolishes HP1Hsα dimerization (data not shown), also failed to show binding to these substrates (Fig. 3, A and B). Thus, both the CD and the CSD play critical roles in binding methylated mononucleosomes as well as methylated nucleosomal arrays.
FIGURE 3.
Functional determinants of HP1Hsα and HP1a for association with nucleosomal substrates. A, EMSA analysis of HP1Hsα and methylated (Methyl) 12-mer nucleosome arrays (8. 0 nm of 177-bp DNA repeats). Arrays were detected by staining with SYBR Gold. Note that half-saturation of wild type HP1Hsα binding occurs at 16 nm, whereas both mutants fail to bind even at 500 nm. B, EMSA analysis of HP1Hsα and 32.0 nm of either unmethylated (Unmeth) or histone H3K9Me3-containing mononucleosomes (Mono). C, chromosomal localization of HP1a with and without the hinge domain. Polytene chromosomes were prepared from larvae rescued by expression of wild type HP1a (left panel) or HP1a lacking a hinge (ΔH; right panel) and stained with antibodies that recognize the LacI tag fused to HP1a. C denotes the centric region; 31 denotes cytological region 31 on chromosome 2L, a euchromatic region known to bind HP1a.
To extend our in vitro observations, we performed in vivo analysis using Drosophila melanogaster, which provides a genetic system to test the function of the mutant HP1 proteins. Our selection of Drosophila was based on the fact that human HP1Hsα rescues lethality caused by mutations in Su(var)2-5, the gene encoding HP1a (37). Transgenic flies expressing tagged wild type and mutant versions of HP1a analogous to those used for the in vitro experiments were generated.
We tested whether these HP1a transgenes could rescue the lethality of Su(var)2-5 trans-heterozygous mutants (Table 1), which die at the late third larval instar stage (38). Both wild type and mutant versions of tagged HP1a were expressed via a heat shock promoter induced by daily heat shock. Western analysis revealed that the mutant proteins were expressed at similar levels to each other and comparable with endogenous levels of HP1a (data not shown). Wild type HP1a partially rescued lethality; based on Mendelian ratios, 42% of the expected class survived to adulthood (Table 1). In contrast, HP1a possessing V26M (CD mutant), which disrupts interaction with histone H3K9Me3, failed to rescue. Likewise, HP1a possessing amino acid substitution I191E (CSD mutant), which causes lack of HP1a homodimerization, also failed to rescue. To determine whether the CD and CSD were sufficient for viability, an HP1a mutant containing a deletion of the hinge region (amino acids 83–131, ΔH) was tested. This mutant gave 18% rescue (Table 1). Thus, the CD and CSD, but not the hinge, are required for essential functions of HP1a.
TABLE 1.
Genetic complementation analyses using Su(var)2-5 (i.e. HP1a) transgenes
| Parental genotypes |
Progeny |
% of expected class | ||
|---|---|---|---|---|
| Female | Male | Curly wings | Straight wings (rescued) | |
| P{HP1a}/P{HP1a}; Su(var)2-504/CyO | Su(var)2-502/CyO | 446 | 64 | 42 |
| Su(var)2-502/CyO | Su(var)2-504/CyO; P{V26M}/P{V26M} | 426 | 0 | 0 |
| Su(var)2-502/CyO | P{I191E}, Su(var)2-504/CyO | 413 | 0 | 0 |
| P{Δhinge}/P{Δhinge}; Su(var)2-502/CyO | Su(var)2-504/CyO | 302 | 19 | 18 |
The fact that the hinge deletion rescued lethality was surprising because chromosome localization has been attributed to the hinge (39). To determine the localization of the hinge deletion, polytene chromosomes were stained with antibodies to HP1a. The pattern of localization was nearly identical to that observed for HP1a in the wild type HP1a rescued flies (Fig. 3C). These data provide an explanation for rescue with the hinge deletion.
HP1Hsα Binding to Nucleosomal Arrays Promotes Chromatin Condensation
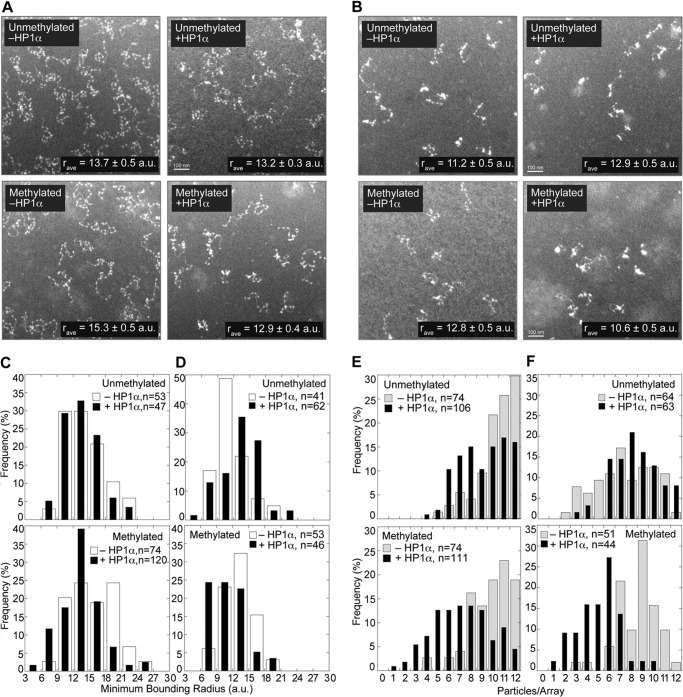
To directly understand the structural implications of HP1Hsα, binding to nucleosome arrays, we visualized bound arrays using electron microscopy (EM). Two ionic strength conditions (8 mm and 50 mm NaCl) were used (Fig. 1A), and the ability to observe 12 nucleosomes per array when the arrays are extended is a testament to the sensitivity of the method. The micrographs were analyzed for changes in array condensation due to HP1Hsα binding using two published methods (40). First, the radius of the smallest circle that encloses a nucleosomal array defined as the minimal bounding radius (r), reported in arbitrary units was used as a unit of measurement. The frequency of each r value is shown in histogram format (Fig. 4, C and D). Second, the total number of visible particles per array was counted (Fig. 4, E and F). The rationale was that condensation would promote association of nucleosomes resulting in a reduced particle count. The total number of particles per array varies due to differences in nucleosomal saturation, nucleosomal overlap from placement on the EM grid, and binding interactions between nucleosomes. The distribution of particles per array is not Gaussian, thus distributions were compared using a non-parametric Kolmogorov-Smirnov test.
FIGURE 4.
Electron microscopy analysis of HP1Hsα -nucleosomal array complexes. A and B, representative EM images of HP1Hsα bound to 12-mer nucleosomal arrays containing unmodified histone H3 and H3K9Me3 at either 8 (A) or 50 mm (B) NaCl. The average minimal bounding radius (r) represents the radius of the smallest circle that contains a given array (in arbitrary length units, a.u.). C and D, histogram of the frequency of r values determined from 8 and 50 mm NaCl conditions, respectively. Because the r values are continuous, arbitrary threshold radii (values under the tick marks) were selected. The fraction of particles with r values between the threshold values was tabulated, where the total number of particles analyzed per sample is indicated by n. This binning of r values was performed to show this distribution graphically but was not used in the statistical analysis. E and F, histogram of the frequency of number of particles per nucleosomal array from 8 and 50 mm NaCl conditions, respectively. Particles were counted from EM images and represent either individual nucleosomes or compacted clusters of nucleosomes that could not be resolved.
At low ionic strength in the absence of histone H3K9Me3, HP1Hsα did not significantly alter the compaction of the arrays (Fig. 4, A and C; p = 0.300). In contrast, arrays containing histone H3K9Me3 showed compaction upon HP1Hsα addition (Fig. 4, A and C; p = 6.7 × 10−5), with the average r value decreasing from 15.3 ± 0.5 arbitrary units to 12.9 ± 0.4 arbitrary units, indicating that HP1Hsα promotes condensation of methylated nucleosomal arrays. Likewise, there was not a statistically significant difference in particle counts between the methylated and unmethylated arrays (Fig. 4, A and E; p = 0.285). Upon addition of HP1Hsα, there was a shift in the distribution toward fewer particles in the unmethylated arrays and a more pronounced shift with the methylated arrays. The distribution change in the unmethylated arrays due to the addition of HP1Hsα was statistically significant (Fig. 4, A and E; p = 0.014), consistent with HP1Hsα being able to promote nucleosome association even in the absence of nucleosome methylation. The distribution change upon the addition of HP1Hsα to the methylated arrays was also statistically significant (Fig. 4, A and E; p = 4.3 × 10−8). Moreover, the difference in the methylated and unmethylated distributions in the presence of HP1Hsα was statistically significant (p = 3.3 × 10−4).
Increased ionic strength has been shown to cause nucleosome array compaction (41). Indeed, with increased ionic strength the methylated and unmethylated arrays were more condensed with respect to visual appearance (Fig. 4, B compared with A), r value (Fig. 4, D compared with C), and particle counts (Fig. 4, F and E). Upon addition of HP1Hsα to these more compacted arrays, the methylated arrays show enhanced compaction (Fig. 4, B and F): the r value and particle count decreased (p = 0.0012 and 6 × 10−11, respectively). This is in contrast to the precompacted unmethylated arrays, where HP1Hsα induces no significant change in particle count (p = 0.148), and results in an increase in r value from 11.2 ± 0.5 to 12.9 ± 0.5 (p = 0.012). Thus, HP1Hsα augmented existing condensation in arrays containing histone methylation. Collectively, the EM analysis shows that HP1Hsα promotes nucleosome associations within an array, especially when arrays are predisposed to condensation.
HP1Hsα Promotes Intra- and Interarray Interactions
Nucleosomal arrays can be induced to undergo reversible strand-to-strand interactions (29), and binding to HP1Hsα might influence this interaction. To directly test for this, differential sedimentation analyses were performed (29). Incubation of unmethylated arrays with increasing amounts of HP1Hsα did not enhance the propensity of the arrays to self-associate (Fig. 5A, left panel). In contrast, incubation of histone H3K9Me3-containing arrays with increasing concentrations of HP1Hsα made the arrays more sensitive to magnesium-induced association (Fig. 5A, middle panel). Additionally, this increased propensity for array self-association required dimerization, as the HP1Hsα defective for dimerization (CSD mutant) did not promote self-association of H3 methylated arrays (Fig. 5A, right panel). These data suggest that, in vitro, HP1Hsα dimers interact with H3 methylated nucleosomes to promote intra- and interarray interactions.
FIGURE 5.
Interarray interactions induced by HP1Hsα. A, differential sedimentation analysis of interarray associations for nucleosomal arrays possessing unmethylated and methylated nucleosomes, with wild type and mutant (Mut) versions of HP1Hsα. The percent of material in the supernatant is plotted as a function of divalent magnesium cation concentration. Error bars represent S.D. from at least three replicates, with stars representing statistically significant differences as determined by the Student's t test analysis with a p cut-off value of 0.05. B, cytological evidence of HP1a bridging distant chromosome sites. A LacI-HP1a fusion protein was expressed in flies possessing lac operator repeats inserted near the telomere on the X chromosome (arrow) in an otherwise wild type background. Third instar larval polytene chromosomes were fixed and stained with antibodies to LacI. HP1a tethering caused chromosome folding and looping in 36% percent of the chromosomes. These loops formed by interactions between the HP1a-tethered site and a distant chromosome site (two right panels). In contrast, tethering a control LacI-GFP to the same genomic site produced isolated linear chromosomes with reduced numbers of such interactions (left panel). T denotes telomeres.
Tethering Drosophila HP1a to specific sites in the genome results in chromosome looping (42, 43), similar to our in vitro observations (Fig. 5B). To determine the requirements for such interactions, we expressed LacI-HP1a fusion proteins in a genetic background with lacO sequences inserted at specific genomic sites (42). Polytene chromosomes were fixed, stained with antibodies against LacI, and examined with light microscopy. In these preparations, 36% of the chromosomes bound by wild type HP1a exhibited a loop and/or folded configuration in which the HP1a tethered site was associated with a distant chromosome site (Fig. 5B). In contrast, only 8% for the control LacI-GFP-bound chromosomes showed the folded configuration, with the remaining chromosomes showing an HP1a tethered site that was not interacting with other chromosome regions (Fig. 5B). Additionally, the HP1a mutant I191E that cannot dimerize displayed only 16% interactions, consistent with the dependence of CSD for in vitro nucleosome array interactions (Fig. 5A, right panel). Taken together, these data suggest that HP1a dimers can facilitate distant interactions on nucleosome templates.
In Silico Modeling of HP1Hsα-nucleosome Interactions
To provide a higher resolution understanding of how HP1Hsα dimers might interact with nucleosomes, computational docking studies were performed. Atomic resolution models were generated for both HP1Hsα and a nucleosome. Then, the CDs of an HP1Hsα dimer were docked onto the two histone H3K9Me3 tails of a mononucleosome and onto two histone H3K9Me3 tails of adjacent nucleosomes. These atomic resolution models showed that the two CDs of an HP1Hsα dimer are capable of bridging two histone H3K9Me3 residues on the same or neighboring nucleosomes. (Fig. 6A).
FIGURE 6.
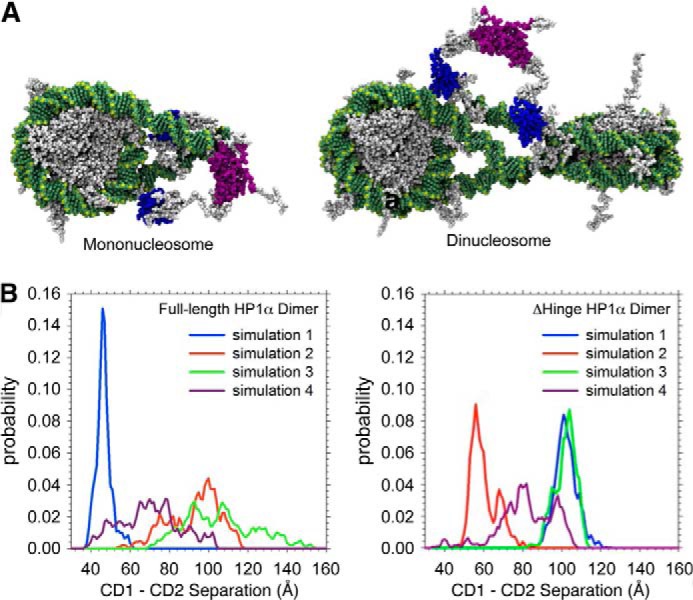
In silico models of HP1Hsα-nucleosome interactions. A, all atom atomic resolution model of an HP1Hsα dimer bound to a mononucleosome (left) and dinucleosome (right). DNA (green) wrapped around nucleosomes (gray) that are bound by HP1HSα (CD, blue; CSD, magenta; unstructured region, white). B, graphical representation of the probability of the distance between CDs of an HP1Hsα dimer obtained by molecular dynamics simulations. Different color lines represent four independent 100-ns simulations. Each simulation used a different starting configuration of the HP1Hsα dimer in which the CD-CD distance was different. The probability of the distance between CDs of a wild type HP1Hsα dimer (left) and a dimer lacking both hinge domains (right) is shown. Both wild type and mutant HP1Hsα dimers exhibit similar ranges that accommodate the bridging of histone H3K9Me3 tails in the atomic resolution models in A.
A key feature of these models is that the CD-CD separation must be sufficient to bridge between two histone H3K9Me3 residues. We wondered whether the flexible hinge played a role in the distance of CD-CD separation. Molecular dynamics simulations of HP1Hsα using a full atom in silico model were performed. Four different initial starting configurations in which the hinge adopted different structures that positioned the CD and CSD at varying distances from one another were used. These simulations provided a determination of the range of motion exhibited between the two CD domains (Fig. 6). The molecular dynamics data demonstrated substantial flexibility of the wild type HP1Hsα dimer; the distance between the two CDs ranged from 40 to 160 Å (Fig. 6, left panel). This range of motion was not significantly altered by deletion of the hinge region (Fig. 6, right panel). These data indicate that both wild type and hinge deletion can adopt similar structures that exhibit similar flexibility in CD-CD distance, consistent with the wild type and hinge deletion having a similar localization pattern in vivo (Fig. 3C).
DISCUSSION
The discovery that the HP1 CD binds histone H3K9Me2/3 led to models in which this interaction provides the structural basis for the condensed structure of pericentric heterochromatin (5–7, 44). Despite studies showing that histone H3K9Me3 methylation in nucleosomal arrays facilitates HP1 binding (15, 16), details regarding the nature of the complex and the consequences of binding remained unknown.
Here, we show that HP1Hsα binds histone H3K9Me3-containing nucleosome arrays (Fig. 2A) and that this binding is facilitated by both K9 methylation and the ability of HP1Hsα to recognize this methylation via a CD (Fig. 3A). In contrast to other studies, we did not find that additional factors such as chromatin remodeling complexes (15) are necessary for robust binding.
The binding of HP1Hsα to histone H3K9Me3-containing arrays affects higher-order chromatin structure. Our EM analyses (Fig. 4) demonstrate that HP1Hsα binding results in intra-array chromatin condensation. From in vitro and in vivo analyses, HP1Hsα binding facilitates associations within and between chromatin strands (Figs. 4 and 5). It is possible that both types of interactions are involved in the formation of heterochromatin.
The presence of histone H3K9Me3 is not essential for HP1Hsα binding; significant HP1Hsα binding was observed to unmethylated arrays and free DNA when NaCl concentrations in both the binding and EMSA buffer were at or below 50 mm (Fig. 2D). However, this methylation-independent binding is diminished relative to methylation-dependent binding when approaching physiological salt concentrations. In previous studies of HP1 binding to unmethylated nucleosomes (16, 17, 35), HP1 binding and/or EMSA running buffers were at ionic strengths below 50 mm, potentially accounting for the tighter non-methylated nucleosomal binding. However, these differences may be due to other parameters that differ between experiments. For our systems, we also note that, though there is more methylation-independent binding at lower salt conditions, the amount of intra-array compaction mediated by HP1Hsα is significantly less than that observed for a methylated array (Fig. 5).
Methyl-independent binding that involves direct DNA association might be an important aspect of the function of HP1 proteins. Indeed, binding of Drosophila HP1a to telomeric DNA was shown to be critical for telomere maintenance (38). This direct DNA interaction might also be a contributing factor to the apparent enhanced affinity of HP1Hsα for histone H3K9Me3-containing arrays relative to histone H3K9Me2/3 mononucleosomes (Fig. 2, A and C). Both nucleosome systems have 177 bp of DNA per nucleosome, with the octamer roughly positioned in the middle. However, due to the fact that the DNA in an array is contiguous, an HP1Hsα molecule might have access to twice as much DNA between nucleosomes in an array versus a mononucleosome.
The mechanisms by which HP1Hsα mediates compaction within and between arrays are not clear. Our computational studies show that the CDs of an HP1Hsα dimer can span 4 to 16 nm (Fig. 6B), and docking studies demonstrate that this distance is sufficient to accommodate association with a histone H3K9Me3 containing mono- and dinucleosome (Fig. 6A). Our in vitro studies show that amino acid substitutions in the CSD, which disrupt dimerization, eliminate binding to methylated nucleosomes (Fig. 3). In addition, the propensity for strand-to-strand interactions is decreased in the absence of a functional CD (Fig. 5A). Collectively, these data are consistent with models in which HP1Hsα dimers can bridge two methylated tails within a mononucleosome or between nucleosomes (Fig. 6A).
Our studies examined binding of HP1Hsα to both mononucleosomes and nucleosomal arrays. The EMSA analyses showed that the half-saturation concentration required for electrophoretic mobility shift is at least 62.5-fold greater for the methylated mononucleosomes than the arrays. Although a direct comparison cannot be made because it is not known how many HP1Hsα molecules are required to cause a gel shift, we assumed that at least one HP1Hsα is bound per array. Given that assumption, our calculations indicate that the 62.5-fold difference in half-shift concentrations for the array is greater than would be expected if each nucleosome in an array bound HP1Hsα similar to a mononucleosome (see “Experimental Procedures”). Thus, our data suggests that methylated arrays enhance HP1Hsα binding relative to methylated mononucleosomes.
We have demonstrated that the presence of histone H3K9Me3 in nucleosomal arrays is sufficient for HP1Hsα binding and that HP1Hsα association is accompanied by changes in higher-order chromatin structure. Other determinants are expected to affect the formation and maintenance of the compact state. These factors include nucleosome arrangement, as ordered nucleosomal arrays are a hallmark of heterochromatin (11, 13), and histone deacetylation, which is required for HP1Hsα association (45).
Acknowledgments
K. Gadhok and A. Peterson are acknowledged for technical assistance with Drosophila experiments. K. Wakeland is acknowledged for help with the statistical analysis of the EM images. Computational time on the TACC supercomputing resource Ranger provided through the National Science Foundation funded TeraGrid and XSEDE programs is acknowledged.
This work was supported, in whole or in part, by Ruth L. Kirschtein National Research Service Award Postdoctoral Fellowship GM08574 (to M. W. V.), National Institutes of Health Grant GM061513 (to L. L. W.), American Cancer Society Research Scholar Grant 1206501 (to M. J. B.), and National Institutes of Health GM79663-03S1 (to M. A. S.-K.).
- CD
- chromo domain
- CSD
- chromo shadow domain.
REFERENCES
- 1. Dialynas G. K., Vitalini M. W., Wallrath L. L. (2008) Linking Heterochromatin Protein 1 (HP1) to cancer progression. Mutat. Res. 647, 13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heitz E. (1928) Das Heterochromatin der Moose. Jahrb. Wiss Botanik. 69, 762–818 [Google Scholar]
- 3. Weiler K. S., Wakimoto B. T. (1995) Heterochromatin and gene expression in Drosophila. Annu. Rev. Genet. 29, 577–605 [DOI] [PubMed] [Google Scholar]
- 4. Lomberk G., Wallrath L., Urrutia R. (2006) The heterochromatin protein 1 family. Genome Biol. 7, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacobs S. A., Taverna S. D., Zhang Y., Briggs S. D., Li J., Eissenberg J. C., Allis C. D., Khorasanizadeh S. (2001) Specificity of the HP1 chromo domain for the methylated N terminus of histone H3. EMBO J. 20, 5232–5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakayama J., Rice J. C., Strahl B. D., Allis C. D., Grewal S. I. (2001) Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292, 110–113 [DOI] [PubMed] [Google Scholar]
- 7. Bannister A. J., Zegerman P., Partridge J. F., Miska E. A., Thomas J. O., Allshire R. C., Kouzarides T. (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410, 120–124 [DOI] [PubMed] [Google Scholar]
- 8. Cowieson N. P., Partridge J. F., Allshire R. C., McLaughlin P. J. (2000) Dimerisation of a chromo shadow domain and distinctions from the chromodomain as revealed by structural analysis. Curr. Biol. 10, 517–525 [DOI] [PubMed] [Google Scholar]
- 9. Brasher S. V., Smith B. O., Fogh R. H., Nietlispach D., Thiru A., Nielsen P. R., Broadhurst R. W., Ball L. J., Murzina N. V., Laue E. D. (2000) The structure of mouse HP1 suggests a unique mode of single peptide recognition by the shadow chromo domain dimer. EMBO J. 19, 1587–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thiru A., Nietlispach D., Mott H. R., Okuwaki M., Lyon D., Nielsen P. R., Hirshberg M., Verreault A., Murzina N. V., Laue E. D. (2004) Structural basis of HP1/PXVXL motif peptide interactions and HP1 localisation to heterochromatin. EMBO J. 23, 489–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wallrath L. L., Elgin S. C. (1995) Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev. 9, 1263–1277 [DOI] [PubMed] [Google Scholar]
- 12. Cryderman D. E., Cuaycong M. H., Elgin S. C., Wallrath L. L. (1998) Characterization of sequences associated with position-effect variegation at pericentric sites in Drosophila heterochromatin. Chromosoma 107, 277–285 [DOI] [PubMed] [Google Scholar]
- 13. Sun F. L., Cuaycong M. H., Elgin S. C. (2001) Long-range nucleosome ordering is associated with gene silencing in Drosophila melanogaster pericentric heterochromatin. Mol. Cell. Biol. 21, 2867–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lachner M., O'Carroll D., Rea S., Mechtler K., Jenuwein T. (2001) Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410, 116–120 [DOI] [PubMed] [Google Scholar]
- 15. Eskeland R., Eberharter A., Imhof A. (2007) HP1 binding to chromatin methylated at H3K9 is enhanced by auxiliary factors. Mol. Cell. Biol. 27, 453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Canzio D., Chang E. Y., Shankar S., Kuchenbecker K. M., Simon M. D., Madhani H. D., Narlikar G. J., Al-Sady B. (2011) Chromodomain-mediated oligomerization of HP1 suggests a nucleosome-bridging mechanism for heterochromatin assembly. Mol. Cell 41, 67–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan J. Y., Rangasamy D., Luger K., Tremethick D. J. (2004) H2A. Z alters the nucleosome surface to promote HP1α-mediated chromatin fiber folding. Mol. Cell 16, 655–661 [DOI] [PubMed] [Google Scholar]
- 18. Cryderman D. E., Grade S. K., Li Y., Fanti L., Pimpinelli S., Wallrath L. L. (2005) Role of Drosophila HP1 in euchromatic gene expression. Dev. Dyn 232, 767–774 [DOI] [PubMed] [Google Scholar]
- 19. de Wit E., Greil F., van Steensel B. (2005) Genome-wide HP1 binding in Drosophila: developmental plasticity and genomic targeting signals. Genome Res. 15, 1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mateescu B., England P., Halgand F., Yaniv M., Muchardt C. (2004) Tethering of HP1 proteins to chromatin is relieved by phosphoacetylation of histone H3. EMBO Rep. 5, 490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fischle W., Wang Y., Jacobs S. A., Kim Y., Allis C. D., Khorasanizadeh S. (2003) Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev. 17, 1870–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luger K., Rechsteiner T. J., Richmond T. J. (1999) Preparation of nucleosome core particle from recombinant histones. Methods Enzymol. 304, 3–19 [DOI] [PubMed] [Google Scholar]
- 23. Li S., Shogren-Knaak M. A. (2008) Cross-talk between histone H3 tails produces cooperative nucleosome acetylation. Proc. Natl. Acad. Sci. U.S.A. 105, 18243–18248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dorigo B., Schalch T., Bystricky K., Richmond T. J. (2003) Chromatin fiber folding: requirement for the histone H4 N-terminal tail. J. Mol. Biol. 327, 85–96 [DOI] [PubMed] [Google Scholar]
- 25. Shogren-Knaak M., Ishii H., Sun J. M., Pazin M. J., Davie J. R., Peterson C. L. (2006) Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 311, 844–847 [DOI] [PubMed] [Google Scholar]
- 26. Blacketer M. J., Feely S. J., Shogren-Knaak M. A. (2010) Nucleosome interactions and stability in an ordered nucleosome array model system. J. Biol. Chem. 285, 34597–34607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sinha D., Shogren-Knaak M. A. (2010) Role of direct interactions between the histone H4 Tail and the H2A core in long range nucleosome contacts. J. Biol. Chem. 285, 16572–16581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Demeler B., van Holde K. E. (2004) Sedimentation velocity analysis of highly heterogeneous systems. Anal. Biochem. 335, 279–288 [DOI] [PubMed] [Google Scholar]
- 29. Schwarz P. M., Felthauser A., Fletcher T. M., Hansen J. C. (1996) Reversible oligonucleosome self-association: dependence on divalent cations and core histone tail domains. Biochemistry 35, 4009–4015 [DOI] [PubMed] [Google Scholar]
- 30. Hess B., Kutzner C., van der Spoel D., Lindahl E. (2008) GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 [DOI] [PubMed] [Google Scholar]
- 31. Humphrey W., Dalke A., Schulten K. (1996) VMD: visual molecular dynamics. J. Mol. Graph. 14, 33-38 [DOI] [PubMed] [Google Scholar]
- 32. Kaustov L., Ouyang H., Amaya M., Lemak A., Nady N., Duan S., Wasney G. A., Li Z., Vedadi M., Schapira M., Min J., Arrowsmith C. H. (2011) Recognition and specificity determinants of the human cbx chromodomains. J. Biol. Chem. 286, 521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiang Z., Soto C. S., Honig B. (2002) Evaluating conformational free energies: the colony energy and its application to the problem of loop prediction. Proc. Natl. Acad. Sci. U.S.A. 99, 7432–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y., Danzer J. R., Alvarez P., Belmont A. S., Wallrath L. L. (2003) Effects of tethering HP1 to euchromatic regions of the Drosophila genome. Development 130, 1817–1824 [DOI] [PubMed] [Google Scholar]
- 35. Zhao T., Heyduk T., Allis C. D., Eissenberg J. C. (2000) Heterochromatin protein 1 binds to nucleosomes and DNA in vitro. J. Biol. Chem. 275, 28332–28338 [DOI] [PubMed] [Google Scholar]
- 36. Jacobs S. A., Khorasanizadeh S. (2002) Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science 295, 2080–2083 [DOI] [PubMed] [Google Scholar]
- 37. Norwood L. E., Grade S. K., Cryderman D. E., Hines K. A., Furiasse N., Toro R., Li Y., Dhasarathy A., Kladde M. P., Hendrix M. J., Kirschmann D. A., Wallrath L. L. (2004) Conserved properties of HP1(Hsα). Gene 336, 37–46 [DOI] [PubMed] [Google Scholar]
- 38. Fanti L., Giovinazzo G., Berloco M., Pimpinelli S. (1998) The heterochromatin protein 1 prevents telomere fusions in Drosophila. Mol. Cell 2, 527–538 [DOI] [PubMed] [Google Scholar]
- 39. Maison C., Bailly D., Roche D., Montes de Oca R., Probst A. V., Vassias I., Dingli F., Lombard B., Loew D., Quivy J. P., Almouzni G. (2011) SUMOylation promotes de novo targeting of HP1α to pericentric heterochromatin. Nat. Genet. 43, 220–227 [DOI] [PubMed] [Google Scholar]
- 40. Francis N. J., Kingston R. E., Woodcock C. L. (2004) Chromatin compaction by a polycomb group protein complex. Science 306, 1574–1577 [DOI] [PubMed] [Google Scholar]
- 41. Hansen J. C., Ausio J., Stanik V. H., van Holde K. E. (1989) Homogeneous reconstituted oligonucleosomes, evidence for salt-dependent folding in the absence of histone H1. Biochemistry 28, 9129–9136 [DOI] [PubMed] [Google Scholar]
- 42. Danzer J. R., Wallrath L. L. (2004) Mechanisms of HP1-mediated gene silencing in Drosophila. Development 131, 3571–3580 [DOI] [PubMed] [Google Scholar]
- 43. Seum C., Spierer A., Delattre M., Pauli D., Spierer P. (2000) A GAL4-HP1 fusion protein targeted near heterochromatin promotes gene silencing. Chromosoma 109, 453–459 [DOI] [PubMed] [Google Scholar]
- 44. Munari F., Soeroes S., Zenn H. M., Schomburg A., Kost N., Schröder S., Klingberg R., Rezaei-Ghaleh N., Stützer A., Gelato K. A., Walla P. J., Becker S., Schwarzer D., Zimmermann B., Fischle W., Zweckstetter M. (2012) Methylation of K9 in histone H3 directs alternative modes of highly dynamic interaction of heterochromatin protein hHP1βwith the nucleosome. J. Biol. Chem. 287, 33756–33765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Honda S., Lewis Z. A., Shimada K., Fischle W., Sack R., Selker E. U. (2012) Heterochromatin protein 1 forms distinct complexes to direct histone deacetylation and DNA methylation. Nat. Struct. Mol. Biol. 19, 471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davey C. A., Sargent D. F., Luger K., Maeder A. W., Richmond T. J. (2002) Solvent-mediated interactions in the structure of the nucleosome core particle at 1.9 [angstrom] resolution. J. Mol. Biol. 319, 1097–1113 [DOI] [PubMed] [Google Scholar]