Abstract
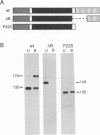
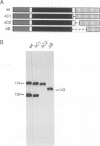
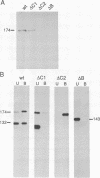
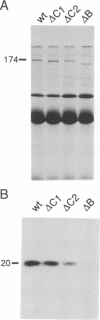
The ninaC locus encodes two unconventional myosins, p132 and p174, consisting of fused protein kinase and myosin head domains expressed in Drosophila photoreceptor cells. NinaC are the major calmodulin-binding proteins in the retina and the NinaC-calmodulin interaction is required for the normal subcellular localization of calmodulin as well as for normal photo-transduction. In the current report, we present evidence for two calmodulin-binding sites in NinaC, C1 and C2, which have different in vitro binding properties. C1 was found to be common to both p132 and p174 while C2 was unique to p174. To address the requirements for calmodulin binding at each site in vivo, we generated transgenic flies expressing ninaC genes deleted for either C1 or C2. We found that the spatial localization of calmodulin depended on binding to both C1 and C2. Furthermore, mutation of either site resulted in a defective photoresponse. A prolonged depolarization afterpotential (PDA) was elicited at lower light intensities than necessary to produce a PDA in wild-type flies. These results suggest that calmodulin binding to both C1 and C2 is required in vivo for termination of phototransduction.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentrop J., Plangger A., Paulsen R. An arrestin homolog of blowfly photoreceptors stimulates visual-pigment phosphorylation by activating a membrane-associated protein kinase. Eur J Biochem. 1993 Aug 15;216(1):67–73. doi: 10.1111/j.1432-1033.1993.tb18117.x. [DOI] [PubMed] [Google Scholar]
- Brockerhoff S. E., Stevens R. C., Davis T. N. The unconventional myosin, Myo2p, is a calmodulin target at sites of cell growth in Saccharomyces cerevisiae. J Cell Biol. 1994 Feb;124(3):315–323. doi: 10.1083/jcb.124.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byk T., Bar-Yaacov M., Doza Y. N., Minke B., Selinger Z. Regulatory arrestin cycle secures the fidelity and maintenance of the fly photoreceptor cell. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1907–1911. doi: 10.1073/pnas.90.5.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler M., Kroschewski R., Stöffler H. E., Behrmann T. Rat myr 4 defines a novel subclass of myosin I: identification, distribution, localization, and mapping of calmodulin-binding sites with differential calcium sensitivity. J Cell Biol. 1994 Jul;126(2):375–389. doi: 10.1083/jcb.126.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. R., Au D., Alexander K. A., Nicolson T. A., Storm D. R. Characterization of the calmodulin binding domain of neuromodulin. Functional significance of serine 41 and phenylalanine 42. J Biol Chem. 1991 Jan 5;266(1):207–213. [PubMed] [Google Scholar]
- Cheney R. E., Mooseker M. S. Unconventional myosins. Curr Opin Cell Biol. 1992 Feb;4(1):27–35. doi: 10.1016/0955-0674(92)90055-h. [DOI] [PubMed] [Google Scholar]
- Conzelman K. A., Mooseker M. S. The 110-kD protein-calmodulin complex of the intestinal microvillus is an actin-activated MgATPase. J Cell Biol. 1987 Jul;105(1):313–324. doi: 10.1083/jcb.105.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolph P. J., Ranganathan R., Colley N. J., Hardy R. W., Socolich M., Zuker C. S. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science. 1993 Jun 25;260(5116):1910–1916. doi: 10.1126/science.8316831. [DOI] [PubMed] [Google Scholar]
- Doza Y. N., Minke B., Chorev M., Selinger Z. Characterization of fly rhodopsin kinase. Eur J Biochem. 1992 Nov 1;209(3):1035–1040. doi: 10.1111/j.1432-1033.1992.tb17379.x. [DOI] [PubMed] [Google Scholar]
- Espindola F. S., Espreafico E. M., Coelho M. V., Martins A. R., Costa F. R., Mooseker M. S., Larson R. E. Biochemical and immunological characterization of p190-calmodulin complex from vertebrate brain: a novel calmodulin-binding myosin. J Cell Biol. 1992 Jul;118(2):359–368. doi: 10.1083/jcb.118.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espreafico E. M., Cheney R. E., Matteoli M., Nascimento A. A., De Camilli P. V., Larson R. E., Mooseker M. S. Primary structure and cellular localization of chicken brain myosin-V (p190), an unconventional myosin with calmodulin light chains. J Cell Biol. 1992 Dec;119(6):1541–1557. doi: 10.1083/jcb.119.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson H. V., Spudich J. A. Molecular evolution of the myosin family: relationships derived from comparisons of amino acid sequences. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):659–663. doi: 10.1073/pnas.90.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer J. A., 3rd The structure and function of unconventional myosins: a review. J Muscle Res Cell Motil. 1994 Feb;15(1):1–10. doi: 10.1007/BF00123827. [DOI] [PubMed] [Google Scholar]
- Hillman P., Hochstein S., Minke B. Transduction in invertebrate photoreceptors: role of pigment bistability. Physiol Rev. 1983 Apr;63(2):668–772. doi: 10.1152/physrev.1983.63.2.668. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Prendergast J. A., Singer R. A. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991 May;113(3):539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Isono K., Pye Q., Pak W. L. Gene encoding cytoskeletal proteins in Drosophila rhabdomeres. Proc Natl Acad Sci U S A. 1987 Feb;84(4):985–989. doi: 10.1073/pnas.84.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. A., Seperack P. K., Strobel M. C., Copeland N. G., Jenkins N. A. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature. 1991 Feb 21;349(6311):709–713. doi: 10.1038/349709a0. [DOI] [PubMed] [Google Scholar]
- Mismer D., Rubin G. M. Analysis of the promoter of the ninaE opsin gene in Drosophila melanogaster. Genetics. 1987 Aug;116(4):565–578. doi: 10.1093/genetics/116.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C., Rubin G. M. The Drosophila ninaC locus encodes two photoreceptor cell specific proteins with domains homologous to protein kinases and the myosin heavy chain head. Cell. 1988 Mar 11;52(5):757–772. doi: 10.1016/0092-8674(88)90413-8. [DOI] [PubMed] [Google Scholar]
- Mooseker M. Myosin superfamily: a multitude of myosins. Curr Biol. 1993 Apr 1;3(4):245–248. doi: 10.1016/0960-9822(93)90346-p. [DOI] [PubMed] [Google Scholar]
- Nagy K. Biophysical processes in invertebrate photoreceptors: recent progress and a critical overview based on Limulus photoreceptors. Q Rev Biophys. 1991 May;24(2):165–226. doi: 10.1017/s0033583500003401. [DOI] [PubMed] [Google Scholar]
- Pak W. L. Molecular genetic studies of photoreceptor function using Drosophila mutants. Prog Clin Biol Res. 1991;362:1–32. [PubMed] [Google Scholar]
- Phillips A. M., Bull A., Kelly L. E. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992 Apr;8(4):631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- Porter J. A., Hicks J. L., Williams D. S., Montell C. Differential localizations of and requirements for the two Drosophila ninaC kinase/myosins in photoreceptor cells. J Cell Biol. 1992 Feb;116(3):683–693. doi: 10.1083/jcb.116.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. A., Montell C. Distinct roles of the Drosophila ninaC kinase and myosin domains revealed by systematic mutagenesis. J Cell Biol. 1993 Aug;122(3):601–612. doi: 10.1083/jcb.122.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. A., Yu M., Doberstein S. K., Pollard T. D., Montell C. Dependence of calmodulin localization in the retina on the NINAC unconventional myosin. Science. 1993 Nov 12;262(5136):1038–1042. doi: 10.1126/science.8235618. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Smith D. P., Stamnes M. A., Zuker C. S. Signal transduction in the visual system of Drosophila. Annu Rev Cell Biol. 1991;7:161–190. doi: 10.1146/annurev.cb.07.110191.001113. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Swanljung-Collins H., Collins J. H. Ca2+ stimulates the Mg2(+)-ATPase activity of brush border myosin I with three or four calmodulin light chains but inhibits with less than two bound. J Biol Chem. 1991 Jan 15;266(2):1312–1319. [PubMed] [Google Scholar]
- Swanljung-Collins H., Collins J. H. Phosphorylation of brush border myosin I by protein kinase C is regulated by Ca(2+)-stimulated binding of myosin I to phosphatidylserine concerted with calmodulin dissociation. J Biol Chem. 1992 Feb 15;267(5):3445–3454. [PubMed] [Google Scholar]
- Titus M. A. Myosins. Curr Opin Cell Biol. 1993 Feb;5(1):77–81. doi: 10.1016/s0955-0674(05)80011-0. [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Spudich J. A. A functional recombinant myosin II lacking a regulatory light chain-binding site. Science. 1993 Dec 17;262(5141):1867–1870. doi: 10.1126/science.8266074. [DOI] [PubMed] [Google Scholar]
- Wolenski J. S., Hayden S. M., Forscher P., Mooseker M. S. Calcium-calmodulin and regulation of brush border myosin-I MgATPase and mechanochemistry. J Cell Biol. 1993 Aug;122(3):613–621. doi: 10.1083/jcb.122.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Harrison D. H., Schlichting I., Sweet R. M., Kalabokis V. N., Szent-Györgyi A. G., Cohen C. Structure of the regulatory domain of scallop myosin at 2.8 A resolution. Nature. 1994 Mar 24;368(6469):306–312. doi: 10.1038/368306a0. [DOI] [PubMed] [Google Scholar]
- Yamada T., Takeuchi Y., Komori N., Kobayashi H., Sakai Y., Hotta Y., Matsumoto H. A 49-kilodalton phosphoprotein in the Drosophila photoreceptor is an arrestin homolog. Science. 1990 Apr 27;248(4954):483–486. doi: 10.1126/science.2158671. [DOI] [PubMed] [Google Scholar]