Background: The circulatory ST6Gal-1 level is inversely related to hematopoietic activity, but the biochemical function of systemic ST6Gal-1 is unknown.
Results: Hematopoietic progenitors do not express self-ST6Gal-1 but are acted upon by remotely produced enzyme.
Conclusion: Distally produced rather than endogenous ST6Gal-1 is the principal modifier of the early hematopoietic progenitor cell surface.
Significance: Extrinsic ST6Gal-1 may be a potent systemic regulator of hematopoiesis.
Keywords: Glycosylation, Hematopoiesis, Plasma, Serum, Sialic Acid, Sialyltransferase, Hematopoietic Stem and Progenitor Cells, st6gal1
Abstract
Glycans occupy the critical cell surface interface between hematopoietic cells and their marrow niches. Typically, glycosyltransferases reside within the intracellular secretory apparatus, and each cell autonomously generates its own cell surface glycans. In this study, we report an alternate pathway to generate cell surface glycans where remotely produced glycosyltransferases remodel surfaces of target cells and for which endogenous expression of the cognate enzymes is not required. Our data show that extracellular ST6Gal-1 sialyltransferase, originating mostly from the liver and released into circulation, targets marrow hematopoietic stem and progenitor cells (HSPCs) and mediates the formation of cell surface α2,6-linked sialic acids on HSPCs as assessed by binding to the specific lectins Sambucus nigra agglutinin and Polysporus squamosus lectin and confirmed by mass spectrometry. Marrow HSPCs, operationally defined as the Lin−c-Kit+ and Lin−Sca-1+c-Kit+ populations, express negligible endogenous ST6Gal-1. Animals with reduced circulatory ST6Gal-1 have marrow Lin−Sca-1+c-Kit+ cells with reduced S. nigra agglutinin reactivity. Bone marrow chimeras demonstrated that α2,6-sialylation of HSPCs is profoundly dependent on circulatory ST6Gal-1 status of the recipients and independent of the ability of HSPCs to express endogenous ST6Gal-1. Biologically, HSPC abundance in the marrow is inversely related to circulatory ST6Gal-1 status, and this relationship is recapitulated in the bone marrow chimeras. We propose that remotely produced, rather than the endogenously expressed, ST6Gal-1 is the principal modifier of HSPC glycans for α2,6-sialic acids. In so doing, liver-produced ST6Gal-1 may be a potent systemic regulator of hematopoiesis.
Introduction
Hematopoiesis is the process through which bone marrow stem cells continuously regenerate all blood cell lineages while simultaneously self-renewing to replenish the stem cell pool. Any insufficiency of hematopoiesis, whether from iatrogenic causes secondary to cancer therapy, exposure to toxins, or unknown etiology, poses significant risks of mortality. A key parameter in the maintenance of the hematopoietic compartment is the residency of the hematopoietic stem and progenitor cells (HSPCs)4 in the appropriate supportive marrow niches (1). Although it is generally held that elaboration of hematopoietic progenitors is dictated principally by cytokines, this process hinges on the critical cell-cell and cell-matrix interactions with the marrow growth niches. By virtue of their presence on cell surfaces, sialyl glycans, which are common structures on mammalian cell surfaces, occupy the critical interphase between cells and their external environment. The sialic acid linkages in sialyl glycans are generated by the sialyltransferases, a conserved subfamily of glycosyltransferases (2). The sialyltransferase ST6Gal-1 constructs α2,6-sialyl linkages on the Galβ1-4GlcNAc termini of these conjugates. ST6Gal-1 is present in most tissues and is abundantly expressed in the liver (3).
Classically, glycosylation is presumed to be a cell-autonomous process in which individual cells express their own complement of glycosyltransferases to construct their cell surface and secreted glycans. However, a sizable pool of extracellular ST6Gal-1 that is primarily liver-originated also exists in circulation (4, 5). Despite the known association between the levels of circulatory ST6Gal-1 with inflammation and certain malignancies (6–8), the physiologic role of this bloodborne pool of sialyltransferase has remained elusive. To investigate the biology of this released pool of glycosyltransferase that is available systemically to distal tissues and cells, we generated a mouse model, St6gal1-dP1, with a partial deficiency of ST6Gal-1 limited to the liver-produced circulatory pool of ST6Gal-1 (9). The St6gal1-dP1 mouse had remarkably robust acute neutrophilic and eosinophilic inflammatory responses and faster recovery time to cyclophosphamide-induced myelosuppression secondary to elevated hematopoietic capability (10, 11). These observations led to the idea that the extracellular ST6Gal-1 in circulation is a systemic regulator of hematopoiesis in the marrow.
In a manner that departs from the canonical process of cell-autonomous glycosylation, we hypothesize that the systemic ST6Gal-1 can modify cell surface glycans on distal target cells. Our data show that marrow HSPC surface glycans are amply decorated with α2,6-linked sialic acids. However, HSPCs do not express appreciable amounts of endogenous ST6Gal-1 to construct these α2,6-sialyl linkages. Extrinsically produced ST6Gal-1, rather than the intrinsically produced enzyme, is the principal mediator of marrow HSPC surface α2,6-sialylation. Together, these new data implicate extrinsic ST6Gal-1 in the non-cell-autonomous construction of HSPC α2,6-sialyl linkages that results in attenuation of marrow hematopoietic activity.
EXPERIMENTAL PROCEDURES
Animals
The Institute Animal Care and Use Committee of Roswell Park Cancer Institute approved all animal studies. The St6gal1-dP1 mouse was generated by a specific disruption to the P1 promoter of the ST6Gal-1 gene, including removal of the 1.2-kb region containing Exon H (9, 10, 12). The St6gal1-dP1 mouse has a limited ST6Gal-1 deficiency restricted to the liver-produced pool of ST6Gal-1 (9). The St6gal1-KO mouse has a globally inactivated ST6Gal-1 gene and was originally produced by Marth and co-workers (13); it was obtained from the Consortium of Functional Glycomics. Both strains have been backcrossed >10 generations into C57BL/6 background. Age- and sex-matched C57BL/6 (CD45.2) or B6.SJL-Ptprc (CD45.1) animals were used as controls and are commonly referenced as “wild type” (WT). Adoptive bone marrow transfers were performed on lethally irradiated (1200 rads) recipient animals. Typically, 5 × 106 unfractionated donor marrow cells in a total volume of 200 μl of buffered saline were introduced by tail vein for each recipient animal. Routine FACS analysis for neutrophils (ly6G), B cells (B220), and T cells (CD3) was combined with anti-CD45.1 and anti-CD45.2 antibodies. Biotin-conjugated Sambucus nigra agglutinin (SNA) (0.2 μg/106 cells; Vector Laboratories) or Polysporus squamosus lectin (PSL) (0.2 μg/106 cells; EY Laboratories) was used followed by streptavidin-allophycocyanin. Direct FITC-conjugated lectins (0.2 μg/106 cells; Vector Laboratories) were also used in some situations with results identical to biotin-conjugated lectins. All antibodies were purchased from BioLegend (San Diego, CA).
HSPC Isolation and ex Vivo Cultivation
Bone marrow cells were collected from femurs of mice, resuspended in RBC lysis buffer (0.8% NH4Cl, 0.1 mm EDTA buffered with KHCO3 to pH 7.4), washed and resuspended in phosphate-buffered saline (PBS) with 0.5% BSA or fetal bovine serum and 2 mm EDTA, and then passed through a 100-μm cell strainer (BD Biosciences). Cells were centrifuged and resuspended in the same buffer (up to 2 × 108 cells/ml), and 50 μl/ml biotin-progenitor cell enrichment mixture was added to the cell suspension. Lineage depletion was accomplished by negative selection using magnetic microparticles according to the manufacturer's protocol (STEMCELL Technologies, Vancouver, British Columbia, Canada). Lin−c-Kit+ (LK) and Lin−Sca-1+c-Kit+ (LSK) cells were isolated from lineage-depleted pools using c-Kit (CD117) microbeads, or alternatively, LSK and LK cells were purified by FACS, yielding a purity routinely >90%. HSPCs were ex vivo cultured as follows: 105 wild-type (C57BL/6) LK cells were placed in 1 ml of serum-free medium (StemSpan® serum-free expansion medium, STEMCELL Technologies). Where indicated (see Fig. 1, G–I), recombinant ST6Gal-1 (3.4 microunits/ml) and/or CMP-Sia (50 μm) (see below) was also included for 3 h prior to addition of the cytokine mixture (50 ng/ml rmSCF, 25 ng/ml rmTPO, 30 ng/ml rmFlt3, 2 ng/ml IL-3, and 20 ng/ml rmG-CSF; purchased from BioVision, Milpitas, CA). Liquid cultures were maintained at 37 °C in 5% CO2 for 72 h.
FIGURE 1.
St6gal1-dP1 animals have elevated hematopoietic capacity. A–C, HSPCs are more abundant in the St6gal1-dP1 marrow. For A, B, and C, bone marrow cells were collected from age- and sex-matched C57BL/6 (W; open bars; n = 8), St6gal1-dP1 (P; hatched bars; n = 6), and St6gal1-KO (K; solid bars; n = 8). The total (Tot) cells of one femur were counted (A). Cells were analyzed by flow cytometry for LK (B) and LSK (C) populations. D–E, St6gal1-dP1 recipients support increased marrow HSPCs. Cells from wild-type (B6.SJL-Ptprc; CD45.1) marrows were adoptively transferred into C57BL/6 (W; CD45.2; n = 5; open bars) or St6gal1-dP1 (P; CD45.2; n = 6; hatched bars) recipients. The recipient marrows were analyzed 75 days after transplantation for total CD45.1+ cells (D) and in LK (E) and LSK (F) fractions. p values were <0.05 (*) and 0.01 (**). Error bars represent S.D. G–I, exogenously added ST6Gal-1 attenuates LK cell proliferation ex vivo. LK cells from wild-type marrows were cultured in the presence of rmSCF, rmTPO, rmFlt3, rmIL-3, and rmG-CSF at an initial seeding of 100,000 LK cells. G shows total cell counts after 72 h without (open bars) or with the additions of recombinant rat ST6Gal-1 (rST6) (hatched bars) or recombinant rat ST6Gal-1 and CMP-Sia (solid bars). G is a representative of this experiment, which was repeated independently four times. H and I show flow cytometry analysis of the study from G cultivated without (H) or with (I) recombinant ST6Gal-1 (rST) and CMP-Sia using anti-c-Kit and CD11b antibodies to visualize LK (c-Kit+CD11−), committed progenitors (c-Kit+CD11+), and differentiated cells (CD11+). J, HSPCs from St6gal1-KO can be sialylated by leakage from dying cells. 105 bone marrow cells of St6gal1-KO mice were hypotonically lysed for 10 min. After centrifugation, the supernatant was isolated and mixed with concentrated PBS to reconstitute the physiologic saline condition. The supernatant was added to 5 × 104 LK cells and incubated with (solid line) or without recombinant ST6Gal-1 (dashed line) for 2 h. As a positive control, LK cells were also incubated with ST6Gal-1 and 100 μm CMP-Sia (shaded area). SNA reactivity was analyzed by flow cytometry.
To test the ability of fresh bone marrow lysates to provide the sugar donor substrate for cell surface remodeling by ST6Gal-1, 105 St6gal1-KO bone marrow cells were hypotonically lysed in 10 μl of water for 10 min whereupon cell debris was removed from the supernatant by centrifugation. Concentrated PBS was added to the supernatant to reconstitute the physiologic saline condition. The reconstituted cell lysate supernatant was added to 5 × 104 St6gal1-KO LK cells in 40 μl of RPMI 1640 medium with or without recombinant ST6Gal-1 (3.4 microunits/ml) enzyme. For a positive control, CMP-Sia (100 μmol) instead of cell lysate was used. The cells were incubated at 37C in 5% CO2 for 2 h and analyzed by flow cytometry for cell surface SNA reactivity.
For colony-forming cell assays, bone marrow nucleated cells in a volume of 0.1 ml were plated in 0.9 ml of methylcellulose medium (MethoCult 3534, STEMCELL Technologies) in duplicate and placed in humidified incubator with 5% CO2 at 37 °C. Colonies containing at least 50 cells were counted 7 days after incubation. For the assessment of passive α2,6-sialyl glycan absorption by cultivated cells, St6gal1-KO (and wild type as a positive control) LK cells were cultivated (a) in RPMI 1640 medium containing 50% heat-treated (56 °C for 60 min) wild-type or St6gal1-KO serum for 3 h and (b) in StemSpan medium containing the above mentioned cytokines and 5-fold excess wild-type (CD45.1) or St6Gal1-KO (as a negative control) whole bone marrow cells for 3 and 7 days. The majority of cells of both genotypes were alive and proliferating after 3 days. On day 7, half of the cells were progeny of LK cells. SNA reactivity of LK cells or their progeny was assessed using anti-CD45.2 antibody by flow cytometry. To measure the absolute numbers of LK and LSK cells, we performed flow cytometry using a lineage mixture (CD11b, CD45R/B220, CD4, CD8, GR1, TER119, and CD5), c-Kit (CD117), and Sca-1 to extract the frequency of LK and LSK cells for each mouse and calculated the absolute numbers using cellularity.
Sialyltransferase Assays
Sialyltransferase assays were carried out under incubation conditions as described previously (9). To measure ST6Gal-1 activity in cells, cells were lysed in a mild lysis buffer (10 mm Tris, 0.1% Nonidet P-40, pH 7.3), and 2 μl of lysate (containing 105 cells) or purified enzyme was incubated for 2 h at 37 °C with 12.5 μm CMP-[3H]Sia and monitored for the transfer of [3H]Sia to a Galβ1–4GlcNAcα-O-Bn acceptor compound (2.5 mm; Toronto Research Co.). [3H]Sia enzymatically transferred to supplied acceptors was measured after recovery and separation from unreacted CMP-[3H]Sia by C18 reverse phase chromatography. Separation of reacted acceptor into α2,6- and α2,3-sialic acid fractions was performed with SNA-agarose (Vector Laboratories) affinity chromatography. The ST6Gal-1 activity corresponded to the SNA-agarose-retained α2,6-sialic acid-positive fraction, and the α2,3-sialyltransferase activity corresponded to those products not retained by SNA-agarose. For all ST6Gal-1 enzymatic studies, 1 unit is the enzymatic activity that generates 1 μmol of Siaα2,6Galβ1–4GlcNAcα-O-Bn from Galβ1–4GlcNAcα-O-Bn/min at 37 °C and pH 6.5. As a reference, the serum ST6Gal-1 activity of mouse is between 1.5 and 4.5 microunits/ml, translating roughly to 0.15–0.45 milliunit/kg of body weight assuming 2 ml of blood in a 20-g mouse.
Recombinant ST6Gal-1 Production
The coding sequence (cDNA) of the secretory domain of rat ST6Gal-1 was amplified from full-length cDNA (pBS RK-7) using standard polymerase chain reaction (PCR) and the following primers: 1) GAT GAC AAG CTT AGC AAG CAA GAC CCT (the HindIII restriction site for cloning was engineered into this primer (underscored)) and 2) GGA TCC TCT AGA TCA ACA ACG AAG AAT GTT (the XbaI restriction site for cloning was engineered into this primer (underscored)). After digestion with the above restriction enzymes, the resulting coding sequence was cloned into HindIII and XbaI sites of p3XFLAG-CMV-8 (Sigma) expression vector, which has a preprotrypsin leader sequence that precedes the 3XFLAG sequence to produce a soluble form of recombinant protein. Stable transfection of Chinese Hamster ovary (CHO) cells with the above expression vector was carried out using FuGENE® HD transfection reagent (Roche Diagnostics) according to the manufacturer's recommendations. CHO cells were cultured in DMEM and 10% FBS. One day after transfection, the medium was replaced. After 24 h, the medium was collected, and the activity of recombinant ST6Gal-1 was measured the same way as mentioned above. A column of M2 anti-FLAG-agarose (Sigma) was used to immunoprecipitate ST6Gal-1 from the medium according to the manufacturer's instructions. The eluted ST6Gal-1 was dialyzed into PBS and lyophilized. Enzymatic activity of reconstituted recombinant ST6Gal-1 was measured as detailed above.
RNA Isolation RT-PCR and Real Time RT-PCR
Total RNA was isolated using TRIzol (Invitrogen) or RNAqueous®-4PCR (Ambion), and cDNA was synthesized from 1 μg of RNA using an iScript reverse transcriptase kit (Bio-Rad) according to the manufacturers' instructions. Real time PCRs using iQ SYBR Green Supermix (Bio-Rad) were performed on the My iQ Single-Color Real-Time PCR Detection System (Bio-Rad). Primer pairs for each gene and mRNA form were designed based on GenBankTM information. Relative mRNA levels are derived from ΔCt, the difference of the threshold cycle value of the target mRNA and the Ct value for RPL32, a ribosomal protein mRNA used as a reference standard using the formula: 1000 × 2ΔCt. The PCR primer pairs for real time RT-PCR analysis were as follows: RPL32, GCGAAACTGGCGGAAACC and ACATTGTGGACCAGGAACTTG; ST3Gal-3, GTATGATAGGCTGGGCTTCC and GCTGGCTTGGAGAACCTG; ST3Gal-4, TCCCATCTC AGAGAAGAAAGAG and GGCAGCTCATAGGTGGATG; ST3Gal-6, GCCAGCTTTCGCCAATCTTC and AGGGCCGAGCTGAAATATGTC; ST6Gal-1, CCAAGCCCAGAAGGATTAGC and ACGCAGATGATGGCAAACAG 3′. The primer pairs for RT-PCR analysis of LK cells were as follows: RPL32, CATGCACACAAGCCATCTCATCA and TCTCACAATGTGTCCTCTAAGAT; ST6Gal-1: CCTGGCCTCCAGACCTAGTAAAGT and TCCCTTTCTTCCACACGCAGATGA.
Glycomics Analysis of Marrow Cells for α2,6- and α2,3-Sialyl Linkages
St6gal1-KO donor cells were transplanted into St6gal1-KO or wild-type recipients as described earlier. Typically, >95% of the cells recovered from the marrow of the chimeras at day 7 originated from the donor. Biotinylated anti-CD45.1 or anti-CD45.2 and magnetic microparticles as described earlier were used to further remove the remaining recipient-derived cells. The purity of the donor-derived recovered cell preparations was again verified by flow cytometry. 107 donor-originated cells from 10–15 recipients were used.
Protein was separated from cellular lipids using a procedure based on methods described previously (14–16). Cells were ruptured in ice-cold water on ice using a Dounce homogenizer. The extraction of lipids was accomplished by adjusting the solvent mixture to a final ratio of chloroform/methanol/water equal to 4:8:3. The extract was mixed at room temperature with end-over-end agitation overnight. The protein-rich insoluble material was then collected by centrifugation at 2000 × g at 4 °C for 15 min. The lipid-rich supernatant was removed, dried with N2 gas, and stored. Four milliliters of chloroform/methanol/water (4:8:3) solution was added to the pellet, and the mixture was briefly sonicated, vortexed, and then mixed at room temperature with end-over-end agitation for 2 h. The protein-rich insoluble material was collected again by centrifugation at 2000 × g at 4 °C for 15 min, and the lipid-rich supernatant was removed, dried with N2 gas, and stored. The material was re-extracted in this fashion a total of three times. To remove contaminates such as detergents and fatty acids, 5 ml of acetone/water in a ratio of 4:1 was added to the proteinaceous pellet, and the tube was sonicated, vortexed, and then stored at −20 °C for 30 min. The solution was then centrifuged at 2000 × g at 4 °C for 15 min. The supernatant was removed, and the procedure was repeated three times with 100% acetone used during the last two extractions. The protein-rich pellet was gently dried under a stream of N2 gas, placed in a vacuum desiccator for 1 h, and then weighed.
To release the N-linked glycans, the protein-rich powder was resuspended in 115 μl of trypsin buffer (0.1 m Tris-HCl, pH 8.2 containing 1 mm CaCl2)/mg of powder by vortexing and sonication and then heated to 100 °C for 5 min. Once cooled to room temperature, trypsin was added in a 20:1 protein/enzyme ratio (5 mg/ml in trypsin buffer). Protease digestion was allowed to proceed for 18 h at 37 °C. A second equimolar aliquot of trypsin was added, and the digest was allowed to proceed for an additional 18 h at 37 °C before the solution was heated to 100 °C for 5 min. Undissolved material was removed by centrifugation, and the supernatant was frozen on dry ice and lyophilized overnight. The dried peptides were resuspended in 250 μl of 5% acetic acid (v/v) and loaded onto a Sep-Pak C18 cartridge column (17) that had been preconditioned by passing 1 ml of methanol through it three times followed by passing 1 ml of 5% acetic acid through it three times. The column was washed by passing 1 ml of 5% acetic acid through it 10 times. Glycopeptides were eluted stepwise, first with 1 ml of 20% isopropyl alcohol in 5% acetic acid, next by 1 ml of 40% isopropyl alcohol in 5% acetic acid, and finally by 1 ml of 100% isopropyl alcohol. The three fractions were pooled and dried using a centrifugal evaporator. A BCA assay (Pierce) was used to determine the protein content of samples before N-linked glycans were released. Glycopeptides were resuspended in 50–100 μl of 50 mm sodium phosphate buffer, pH 7.5 and digested with peptide-N-glycosidase F for 18 h at 37 °C. Released oligosaccharides were separated from trypsin and peptides using a Sep-Pak C18 cartridge column that was preconditioned as described above. The digest solution was applied to the column, the digest tube was then rinsed with 200 μl of 5% acetic acid three times, and this solution was loaded onto the column to ensure that all sample was transferred. The column run-through and three additional washes of 1 ml of 5% acetic acid containing released N-linked glycans were collected, frozen on dry ice, and lyophilized.
In some instances, the released oligosaccharides were further enriched into acidic, sialic acid-containing, N-linked glycans using a modified procedure based on a glycosaminoglycan purification protocol described previously by Shi and Zaia (18). A macrospin column (Harvard Apparatus) with DEAE resins was saturated by washing with 300 μl of elution buffer (2 m NH4HCO3) and then 3 × 300 μl of loading buffer (5 mm Tris-HCl, pH 8). Released oligosaccharides were dissolved in 300 μl of loading buffer, applied to the column, and allowed to adhere for 10 min. Sample solution was repipetted through the column once to ensure complete binding to the resin. The column was washed with 3 × 300 μl of loading buffer, and the non-binding, non-acidic, N-linked glycans were collected, frozen on dry ice, and lyophilized overnight. The column was washed with 6 × 300 μl of loading buffer and then 300 μl of H2O. Finally, acidic N-linked glycans were eluted with 6 × 300 μl of elution buffer, frozen on dry ice, and lyophilized overnight. After drying, water was added, and samples were relyophilized to remove residues of the volatile salt NH4HCO3. This was repeated until no salt was visible.
Released oligosaccharides were permethylated as described by Anumula and Taylor (19) for analysis by mass spectrometry (MS). Briefly, the dried eluate was dissolved with dimethyl sulfoxide and methylated with NaOH and methyl iodide. The reaction was quenched with water, and per-O-methylated carbohydrates were extracted with methylene chloride and dried under N2. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF/MS) was performed using an AB SCIEX TOF/TOF 5800 (Applied Biosystems/MDS Analytical Technologies) in positive ion reflector mode. The permethylated glycans were dissolved with methanol and crystallized with α-dihyroxybenzoic acid (20 mg/ml in 1:1 methanol/water) matrix, and then 1 μl of the solution was spotted on the sample plate. For the nanospray ionization-linear ion trap MS, the permethylated glycans were dissolved in 1 mm lithium carbonate in 50% methanol. The samples were infused directly into an LTQ Orbitrap Discovery mass spectrometer (Thermo Scientific) at a flow rate of 0.5 μl/min to obtain nanospray ionization. The full mass spectrum of each sample was obtained in the positive ion mode. MS5 analysis of selected parent ions was performed using collision energies between 35 and 45% and an m/z range from 55 to 2000 to ascertain sialic acid-galactose linkages as described by Anthony et al. (20). Total ion mapping was performed using the XCalibur software package (version 2.0) as described by Aoki et al. (14) to obtain automated MS and MS/MS spectra. The m/z range from 300 to 2000 was scanned using 40% collision energy.
RESULTS
Systemic ST6Gal-1 and Marrow Blood Cell Production
Earlier, we observed that increased production of inflammatory cells was associated with the depressed circulatory ST6Gal-1 levels in the St6gal1-dP1 mouse, leading to the idea that systemic ST6Gal-1 is a systemic regulator of hematopoiesis in the marrow. The increased hematopoietic capacity is recapitulated in Fig. 1, A–C, which show that HSPCs, functionally represented by the LK and LSK populations, are more abundant in the St6gal1-dP1 than in the C57BL/6 wild-type marrow. Curiously, the LK and LSK populations were not similarly altered in the globally ST6Gal-1-deficient animal, St6gal1-KO. This observation is noteworthy because of the anticipated additional defects in the inability to express ST6Gal-1 in other cell and tissue compartments. For example, the inability to express endogenous ST6Gal-1 by the stromal and other supportive cells might fundamentally alter the marrow niches dictating hematological homeostasis. Moreover, aberrantly sialylated cytokines or other systemic factors might alter signaling interactions with the HSPCs or their supportive niche cells. Because of the anticipated complexities in interpreting information from a global ST6Gal-1 deficiency, HSPC homeostasis in the St6gal1-KO model was not examined further. Rather, we restricted our examination to conditions that alter only the extracellular pool of ST6Gal-1 enzyme. Therefore, to test directly the hypothesis that the more robust hematopoiesis in the St6gal1-dP1 mouse is due to an altered systemic parameter, e.g. circulatory ST6Gal-1, bone marrow chimeras were constructed using the same preparation of wild-type marrow cells to repopulate the hematopoietic compartments of wild-type or St6gal1-dP1 recipients. This is summarized in Fig. 1, D–F, which show that increased marrow cellularity (D), greater LK (E), and greater LSK (F) segregated with the St6gal1-dP1 recipients.
We had posited that extrinsic or extracellularly derived ST6Gal-1 is a potent, systemic attenuator of blood cell generation. Validation of this hypothesis is shown by ex vivo cultures of wild-type LK cells maintained for 72 h that were induced to proliferate to 10 times the original cell numbers from 105 cells to 106 cells. Addition of recombinant ST6Gal-1 to the approximate level found in circulation during an acute phase response (see “Experimental Procedures”) resulted in strikingly suppressed proliferation, which was even more profound with the co-addition of 50 μm CMP-Sia, the ST6Gal-1 sugar donor substrate. These results are shown in Fig. 1G, which was one of four separately performed experiments, all with identical outcome. These observations suggest the catalytic action of the exogenous ST6Gal-1 on the LK cells. Furthermore, exogenously added CMP-Sia is not an absolute requirement possibly because some native CMP-Sia, for example due to leakage from dying cells, may be available. Ex vivo proliferation of wild-type LK cells was accompanied by cellular differentiation as reflected by loss of the cell surface marker c-Kit and acquisition of the lineage marker CD11b (Fig. 1H). Loss of c-Kit and gain of CD11b were halted by exogenously added ST6Gal-1 with CMP-Sia (Fig. 1I), and this block was reproduced but to a lesser degree when ST6Gal-1 was added without CMP-Sia (data not shown). To test directly the idea that functionally useful amounts of sialic acid donor substrate might be available from natural leakage or from dying cells in the ex vivo assay, St6gal1-KO marrow cells, which are SNA-negative, were incubated with fresh hypotonically lysed St6gal1-KO marrow cells with added ST6Gal-1. As shown in Fig. 1J, St6gal1-KO cells acquired cell surface SNA reactivity in the presence of cell lysate to a comparable degree as when CMP-Sia was used.
HSPC Surface α2,6-Sialylation Depends on Extrinsic ST6Gal-1
If HSPC α2,6-sialylation depends on extrinsic or distally produced ST6Gal-1, the extent of α2,6-sialylation HSPC populations should reflect the level of the systemic ST6Gal-1 pool in circulation. Therefore, we predict that HSPCs from St6gal1-dP1 mice, compared with those from wild-type mice, should have reduced SNA reactivity. The lectin SNA was used to illuminate the α2,6-sialyl structures. Cells from the St6gal1-KO mouse, which is globally null for ST6Gal-1, were similarly analyzed as a control and showed completely absent SNA reactivity that reaffirmed the specificity of lectin for ST6Gal-1 products. At steady state, no overt differences in SNA reactivity were noted between St6gal1-dP1 and wild-type total nucleated marrow cells (Fig. 2A), which was not unexpected because 1) ST6Gal-1 expression of total bone marrow cells of St6gal1-dP1 is similar to wild type (11) and 2) HSPCs are a very small proportion of the total marrow cells. To visualize more specifically HSPCs, LK and LSK cells, comprising only 1 and 0.1% of total marrow cells, respectively, were examined. As shown clearly in Fig. 2, B and C, St6gal1-dP1 LK and LSK cells had pronounced reductions in SNA reactivity. Reduced SNA reactivity was more pronounced in LSK than LK cells, corresponding to the degree of enrichment of HSPCs in these respective cell populations. There is still ample SNA reactivity in the LK and LSK populations in the St6gal1-dP1 mice probably because suppression of systemic circulatory ST6Gal-1 is only partial, and these mice retain 25% of the circulatory ST6Gal-1 levels observed in base line of WT mice (9) These observations suggest that HSPCs, but not the more differentiated marrow cells, depend on extrinsic ST6Gal-1 for their surface α2,6-sialyl structures.
FIGURE 2.

HSPCs from St6gal1-dP1 have reduced cell surface SNA reactivity. A–C, flow cytometry using SNA to visualize cell surface α2,6-sialyl structures was performed on total (A), LK (B), and LSK (C) bone marrow cells from C57BL/6 (black line), St6gal1-dP (dashed black line), and St6gal1-KO mice (dashed gray line). D, bone marrow cells from St6gal1-KO, which are inherently SNA-negative, were transplanted into irradiated wild-type (K>W), St6gal1-dP1 (K>P), and St6gal1-KO (K>K) recipients. The SNA profiles of the donor-derived nucleated cells with the recipient marrows were analyzed 1 week after transplantation.
To test the idea that hematopoietic progenitor α2,6-sialylation is dependent on non-hematopoietic cell factors (e.g. extrinsic ST6Gal-1), St6gal1-KO marrow cells, which have an inactive endogenous ST6Gal-1 gene, were transplanted into St6gal1-KO, St6gal1-dP1, or wild-type recipients. At day 7 after transplantation, the point when the transplanted hematopoietic compartments are still in the state of regeneration, the marrow cells were harvested and assessed for SNA reactivity (Fig. 2D). St6gal1-KO cells that cannot express endogenous ST6Gal-1 became highly SNA-reactive when placed in wild-type hosts. Cells from the same batch of St6gal1-KO donors had reduced 4SNA reactivity when placed in St6gal1-dP1 hosts in accordance with the reduced systemic ST6Gal-1 levels. St6gal1-KO cells remained absolutely SNA-negative when repopulating St6gal1-KO recipients. The lectin PSL was also used to verify the interpretation of the SNA analysis. The PSL data were essentially identical to that obtained by SNA (data not shown). Both SNA and PSL showed stringent specificity for α2,6-linked sialic acids (21, 22). The specificity of the commercially available SNA and PSL was further validated against an extensive panel of >600 glycans by Lara K. Mahal, and this information is public data on the website of The Consortium for Functional Glycomics.
To assess the extent in which extrinsic or non-self ST6Gal-1 contributes to HSPC sialylation, the endogenous or intrinsic expression of self-ST6Gal-1 was assessed. Fig. 3A shows that, despite ample cell surface SNA reactivity of LSK and LK cells, native ST6Gal-1 mRNA was essentially undetectable in these cells. Total bone marrow, peritoneal macrophages, splenic B cells, and liver were included as reference controls. However, LSK and LK cells had significant quantities of mRNA for other sialyltransferases, including ST3Gal-4 (Fig. 3B), ST3Gal-3 (Fig. 3C), and ST3Gal-6 (Fig. 3D). These sialyltransferases were chosen specifically because they share the same acceptor substrate, Galβ1–4GlcNAc, as ST6Gal-1. The absence of endogenous ST6Gal-1 expression in HSPCs was further confirmed by in vitro enzyme assays showing lack of α2,6-sialyltransferase activities toward Galβ1–4GlcNAc-R, whereas a low but measurable α2,3 activity could be detected (Fig. 3, E and F, respectively). We also analyzed those cells for mRNA expression of ST6Gal-2, another sialyltransferase that constructs α2,6-sialyl linkages on the Galβ1–4GlcNac termini of these conjugates. St6gal2 was absent in all hematopoietic cell types we analyzed (data not shown). The data strongly imply that self-produced ST6Gal-1 does not contribute significantly to the assembly of HSPC α2,6-sialic acids. By inference, the preponderance of the α2,6-sialyl structures on these cells must be constructed by an alternate mechanism necessitating distally produced ST6Gal-1. However, when induced to differentiate, wild-type LK cells rapidly acquired intrinsic expression of native ST6Gal-1 (Fig. 3G). This observation is consistent with the high levels of endogenous ST6Gal-1 expression in fully differentiated blood cells, including B cells, T cells, macrophages, and dendritic cells.
FIGURE 3.
HSPCs from wild-type C57BL/6 mice have negligible endogenous ST6Gal-1. A–D, undetectable levels of ST6Gal-1 mRNA in WT marrow LK and LSK cells. Real time RT-PCR analysis was performed for ST6Gal-1, ST3Gal-3, ST3Gal-4, and ST3Gal-6, respectively, using RNA from WT LSK cells, LK cells, total bone marrow nucleated cells (BM), peritoneal macrophages (mac), splenic B220+ cells (B), and liver tissue homogenate (liv). E and F, absence of endogenous ST6Gal-1 enzymatic activity in WT LSK and LK cells. Sialyltransferase activities were measured by following transfer of CMP-[3H]Sia to Galβ1–4GlcNAc-O-Bn (LacNAc). The Siaα2,6 products (E) were separated from Siaα2,3 products (F) by SNA-agarose chromatography. Error bars represent S.D. G, HSPCs from wild-type mice acquire intrinsic ST6Gal-1 activity during differentiation. LK cells from wild-type mice were incubated in the presence of rmSCF, rmTPO, rmFlt3, rmIL-3, and rmG-CSF for 6 days. A fraction of cells was used to extract RNA before (LK) and every day during incubation. RT-PCR was performed using 50 ng of cDNA to analyze ST6Gal-1 expression. Expression of the ribosomal protein RPL32 (RPL) was analyzed as a control.
To test the hypothesis that extrinsic or non-self ST6Gal-1 is the principal mediator for α2,6-sialylation of marrow HSPCs, additional bone marrow chimeras were constructed using either wild-type or St6gal1-KO donor cells to repopulate the marrow of WT or St6gal1-KO recipients (Fig. 4). The total absence of signal in the recovered cells of the St6gal1-KO into St6gal1-KO transplants once again affirms the stringency of SNA as a probe for ST6Gal-1 function; the SNA profile in the wild type into wild-type transplants serves as a control for nominal α2,6-sialylation signal. At day 7 after transplantation when total bone marrow cells were examined (Fig. 4A), the recovered St6gal1-KO cells as shown previously were SNA-positive when raised in a wild-type recipient but SNA-negative when raised in an St6gal1-KO recipient. Wild-type donor cells are only SNA-positive when recovered from a wild-type recipient. The lectin PSL was also used to verify the interpretation of the SNA analysis. The PSL data were essentially identical to data obtained using SNA (Fig. 4B) Strikingly, the same wild-type cells had severely decreased SNA reactivity when raised in an ST6Gal-1-deficient environment (e.g. St6gal1-KO recipients). However, it was noted that the SNA reactivity was not reduced to that of the negative St6gal1-KO into St6gal1-KO control, suggesting a possibly minor role for the endogenously expressed ST6Gal-1 in sialylation. To examine further this issue, the SNA reactivity of LK cells was examined to assess specifically the contributions of endogenous versus extrinsic ST6Gal-1 in cell surface sialylation of HSPCs. As shown in Fig. 4C, cell surface SNA reactivity of wild-type LK cells was completely dependent on the extrinsic ST6Gal-1 status of the recipients. LK cells, regardless of their endogenous ability to express self-ST6Gal-1, were completely SNA-negative when raised in an ST6Gal-1-null host.
FIGURE 4.
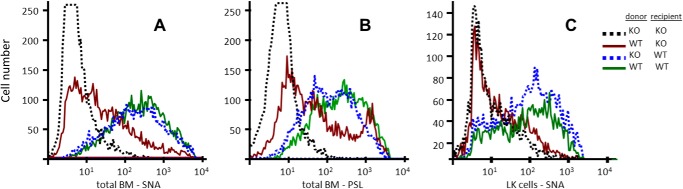
Marrow hematopoietic cell surface α2,6-sialylation completely depends on recipient ST6Gal-1 status in bone marrow chimeras. Bone marrow cells from St6gal1-KO (KO) or WT donors, both of which were CD45.2, were transplanted into irradiated WT CD45.1 recipients. The WT into KO chimera was generated using CD45.1 WT cells and CD45.2 KO recipients. The KO donor into KO chimera used CD45.2 donors and recipients. Either total bone marrow (BM) nucleated cells (A and B) or the purified LK fraction (C) carrying the appropriate donor congenic marker was profiled for SNA (A and C) or PSL (B) reactivity by flow cytometry.
Modification of HSPC Cell Surface by Extrinsic ST6Gal-1
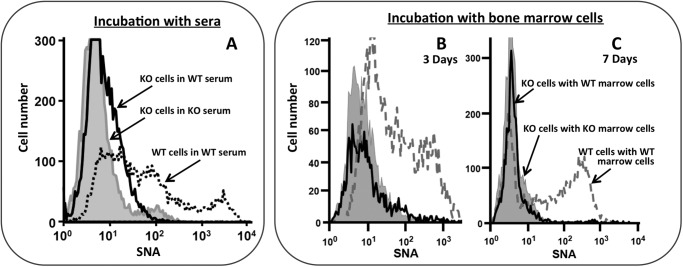
The surface SNA reactivity acquired by the adoptively transferred St6gal1-KO LK cells might be due to absorption of extracellular α2,6-sialyl glycans from their wild-type hosts. If LK cells do absorb external α2,6-sialyl glycans, the St6gal1-KO LK cells should rapidly acquire surface SNA reactivity upon incubation with normal serum, which is extremely rich in α2,6-sialic acids. Wild-type serum was heat-treated at 56 °C for 1 h and tested to ensure inactivation of the native ST6Gal-1 activity (data not shown). Fig. 5A shows that despite ex vivo incubation with 50% heat-treated wild-type serum for up to 3 h St6gal1-KO LK cells remain completely SNA-unreactive and essentially identical to the profile obtained when incubated with St6gal1-KO serum, which does not have α2,6-sialic acids. The SNA profile of wild-type LK cells identically incubated with wild-type serum is also shown for comparative reference.
FIGURE 5.
LK cell surface SNA reactivity is not acquired by scavenging foreign SNA-positive material. A, LK cells from St6gal1-KO (KO) were incubated ex vivo in RPMI 1640 medium with 50% WT (C57BL/6) or KO mouse serum for 3 h (solid line and shaded gray area, respectively). WT LK cells were similarly incubated in the presence of WT serum and are shown as reference (dashed line). B and C, LK cells from St6gal1-KO (KO), which expressed CD45.2, were incubated ex vivo in the presence of 5-fold excess total bone marrow cells from CD45.1 WT (solid line) or KO (shaded area). After 3 or 7 days (B and C, respectively), the CD45.2 St6gal1-KO cells were examined for cell surface SNA reactivity by flow cytometry. WT LK cells similarly incubated with WT marrow cells are shown as reference (dashed line). The incubations were performed in the presence of rmSCF, rmTPO, rmFlt3, rmIL-3, and rmG-CSF as specified under “Experimental Procedures.”
Although St6gal1-KO LK cells do not pick up α2,6-sialyl glycans from the blood, we further examined the possibility that these glycans may have been transferred from other cells within the environment of the bone marrow. To discount this possibility, St6gal1-KO cells were cultivated with 5-fold excess total wild-type bone marrow cells. St6gal1-KO cells were from CD45.2 animals, which can be distinguished from the co-cultivated wild-type marrow cells that are CD45.1. The culture medium included cytokines to allow ex vivo proliferation and differentiation (see “Experimental Procedures”). After 3 or 7 days, the St6gal1-KO cells were assessed for SNA reactivity (Fig. 5, B and C, respectively). The St6gal1-KO cells did not acquire cell surface SNA reactivity despite prolonged incubation with excess ST6Gal-1-intact marrow cells. The dashed line again represents wild-type LK cells incubated with wild-type bone marrow cells, serving as a reference for a nominal SNA profile. Together, these data strongly indicate that marrow HSPCs do not gain SNA reactivity by simple absorption of prefabricated α2,6-sialyl glycans. Rather, HSPCs acquire SNA reactivity through the remodeling of their surface glycans by extrinsic ST6Gal-1.
Confirmation That α2,6-Sialyl Structures on St6gal1-KO Cells Are Constructed by Extrinsic ST6Gal-1
MSn-based glycomics was performed to definitively demonstrate the presence of α2,6-sialyl linkages on St6gal1-KO hematopoietic cells after transplantation into wild-type recipients. Donor-derived cells were recovered from the marrow of the bone marrow chimeras after adoptive transfer by a procedure identical to that presented earlier in Fig. 4. St6gal1-KO donor-derived cells transplanted into wild-type recipients were compared with St6gal1-KO donor-derived cells transplanted into St6gal1-KO recipients. Native WT and St6gal1-KO marrow cells were also similarly analyzed as controls.
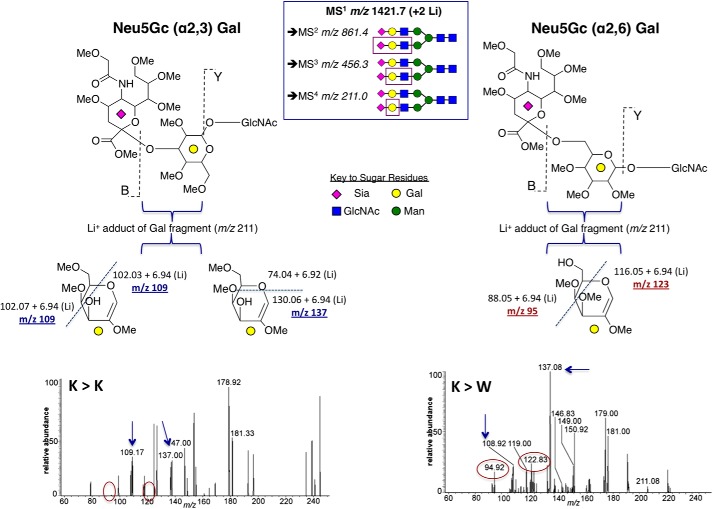
MALDI-TOF/MS by nanospray ionization-MS total ion mapping and MS5 fragmentation was used to investigate the peptide-N-glycosidase F-released N-linked glycans where selected sialic acid-containing glycans were chosen for nanospray ionization-MS5 sialic acid-galactose linkage fragmentation analysis as described under “Experimental Procedures.” The complete comparative analysis is summarized in supplemental Tables S1 and S2 and Figs. S1–S3. Fig. 6 summarizes the analysis of the MS1 m/z 1421.7 (+2 lithiums) ion, the Sia2Gal2Man3GlcNAc4 structure from the St6gal1-KO cells recovered from wild-type and St6gal1-KO transplants, to probe for the presence of the MS5 m/z 95 and 123 ions that are diagnostic for 6-O- (as in Sia-α2,6-Gal-GlcNAc) but not 3-O-substituted galactose in the Sia-Gal-GlcNAc fragments. The data show the presence of α2,3-linked sialic acids on the St6gal1-KO recovered from either the wild-type or St6gal1-KO recipients as expected. On the other hand, α2,6-linked sialic acids were clearly present in the St6gal1-KO cells recovered from wild-type recipients and clearly absent in those cells recovered from St6gal1-KO recipients.
FIGURE 6.
MS analysis for Siaα2,3Gal and Siaα2,6Gal on N-linked glycans of transplanted St6gal1-KO hematopoietic cells. St6gal1-KO bone marrow cells were adoptively transferred into lethally irradiated St6gal1-KO or wild-type recipients (K>K or K>W, respectively). Bone marrow St6gal1-KO cells, which were CD45.2, were recovered from the wild-type CD45.1 hosts and subjected to glycomics analysis for the presence of Siaα2,6Gal on N-linked glycans as described under “Experimental Procedures.” Lithium adducts of the parent MS1 m/z 1421.7 ion, which is the biantennary structure containing two sialic acids in the N-glycolylneuraminic acid (Neu5Gc) form, was chosen for in-depth analysis (inset box) to generate sequentially the fragmentation ions on MS2 m/z 861, MS3 m/z 456, and MS4 m/z 211 that corresponded to boxed areas of the parent glycan structure in the inset. Fragmentation of the “B” and “Y” bonds of the permethylated glycans generated the MS4 m/z 211 ion, representing the penultimate Gal residue. Further fragmentation generated the diagnostic MS5 scar ions; dashed lines denote the labile fragmentation sites. For Neu5Gcα2,3Gal, the MS5 m/z 109 and m/z 137 fragments (arrows) were produced by separate fragmentation events of the MS4 m/z 211 ion. For Neu5Gcα2,6Gal, the MS5 m/z 95 and m/z 123 fragments (circled) were produced by the same fragmentation event on the MS4 m/z 211 ion. The α2,6-NeuGc-specific ions m/z 95 and m/z 123 (circled) were present only on St6gal1-KO cells recovered from wild-type hosts (K>W) but not from those recovered from St6gal1-KO hosts (K>K).
DISCUSSION
The interaction of the hematopoietic stem and progenitor cells with their environment is the primary conduit through which systemic needs are communicated to produce end effector cells and to ensure against premature stem cell depletion by appropriate self-renewal. The role of terminal glycosylation in cell-cell interactions and in signal transduction is well documented, particularly in immune cells, e.g. during selectin-mediated homing of leukocytes to lymph nodes and inflamed sites (23, 24), in galectin-mediated apoptotic selection during lymphocyte maturation (25, 26), and in altered integrin and death receptor signaling by α2,6-sialylation (27–30). Lactosaminyl glycan-based epitopes and their selectin receptors are also important for the homing of stem cells to bone marrow niches (31, 32), and recent indications suggest that differential glycan recognition may serve as a checkpoint between hematopoietic stem cell (HSC) quiescence and proliferation (33). Selectin and lactosaminyl glycan epitopes have also been implicated in breast cancer stem cell dissemination and metastatic lodgment (34, 35). Just how these critical cell surface glycans are regulated biosynthetically remains poorly understood. Classically, cell surface glycans are constructed by glycosyltransferases expressed within each individual cell. The data here present the idea that certain glycosyltransferases from distal origins can remodel critical glycan structures on target cells that need not endogenously express these glycosyltransferases. In doing so, these non-self glycosyltransferases can convey important systemic instructions to the target cells.
Our data demonstrate that external application of ST6Gal-1 arrests HSPC proliferation and differentiation in vitro, an observation that recapitulates the in vivo inverse correlation between circulatory levels of ST6Gal-1 and marrow hematopoiesis, including a link between circulatory ST6Gal-1 insufficiency to faster hematopoietic recovery following cyclophosphamide-induced myeloablation (10, 11). Although the mechanistic basis of how extrinsic ST6Gal-1 arrests HSPC proliferation is not yet known, what is clear from the present study is that extrinsically produced or non-self ST6Gal-1 is used to decorate HSPC surfaces with α2,6-sialyl glycans. The possibility that HSPCs might have acquired these α2,6-sialyl glycans by absorption from the surroundings is highly unlikely. St6gal1-KO LK cells did not acquire cell surface SNA reactivity even after prolonged incubation with wild-type serum or when co-cultivated with excess wild-type bone marrow cells, both of which are highly enriched for α2,6-sialylated material. Taken together, the data are consistent with the notion that the α2,6-sialyl linkages on HSPCs are assembled directly by extrinsic ST6Gal-1.
At present, there is no information on how the extrinsically produced ST6Gal-1 assembles the α2,6-sialyl glycans on the surfaces of HSPCs. One possibility is that HSPCs take up the foreign enzymes and use them to construct the glycans within the intracellular endoplasmic reticulum-Golgi secretory apparatus. An alternative possibility is that the extrinsic ST6Gal-1 mediates the extracellular attachment of α2,6-sialyl residues directly onto cell surface glycans. The idea of extracellular glycosylation is not new, but acceptance for this unconventional mode of glycosylation has been hampered by the widespread notion that there is no extracellular supply of the sugar-nucleotide donor substrates necessary for glycosylation. However, sugar-nucleotides are likely present in the extracellular milieu at least under certain conditions as exemplified by recent reports that cargos of sugar-nucleotides are present in platelets that are released into circulation upon platelet activation (36). Other cells may also release sugar-nucleotides. Consistent with this notion is our observation that partial suppression of LK cell proliferation was achieved by recombinant ST6Gal-1 without exogenously adding CMP-Sia (see Fig. 1G). Under the same ex vivo conditions, we were able to demonstrate that freshly prepared cell lysates can confer to St6gal1-KO LK cells a comparable degree of SNA reactivity as CMP-Sia in the presence of recombinant ST6Gal-1 (Fig. 1J). Moreover, we also noticed that activated platelets are highly efficient suppliers of the sialic acid donor substrate for the external modification of target cell surface glycans (data not shown).
A plethora of glycosyltransferase activities are normally present in systemic circulation, although the physiologic value for their presence in the extracellular milieu has not been recognized. One of the most prominent systemic glycosyltransferases is ST6Gal-1, which our data show is a potent regulator of marrow hematopoietic behavior. The α2,6-sialylation of the cell surface of HSPCs is not mediated by self-expressed ST6Gal-1. Rather, HSPC α2,6-sialylation is dependent on distally produced non-self ST6Gal-1. Early work has documented that extracellular, circulatory ST6Gal-1 is governed by glucocorticoids and interleukins such as IL-6 (37, 38). Acute local inflammation is also accompanied by depression of circulatory ST6Gal-1 presumably to allow increased production of inflammatory cells (10). Our present data reveal a totally novel glycosylation mechanism by which distally produced glycosyltransferases such as the systemic ST6Gal-1 can remodel glycan surfaces of hematopoietic stem and progenitor cells. Many other glycan-constructing enzymes also circulate systemically in the blood. Our current observations introduce the possibility that these other systemic glycosyltransferases can also remodel the glycan architecture of distal target cell surfaces. In the canonical glycosylation process, glycosyltransferases are compartmentalized within the endoplasmic reticulum-Golgi secretory apparatus where they assemble glycans on neoglycoproteins and glycolipids in transit. However, prokaryotes also express glycosyltransferases, and they assemble glycan molecules without the benefit of endoplasmic reticulum-Golgi. The glycosyltransferases in our systemic circulation may have descended from the system of glycosylation that evolutionarily predated the secretory apparatus.
We propose that cell surface glycan architecture is shaped not only by the endogenously expressed glycosyltransferases acting via the canonical intracellular secretory-glycosylation pathway but also by the glycan-modifying enzymes present in the extracellular milieu. The data presented here show clearly that murine hematopoietic progenitors do not express endogenous ST6Gal-1, and their surface α2,6-sialic acid linkages require extrinsically and distally produced ST6Gal-1. It is not clear at this point the extent of the role of extrinsic glycosyltransferases in cell surface glycan remodeling. A number of reports have documented that some stem and progenitor cells, unlike the murine LK cells, are high in both endogenous ST6Gal-1 and cell surface SNA reactivity (39). An earlier observation by Hui and Le Marer (40) also indicated that ST6Gal-1 is expressed in human CD34+ cells, which are generally regarded as human hematopoietic stem and early progenitors. It is not clear whether this reflects an inherent divergence between the human and mouse hematopoietic progenitors. However, both human CD34+ and murine LK cells are extremely heterogeneous populations, and any differences in endogenous ST6Gal-1 status may be due to different cell constituents or the state of differentiation of the progenitors. In this regard, murine LK cells rapidly acquire endogenous ST6Gal-1 expression upon exposure to G-CSF and IL-3, indicating that endogenous ST6Gal-1 expression may be among the first events of further differentiation of the hematopoietic early progenitors, even before acquisition of cell surface lineage-specific markers (see Fig. 3G). It is also tantalizing to speculate that endogenous ST6Gal-1 is normally expressed in true HSCs but is silenced upon transition of the HSCs into early uncommitted progenitors. Silencing of endogenous ST6Gal-1 during HSC development may promote sensitivity to distally produced ST6Gal-1. This process facilitates the conveyance of systemic cues to the developing progenitors regardless of whether the extrinsic enzyme is taken up by the HSPCs or acts extracellularly to modify cell surface glycans. Because true HSCs are less than 0.2% of the LK cell population that were examined, it would be unlikely that the general survey of LK cells, as conducted here, could have revealed the true endogenous ST6Gal-1 status of HSCs. The true ST6Gal-1 and α2,6-sialylation status of true HSCs, defined as the hematopoietic cells with long term repopulation potential, e.g. those defined as Lin−, c-Kit+, Sca-1+, CD150+, CD48+, CD135−, and CD34− (41), remains to be determined. Finally, it is also not unexpected that globally absolute ST6Gal-1 insufficiency presents a complex collection of biologic consequences. The absence of expanded hematopoietic progenitor populations in the St6gal1-KO marrow may be the result of offsetting phenotypes for example. This idea is further supported by our observation that lethally irradiated St6gal1-KO animals are inefficiently rescued by bone marrow transplantation, a phenotype not present in the St6gal1-dP1 mouse.5 The additional disruptions to the hematopoietic environment caused by global ST6Gal-1 insufficiency are beyond the scope of the present study. However, what is clear is the concept that functionally important α2,6-linked sialic acids on lactosaminyl structures are assembled by the intricate interplay between endogenously expressed ST6Gal-1 and those distally produced extrinsic enzymes that are supplied systemically.
Supplementary Material
Acknowledgments
The core facilities of Roswell Park Cancer Institute used in this work were supported in part by National Cancer Institute Cancer Center Support Grant CA15056. Glycomics analysis was supported by National Institutes of Health/National Center for Research Resources Grant 8P41GM103490 to the Complex Carbohydrate Research Center, University of Georgia.
This work was supported, in whole or in part, by National Institutes of Health Grant P01HL107146, a Program of Excellence in Glycosciences grant, and RO1056082 (to J. T. Y. L.).

This article contains supplemental Figs. S1–S3 and Tables S1 and S2.
M. Nasirikenari and J. T. Y. Lau, unpublished observations.
- HSPC
- hematopoietic stem and progenitor cell
- SNA
- S. nigra agglutinin
- PSL
- P. squamosus lectin
- LK
- Lin−c-Kit+
- LSK
- Lin−Sca-1+c-Kit+
- Sia
- sialic acid
- rm
- recombinant mouse
- SCF
- stem cell factor
- TPO
- thrombopoietin
- G-CSF
- granulocyte colony-stimulating factor
- Bn
- benzyl
- HSC
- hematopoietic stem cell.
REFERENCES
- 1. Wang L. D., Wagers A. J. (2011) Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat. Rev. Mol. Cell Biol. 12, 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Audry M., Jeanneau C., Imberty A., Harduin-Lepers A., Delannoy P., Breton C. (2011) Current trends in the structure-activity relationships of sialyltransferases. Glycobiology 21, 716–726 [DOI] [PubMed] [Google Scholar]
- 3. Martin L. T., Marth J. D., Varki A., Varki N. M. (2002) Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J. Biol. Chem. 277, 32930–32938 [DOI] [PubMed] [Google Scholar]
- 4. Kitagawa H., Paulson J. C. (1994) Differential expression of five sialyltransferase genes in human tissues. J. Biol. Chem. 269, 17872–17878 [PubMed] [Google Scholar]
- 5. Jamieson J. C., Lammers G., Janzen R., Woloski B. M. (1987) The acute phase response to inflammation: the role of monokines in changes in liver glycoproteins and enzymes of glycoprotein metabolism. Comp. Biochem. Physiol. B 87, 11–15 [DOI] [PubMed] [Google Scholar]
- 6. Yasukawa Z., Sato C., Kitajima K. (2005) Inflammation-dependent changes in α2,3-, α2,6-, and α2,8-sialic acid glycotopes on serum glycoproteins in mice. Glycobiology 15, 827–837 [DOI] [PubMed] [Google Scholar]
- 7. Berge P. G., Wilhelm A., Schriewer H., Wüst G. (1982) Serum-sialyltransferase activity in cancer patients. Klin. Wochenschr. 60, 445–449 [DOI] [PubMed] [Google Scholar]
- 8. Weiser M. M., Wilson J. R. (1981) Serum levels of glycosyltransferases and related glycoproteins as indicators of cancer: biological and clinical implications. Crit. Rev. Clin. Lab. Sci. 14, 189–239 [DOI] [PubMed] [Google Scholar]
- 9. Appenheimer M. M., Huang R.-Y., Chandrasekaran E. V., Dalziel M., Hu Y. P., Soloway P. D., Wuensch S. A., Matta K. L., Lau J. T. (2003) Biologic contribution of P1 promoter-mediated expression of ST6Gal I sialyltransferase. Glycobiology 13, 591–600 [DOI] [PubMed] [Google Scholar]
- 10. Nasirikenari M., Chandrasekaran E. V., Matta K. L., Segal B. H., Bogner P. N., Lugade A. A., Thanavala Y., Lee J. J., Lau J. T. (2010) Altered eosinophil profile in mice with ST6Gal-1 deficiency: an additional role for ST6Gal-1 generated by the P1 promoter in regulating allergic inflammation. J. Leukoc. Biol. 87, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nasirikenari M., Segal B. H., Ostberg J. R., Urbasic A., Lau J. T. (2006) Altered granulopoietic profile and exaggerated acute neutrophilic inflammation in mice with targeted deficiency in the sialyltransferase ST6Gal I. Blood 108, 3397–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu Y. P., Dalziel M., Lau J. T. (1997) Murine hepatic β-galactoside α2,6-sialyltransferase gene expression involves usage of a novel upstream exon region. Glycoconj. J. 14, 407–411 [DOI] [PubMed] [Google Scholar]
- 13. Hennet T., Chui D., Paulson J. C., Marth J. D. (1998) Immune regulation by the ST6Gal sialyltransferase. Proc. Natl. Acad. Sci. U.S.A. 95, 4504–4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aoki K., Perlman M., Lim J. M., Cantu R., Wells L., Tiemeyer M. (2007) Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J. Biol. Chem. 282, 9127–9142 [DOI] [PubMed] [Google Scholar]
- 15. Seppo A., Moreland M., Schweingruber H., Tiemeyer M. (2000) Zwitterionic and acidic glycosphingolipids of the Drosophila melanogaster embryo. Eur. J. Biochem. 267, 3549–3558 [DOI] [PubMed] [Google Scholar]
- 16. Svennerholm L., Fredman P. (1980) A procedure for the quantitative isolation of brain gangliosides. Biochim. Biophys. Acta 617, 97–109 [DOI] [PubMed] [Google Scholar]
- 17. Dell A., Khoo K. H., Panico M., McDowell R. A., Etienne A. T., Reason A. J., Morri H. R. (1993) Glycobiology: a Practical Approach, pp. 187–222, Oxford University Press, Oxford [Google Scholar]
- 18. Shi X., Zaia J. (2009) Organ-specific heparan sulfate structural phenotypes. J. Biol. Chem. 284, 11806–11814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anumula K. R., Taylor P.B. (1992) A comprehensive procedure for preparation of partially methylated alditol acetates from glycoprotein carbohydrates. Anal. Biochem. 203, 101–108 [DOI] [PubMed] [Google Scholar]
- 20. Anthony R. M., Nimmerjahn F., Ashline D. J., Reinhold V. N., Paulson J. C., Ravetch J. V. (2008) Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320, 373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toma V., Zuber C., Winter H. C., Goldstein I. J., Roth J. (2001) Application of a lectin from the mushroom Polysporus squamosus for the histochemical detection of the NeuAcα2,6Galβ1,4Glc/GlcNAc sequence of N-linked oligosaccharides: a comparison with the Sambucus nigra lectin. Histochem. Cell Biol. 116, 183–193 [DOI] [PubMed] [Google Scholar]
- 22. Shibuya N., Goldstein I. J., Broekaert W. F., Nsimba-Lubaki M., Peeters B., Peumans W. J. (1987) The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(α2–6)Gal/GalNAc sequence. J. Biol. Chem. 262, 1596–1601 [PubMed] [Google Scholar]
- 23. Arata-Kawai H., Singer M. S., Bistrup A., Zante A. v., Wang Y.-Q., Ito Y., Bao X., Hemmerich S., Fukuda M., Rosen S. D. (2011) Functional contributions of N- and O-glycans to L-selectin ligands in murine and human lymphoid organs. Am. J. Pathol. 178, 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kobayashi M., Hoshino H., Suzawa K., Sakai Y., Nakayama J., Fukuda M. (2012) Two distinct lymphocyte homing systems involved in the pathogenesis of chronic inflammatory gastrointestinal diseases. Semin. Immunopathol. 34, 401–413 [DOI] [PubMed] [Google Scholar]
- 25. Ilarregui J. M., Bianco G. A., Toscano M. A., Rabinovich G. A. (2005) The coming of age of galectins as immunomodulatory agents: impact of these carbohydrate binding proteins in T cell physiology and chronic inflammatory disorders. Ann. Rheum. Dis. 64, iv96–iv103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rabinovich G. A., Baum L. G., Tinari N., Paganelli R., Natoli C., Liu F.-T., Iacobelli S. (2002) Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 23, 313–320 [DOI] [PubMed] [Google Scholar]
- 27. Seales E. C., Shaikh F. M., Woodard-Grice A. V., Aggarwal P., McBrayer A. C., Hennessy K. M., Bellis S. L. (2005) A protein kinase C/Ras/ERK signaling pathway activates myeloid fibronectin receptors by altering β1 integrin sialylation. J. Biol. Chem. 280, 37610–37615 [DOI] [PubMed] [Google Scholar]
- 28. Woodard-Grice A. V., McBrayer A. C., Wakefield J. K., Zhuo Y., Bellis S. L. (2008) Proteolytic shedding of ST6Gal-I by BACE1 regulates the glycosylation and function of α4β1 integrins. J. Biol. Chem. 283, 26364–26373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Z., Swindall A. F., Kesterson R. A., Schoeb T. R., Bullard D. C., Bellis S. L. (2011) ST6Gal-I regulates macrophage apoptosis via α2–6 sialylation of the TNFR1 death receptor. J. Biol. Chem. 286, 39654–39662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Swindall A. F., Bellis S. L. (2011) Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J. Biol. Chem. 286, 22982–22990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dimitroff C. J., Lee J. Y., Rafii S., Fuhlbrigge R. C., Sackstein R. (2001) CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J. Cell Biol. 153, 1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sackstein R., Merzaban J. S., Cain D. W., Dagia N. M., Spencer J. A., Lin C. P., Wohlgemuth R. (2008) Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat. Med. 14, 181–187 [DOI] [PubMed] [Google Scholar]
- 33. Winkler I. G., Barbier V., Nowlan B., Jacobsen R. N., Forristal C. E., Patton J. T., Magnani J. L., Lévesque J. P. (2012) Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med. 18, 1651–1657 [DOI] [PubMed] [Google Scholar]
- 34. Stübke K., Wicklein D., Herich L., Schumacher U., Nehmann N. (2012) Selectin-deficiency reduces the number of spontaneous metastases in a xenograft model of human breast cancer. Cancer Lett. 321, 89–99 [DOI] [PubMed] [Google Scholar]
- 35. Zen K., Liu D.-Q., Guo Y.-L., Wang C., Shan J., Fang M., Zhang C.-Y., Liu Y. (2008) CD44v4 is a major E-selectin ligand that mediates breast cancer cell transendothelial migration. PLoS One 3, e1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wandall H. H., Rumjantseva V., Sørensen A. L., Patel-Hett S., Josefsson E. C., Bennett E. P., Italiano J. E., Jr., Clausen H., Hartwig J. H., Hoffmeister K. M. (2012) The origin and function of platelet glycosyltransferases. Blood 120, 626–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X. C., Smith T. J., Lau J. T. (1990) Transcriptional regulation of the liver β-galactoside α2,6-sialyltransferase by glucocorticoids. J. Biol. Chem. 265, 17849–17853 [PubMed] [Google Scholar]
- 38. Dalziel M., Lemaire S., Ewing J., Kobayashi L., Lau J. T. (1999) Hepatic acute phase induction of murine β-galactoside α2,6 sialyltransferase (ST6Gal-1) is IL-6 dependent and mediated by elevation of Exon H-containing class of transcripts. Glycobiology 9, 1003–1008 [DOI] [PubMed] [Google Scholar]
- 39. Swindall A. F., Londoño-Joshi A. I., Schultz M. J., Fineberg N., Buchsbaum D. J., Bellis S. L. (2013) ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Res. 73, 2368–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hui N., Le Marer N. (2001) α-2,6-Sialylation regulation in CD34+ progenitor cells in the human bone marrow and granulocyte colony-stimulating factor mobilization. J. Hematother. Stem Cell Res. 10, 661–668 [DOI] [PubMed] [Google Scholar]
- 41. Wilson A., Laurenti E., Oser G., van der Wath R. C., Blanco-Bose W., Jaworski M., Offner S., Dunant C. F., Eshkind L., Bockamp E., Lió P., Macdonald H. R., Trumpp A. (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.