Abstract
Purpose
Periodontitis is an infectious disease caused predominantly by gram-negative anerobes. The host inflammatory response to these bacteria causes alveolar bone loss that is characterized as periodontitis. Omega-3 fatty acids (ω-3 FAs) have anti-inflammatory properties, thus have been used to treat some chronic inflammatory diseases such as cardiovascular disease and rheumatoid arthritis. We aimed to evaluate the effect of dietary supplementation with ω-3 FAs as a host modulating agent in patients with chronic periodontitis.
Methods
Sixty otherwise healthy subjects with moderate and severe chronic periodontitis were enrolled in our randomised, double-blind, placebo-controlled trial. The control group (CG, n=30) was treated with scaling and root planing (SRP) and given a placebo; the treatment group (TG, n=30) was treated with SRP and dietary supplementation of ω-3 FAs (one 300 mg tablet daily for 12 weeks). Periodontal clinical parameters and serum C-reactive protein (CRP) levels were evaluated in all patients at baseline, a 6-week and 12-week period after treatment.
Results
A significant reduction in the gingival index, sulcus bleeding index, pocket depth, and clinical attachment level was found in the TG compared to the CG at a 12-week period. However, no statistically significant changes in serum CRP levels were found.
Conclusions
Our findings suggest that ω-3 FAs can successfully reduce gingival inflammation, pocket depth, and attachment level gain. Dietary supplementation with ω-3 FAs may have potential benefits as a host modulatory agent in the prevention and/or adjunctive management of chronic periodontitis.
Graphical Abstract
Keywords: Chronic periodontitis, C-reactive protein, Omega-3 fatty acids
INTRODUCTION
Periodontitis is a chronic inflammatory disease characterized by the loss of tooth-supporting structures, including connective tissue attachment and alveolar bone. Tissue destruction in periodontitis can occur because of the interaction between dental plaque bacteria and the immune inflammatory response, which is induced by mediators such as arachidonic acid (AA) metabolites, cytokines, and enzymes. Lipopolysaccharide, a major virulence factor of gram-negative periodontopathogens, induces the release of AA metabolites via the cyclooxygenase and lipoxygenase pathways [1].
Therefore, periodontal diseases are associated with specific pathogenic bacteria; however, most of the tissue damage is caused by the host response to infection, not by the infectious agents directly [2]. Accordingly, host modulatory therapy (HMT) has emerged as a new concept for the treatment of periodontal diseases. HMT aims to reduce tissue destruction and stabilize or even regenerate the periodontium by modifying or down-regulating the destructive aspects of the host response and by up-regulating the protective or regenerative responses [3]. HMT is delivered systemically or locally as part of patient's periodontal therapy and used as an adjunct to conventional periodontal treatment [4]. The goal of HMT is to reduce inflammatory processes as well as enhance wound healing and periodontal stability without impairing normal defense mechanisms or inducing inflammation [5].
A variety of different drug classes have been evaluated as host modulation agents, including nonsteroidal anti-inflammatory drugs, tetracyclines, and bisphosphonates [6]. These drugs have their own limitations and adverse effects when prescribed for HMT [6-10]. However, omega-3 polyunsaturated fatty acids (ω-3 PUFAs), including docosahexaenoic acid (DHA; C22:6, n-3) and eicosapentaenoic acid (EPA; C20:5, n-3), were shown to have therapeutic value and anti-inflammatory and protective effects in rheumatoid arthritis, cystic fibrosis, ulcerative colitis, asthma, atherosclerosis, cancer, cardiovascular disease, and periodontitis [11]. At first, the beneficial effects of ω-3 PUFAs were attributed to a decrease in the production of classic inflammatory mediators such as AA-derived eicosanoids (prostaglandin E2) and inflammatory cytokines [12]. However, later work revealed that a novel series of lipid mediators, resolvins and protectins, is enzymatically converted by ω-3 PUFAs, which serve as substrates for the reaction [11,13-15].
C-reactive protein (CRP) is a serological marker of systemic inflammation that has been associated with an increased risk of various systemic diseases, including cardiovascular disease [16] and adverse pregnancy outcomes [17]. Periodontitis has also been linked to elevated CRP levels in adults. Serum CRP levels were shown to be higher in patients with periodontal disease and positively correlated with clinical parameters, including bleeding on probing (BOP), clinical attachment level (CAL), and the percentage of sites with pocket depths (PDs) ±4 mm. Thus, CRP might be a possible mediator for the association between periodontitis and systemic diseases [18].
The goal of the present study was to test an innovative therapy option for patients affected with generalized moderate and severe chronic periodontitis. Our hypothesis was that dietary supplementation with ω-3 PUFAs has the potential to induce a measurable clinical outcome and pharmacologically interrupt the inflammatory cascade. Therefore, we performed a randomised controlled trial of daily dietary ω-3 PUFA supplementation as an adjunct of standard periodontal therapy (scaling and root planning, SRP). Clinical outcomes of active versus placebo therapies were measured in addition to the quantification of serum CRP biomarkers.
MATERIALS AND METHODS
This parallel, double-blind, placebo-controlled, randomised trial was carried out on eligible patients who reported to the Outpatient Department of the Tatyasaheb Kore Dental College and Research Centre, New Pargaon. The institutional ethical committee approved this study and each patient was enrolled after providing an informed consent. This clinical trial is registered at www.clinicaltrials.gov (registration number NCT01997853).
Sample size
The study was powered at 80% to detect a mean PD difference of 0.8 mm after treatment assuming a 30% within-group change in the primary outcomes PD and CAL. The minimum required sample size was calculated to be 24 patients for each group; to compensate for potential dropouts, 30 patients were recruited for each group.
Eligibility criteria
Sixty patients with moderate (≥2 interproximal sites with a CAL ≥4 mm on different teeth or ≥2 interproximal sites with PDs ≥5 mm on different teeth) or severe (≥2 interproximal sites with a CAL ≥6 mm on different teeth and ≥1 interproximal site with PDs ≥5 mm) chronic periodontitis, as defined by the 2007 criteria [19], were selected for this study. Patients who were nonsmokers, between the age of 30 to 60 years, and without any systemic illnesses, allergies, current pregnancy, history of drug intake, and periodontal treatment in past 6 months were eligible for participation. Participants were recruited from July 2012 to May 2013 and attended clinic visits at baseline, a 6- and 12-week period after randomization/treatment initiation until August 2013.
Randomization
Two groups (the treatment group [TG] and control group [CG]) of 30 participants each were created through randomization in accordance with the 2010 CONSORT (consolidated standards for reporting of trials) guidelines [20]. The entire trial was supervised and monitored by a data monitoring group comprised of two researchers. Patient allocation was done using a random key generator (GraphPad QuickCalcs, GraphPad Software Inc., San Diego, CA, USA). Individuals completely blinded to the study allocated and examined all participants, thus ensuring double blinding.
Each participant was given a number so the data monitoring group could track whether the patient was in the TG or CG. All investigators and outcome assessors were blinded to patient allocation.
Intervention
After the patient allocation, periodontal parameters such as plaque index (PI), gingival index (GI), oral hygiene index-simplified (OHIS), BOP, PD, and CAL were recorded at baseline (before treatment). Serum CRP, PD, and CAL were primary outcome measures, whereas the PI, GI, OHIS, and sulcus bleeding index (SBI) were secondary outcome measures. The same periodontal parameters were recorded at a 6- and 12-week period after treatment in both the TG and CG. A UNC-15 periodontal probe was used to measure the PD and CAL. Four sites per tooth (mesiobuccal, buccal, distobuccal, and palatal or lingual) were checked for their PI, GI, SBI, PD, and CAL. The results of all readings were averaged by the sum of the total number of surfaces recorded. All the clinical parameters except OHIS were recorded for all available teeth. At each visit, serum CRP levels were measured. Initial therapy was performed on all patients at baseline and consisted of a full-mouth SRP; local anesthesia was used whenever deemed appropriate. SRP was performed by hand and with ultrasonic instrumentation as necessary, while oral hygiene instructions were given. The protocol called for SRP to be completed within a 14-day interval. The same periodontist performed all initial therapy procedures. After the completion of the initial therapy session, patients were referred to the data monitoring group to receive their drugs; the data monitoring group was unaware of patient data, which ensured nonbiased evaluations.
The TG received 300 mg of ω-3 PUFAs (Mega-3, Dr. Reddy's Laboratories Ltd., Hyderabad, India) orally as one capsule to be taken once daily for 12 weeks. Each ω-3 PUFA capsule contained 180 mg and 120 mg of EPA and DHA, respectively. The CG received a placebo capsule containing 300 mg of liquid paraffin once daily for 12 weeks. Liquid paraffin is an inert compound used as a lubricant laxative and the typical laxative dose ranges between 15 and 30 mg/day. In this study, the 300 mg/day dose was not expected to have a laxative effect, while any laxative effect was considered to be the same as that of the ω-3 PUFA capsules. Both capsules were dispensed in similar packets ensuring that patients were blinded to their study group. Compliance was checked after 6 weeks when patients were asked to bring their pack of capsules with them to the follow-up visit. Patients with the capsules remaining at the 6-week period were excluded from the study and considered dropouts.
Both groups received thorough oral hygiene instructions, but prescription mouthwash was later provided. During the 6-week and 12-week follow-up visits, the periodontal parameters were recorded and oral hygiene instructions were reinforced.
All examinations were performed by a single dentist who had already received a proper training from a periodontist. At the start of the data collection, 10% of all measurements were repeated by the examiner to evaluate intraexaminer consistency. The reproducibility and concordance of clinical measurements were calculated using the κ index. For probing depths, the κ index was 0.87, which is considered a strong positive association, thereby proving the efficacy of the calibration.
Statistical analysis
Descriptive and inferential statistical analyses were performed. Continuous variables are presented as mean±standard deviation, while categorical measurements are presented as number (percentage). Significance was assessed at a 5% level. The following assumptions about the data were made: (1) dependent variables should be normally distributed, (2) samples drawn from the population should be random, and (3) each case should be independent of one another.
Two tailed, independent Student t-tests were used to investigate the significance of study parameters on a continuous scale between the two groups (intergroup variability). Levene's test was performed to assess the homogeneity of variance between the two groups and two tailed, dependent Student t-tests were used to determine the significance of study parameters on a continuous scale within each group (intragroup variability). Chi-square and Fisher exact tests were used to test for significance among categorical variables as appropriate. Pearson correlations between the changes in clinical variables and CRP (at baseline and 12 weeks after baseline) were also performed.
SAS 9.2 (SAS Institute Inc., Cary, NC, USA), SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA), STATA release 10.1 (StataCorp LP., College Station, TX, USA), MedCalc 9.0.1 (MedCalc, Ostend, Belgium), Systat 12.0 (Systat Software Inc., Chicago, IL, USA), and R ver.2.11.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for all data analyses.
RESULTS
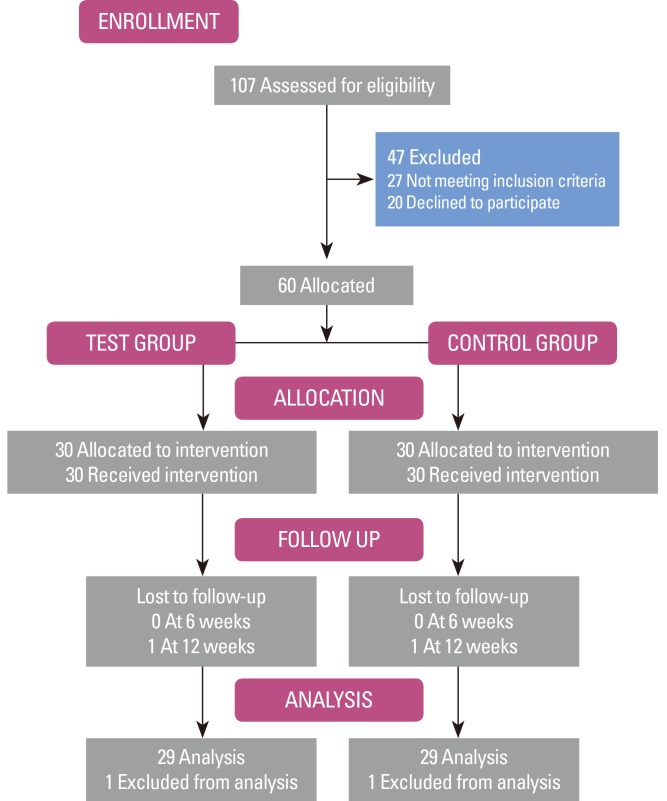
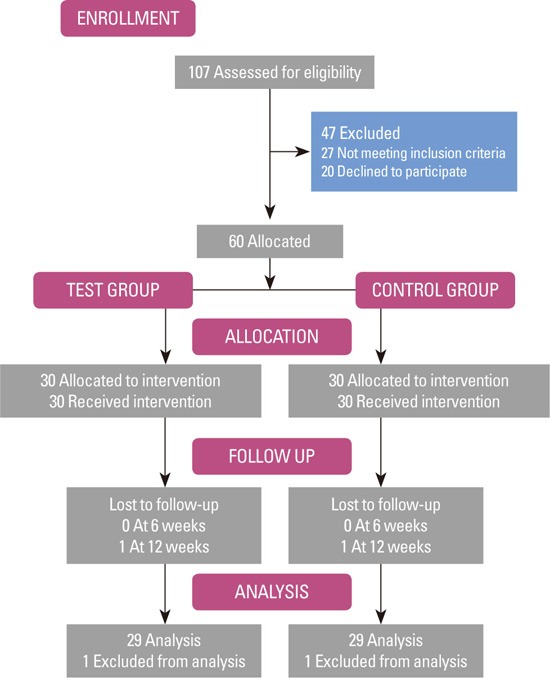
The primary analysis calculated intention-to-treat and involved all patients. One patient from each group was lost to follow-up after the second visit, and hence was excluded from the study. A schematic diagram of the study population is shown in Fig. 1. Between the groups, no statistical differences by age and sex were found (P>0.05) (Table 1). None of the patients had any known systemic conditions, infections, or any other factors known to influence their CRP level. In addition, none of the patients reported any change in their general health or medical status during the study period.
Figure 1.
Schematic diagram of the study population.
Table 1.
Demographics of subject population and baseline periodontal parameters
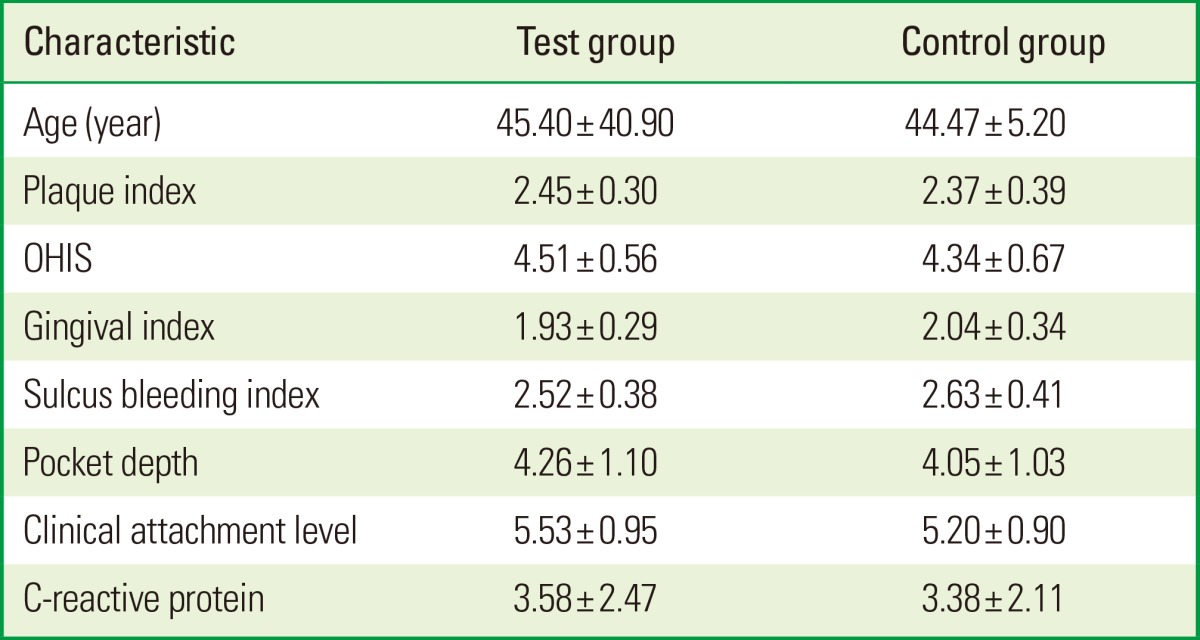
Values are presented as mean±standard deviation. Student t-test.
OHIS: oral hygiene index-simplified.
There were no significant differences between groups (P>0.05).
As previously stated, all clinical and biochemical parameters were assessed in both groups at the baseline and again in 6 and 12 weeks after treatment. At baseline, no significant differences between the groups were found among any of the clinical parameters and serum CRP levels (Table 1). After thorough SRP followed by regular maintenance, the level of plaque control in all the patients was satisfactory. Throughout the study, plaque accumulation was minimal with no significant differences between the two groups.
No statistically significant difference was found for PI between the TG and CG after 6 and 12 weeks. Reduction in the OHIS scores was highly significant in the CG compared to the TG after 6 weeks and 12 weeks (P=0.004). The GI, SBI, and PD significantly decreased in the TG compared to the CG after 6 weeks and 12 weeks (P<0.001). However, the CAL was significantly different only after 12 weeks in the TG compared to the CG (P<0.001). CRP scores were not statistically significant between groups at any point in the study (Table 2).
Table 2.
Full-mouth values for periodontal measurements before and after treatment
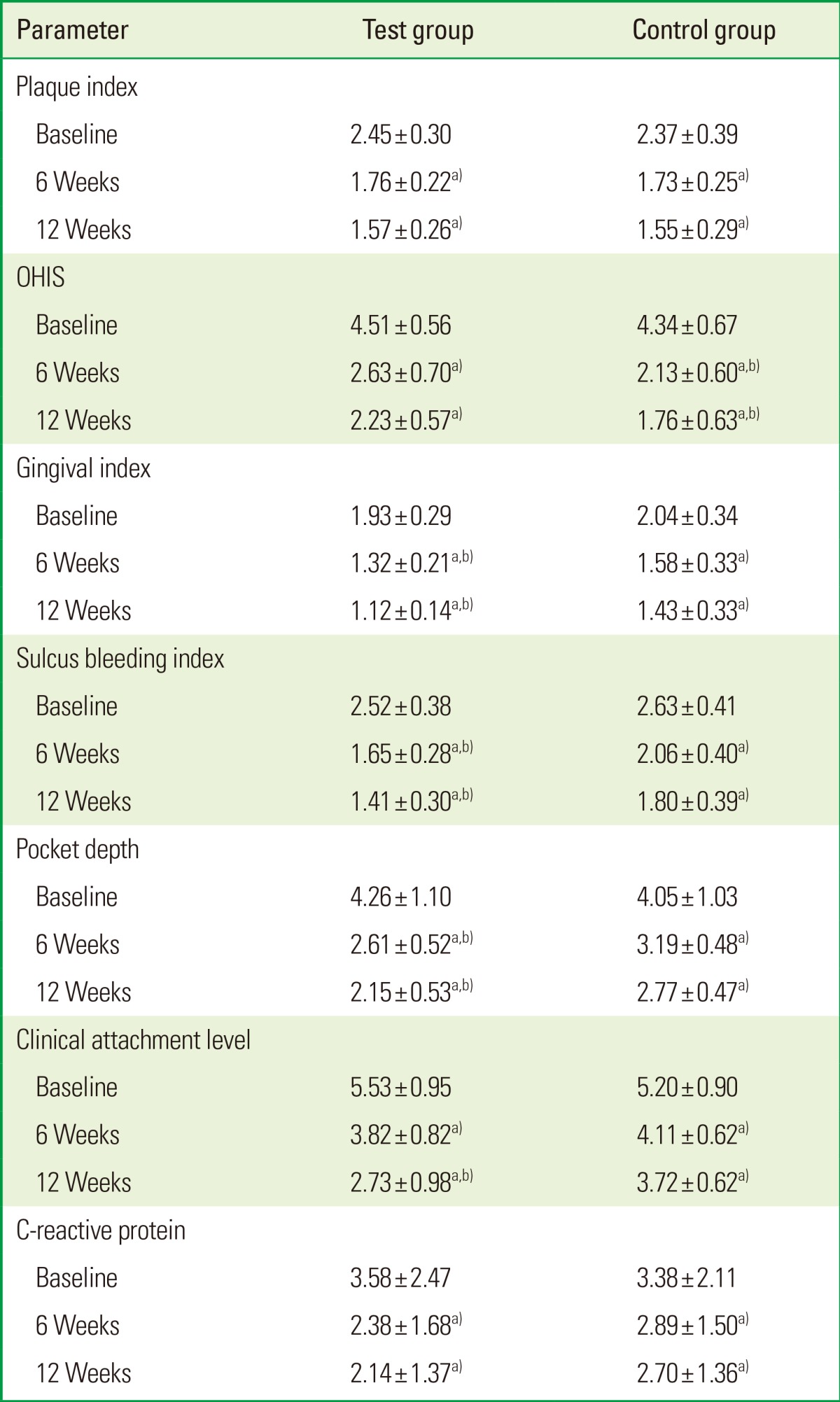
a)P<0.05 compared to baseline. b)P<0.05 compared to the control group.
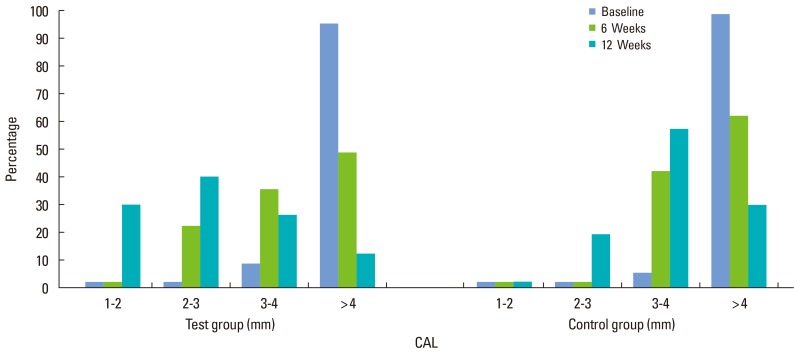
The data were further analyzed to determine changes to the distribution of the CAL before and after treatment. The TG had a significantly greater CAL than the placebo CG. In the CG, the percentage of patients with a CAL between 2 and 3 mm was 0% versus 20% in the TG at the 6-week mark (P=0.43); After 12 weeks, the percentage in the CG versus the TG was 17.2% and 37.9%, respectively (P<0.001). These findings suggest that approximately 20% of sites showed an improved CAL in the TG compared to the CG (Table 3, Fig. 2).
Table 3.
Categorical analysis of clinical attachment level (CAL) in two groups in three different time periods
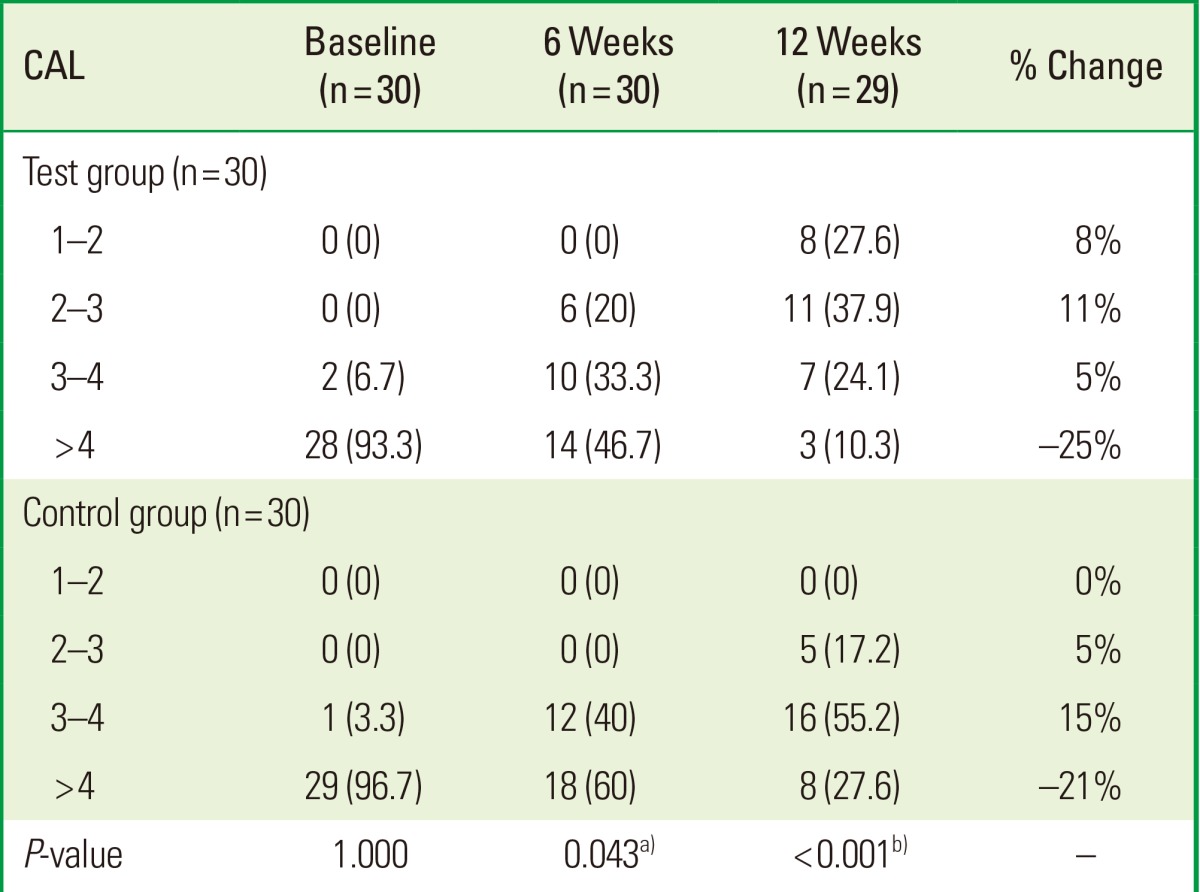
Values are presented as number (%).
a)Moderately significant (0.01<P≤0.05). b)Strongly significant (P≤0.01).
Figure 2.
The categorical distribution of the clinical attachment level (CAL) for each group at different periods.
The results of the Pearson correlation analyses indicate that changes to CRP levels were associated with CAL only in both the TG and CG after treatment (TG: r=0.361, P=0.056; CG: r=0.549, P=0.002) (Table 4).
Table 4.
Pearson correlation of changes in clinical variables with changes in CRP
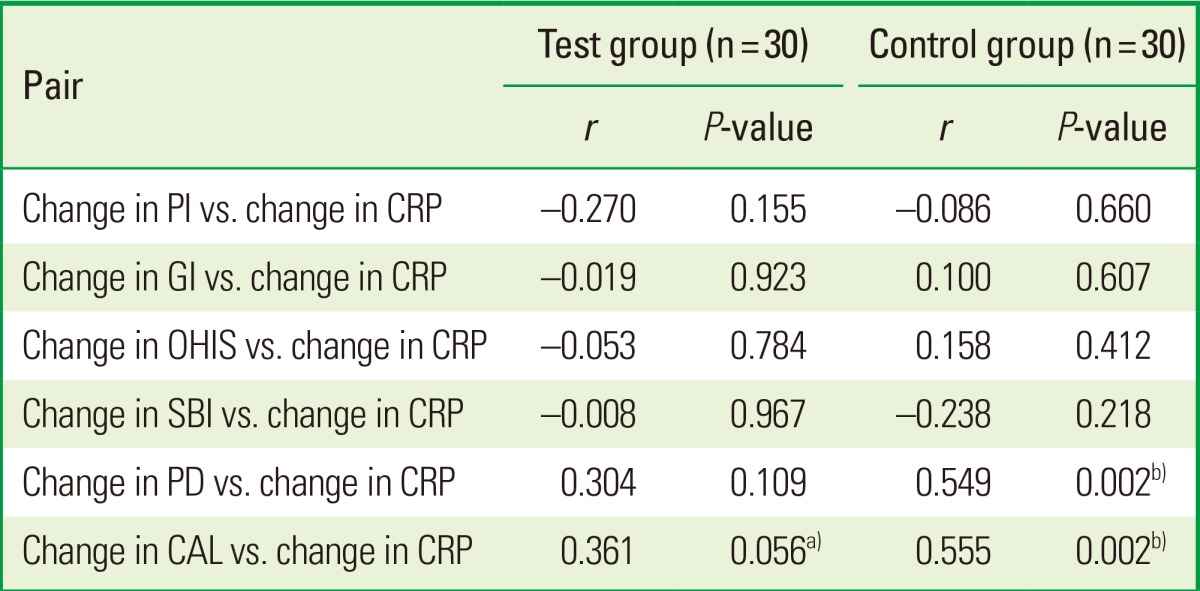
Change in variable is computed based on baseline and 12th week measurement.
PI: plaque index, CRP: C-reactive protein, GI: gingival index, OHIS: oral hygiene index-simplified, SBI: sulcus bleeding index, PD: pocket depth, CAL: clinical attachment level.
a)Suggestive significant (0.05<P<0.10). b)Strongly significant (P≤0.01).
DISCUSSION
In this study, HMT, an adjunct to nonsurgical treatment of periodontitis, using dietary ω-3 PUFA supplementation in humans was successfully performed. A 300-mg gelatin capsule of ω-3 PUFAs (EPA and DHA) administered daily was found to significantly improve the outcomes of standard SRP as defined by PD, CAL, PI, GI, OHIS, and SBI. In addition, serum CRP levels were reduced in the TG; however, these changes were not significant compared to CG.
The beneficial actions of dietary ω-3 PUFA supplementation were demonstrated in various inflammatory conditions in humans including rheumatoid arthritis, cardiovascular disease, and inflammatory bowel disease and in a wide variety of animal models of inflammatory disease [21,22]. Data on the relationship of anti-inflammatory molecules with chronic inflammation in periodontal disease are limited, yet one study suggested that hyperlipidemia may be associated with periodontitis [23]. The molecular basis for the anti-inflammatory effect of ω-3 PUFAs appears to lie in the enzymatic pathways of inflammation resolution by inhibiting the production of nuclear transcription factors and cytokines [11,24]. Inflammation resolution is an active process mediated by the metabolism of AA through lipoxygenase transformation circuits leading to the production of lipoxins, endogenous anti-inflammatory and proresolution lipid mediators. The same enzyme system metabolizes EPA and DHA into resolvins of the E and D series, respectively. In addition, the beneficial actions of resolvins in a variety of inflammatory conditions have been well described [11]. The effect of ω-3 PUFAs, presumably through the actions of resolvins, on inflammatory biomarkers is diverse [11]. For example, reduction of upstream proinflammatory cytokines has been reported, which directs neutrophils to apoptosis and the nonphlogistic (literally non-heat-producing or noninflammatory) recruitment of monocytes. The administration of ω-3 PUFAs demonstrated an increased level of circulating resolvins [13], and the potency of pure resolvins in systemic disease was demonstrated to be greater than that of dietary supplementation in an animal model of diabetes [25].
The prevention and treatment of periodontitis with resolvins and lipoxins were described in animal studies [26]. These molecules are not available for human use; however, dietary supplementation with ω-3 PUFAs in humans is known to increase the circulating level of resolvins [13,14], which suggests a potential therapeutic modality. Marion-Letellier et al. [24] found that DHA, EPA, gamma-linolenic acid, and alpha-linolenic acid increased the level of peroxisome proliferator-activated receptor-gamma and reduced the production of the proinflammatory cytokines interleukin-8 and interleukin-6.
In the present study, GI and SBI, which are standard clinical indices indicating the inflammatory progress of chronic periodontitis, were significantly reduced in the TG compared to the CG (P<0.001). Throughout the study, minimal plaque accumulation was evident with no significant differences between the two groups. However, OHIS was significantly reduced in the CG compared to the TG (P<0.004). Although oral hygiene maintenance was better in the CG than the TG, GI, and SBI were significantly improved in the TG compared to the CG. Several clinical trials have shown that ω-3 PUFAs may significantly reduce the inflammatory burden on the body, which supports our findings.
Alam et al. [27] stated that rats fed with a diet rich in ω-3 PUFAs exhibited a reduced gingival tissue level of AA, prostaglandin E2, and leukotriene C4. Furthermore, Campan et al. [28] demonstrated in a human model that the gingival tissue level of AA, prostaglandin E2, and leukotriene B4 (LTB4) was reduced in the ω-3 PUFAs group, whereas the level for each increased in the placebo group. Vardar et al. [29] have demonstrated a significant decrease in the gingival tissue level of prostaglandin E2, prostaglandin F2 α, LTB4, and platelet activating factor in rats administered ω-3 PUFAs. In a randomized, double-blind, placebo-controlled trial by Elkhouli [30], treatment of a grade II furcation defect with a bone graft augmented by the systemic administration of ω-3 PUFAs resulted in successful reduction of gingival inflammation, reduction in PD, and increased CAL. This was also accompanied by a significant modulatory effect on interleukin-1β and interleukin-10 levels in those treated with ω-3 PUFAs compared to those treated with a bone graft and placebo [30]. Similarly, in a human clinical trial by El-Sharkawy et al. [31], subjects with chronic periodontitis demonstrated a reduced salivary level of receptor activator of nuclear factor kappa-B ligand and matrix metalloprotease-8 after ω-3 PUFA administration compared to the placebo group. Importantly, no adverse effects have been reported after the administration of ω-3 PUFAs. Because patients tolerate ω-3 PUFAs well and ω-3 PUFAs have anti-inflammatory properties, their use in the treatment of periodontal diseases is justified.
In a systematic review and meta-analysis on CRP in relation to periodontitis, Paraskevas et al. [18] have concluded that the serum CRP level in patients with periodontitis is elevated compared with healthy individuals and that periodontal therapy results in the lowering of CRP levels. Hence, we used the serum CRP level as a biochemical parameter to evaluate the effect of ω-3 PUFAs as an adjunctive therapy option used in conjunction with SRP.
Vardar-Sengul et al. [32] demonstrated that both the prophylactic and therapeutic use of ω-3 PUFAs in experimental periodontitis did not change the serum CRP level when compared to a placebo group in a rodent model. In their previous study, they also demonstrated that ω-3 PUFAs have significant inhibitory effects on AA metabolites in gingival tissue [29]. The present study failed to exhibit a significant effect of ω-3 PUFAs on the serum CRP level. Therefore, ω-3 PUFAs seem to have no effect on circulating CRP levels, but a local change may be expected of CRP levels in gingival crevicular fluid, saliva, and gingival tissues in patients with periodontitis after treatment with ω-3 PUFAs.
Differences in the sample size, dose of ω-3 PUFAs, and the short duration of treatment with ω-3 PUFAs may additionally explain the lack of differences between our study groups in terms of circulating levels of CRP and other studies. In this study, ω-3 PUFAs were used in the TG for 12 weeks; administration of ω-3 PUFAs for longer than 12 weeks with different doses may be required to reveal a significant effect on serum CRP levels. However, Mori et al. [33] reported that supplementation with purified EPA or DHA for 6 weeks in nonsmoking diabetic subjects did not lead to any significant change in the serum CRP level compared to placebo controls. Madsen et al. [34] administered ω-3 PUFAs at two different doses (6.6 g/day or 2.0 g/day) to healthy volunteers for 12 weeks and found no change in serum CRP concentrations after either dose of ω-3 PUFAs. Geelen et al. [35] evaluated the effects of ω-3 PUFAs in a placebo-controlled, double-blind study with 84 middle-aged subjects. The subjects were given capsules of either ω-3 PUFAs or a placebo for 12 weeks, and the results indicated no significant differences in serum CRP concentrations [35]. Further studies investigating the optimal dose and administration period of these agents for positive effects on bone metabolism and/or systemic inflammation markers are required.
In a clinical trial on patients with chronic periodontitis, PD and CAL were significantly improved after ω-3 PUFAs were administered compared to the placebo group [31]; these findings have been corroborated by Elkhouli [30]. Moreover, in a clinical trial involving patients with chronic periodontitis, combination therapy with ω-3 and ω-6 PUFAs significantly reduced PD compared to patients who were given a placebo [36].
In the present study, PD and CAL were significantly improved in the TG compared to the CG after 6 weeks and 12 weeks (P<0.001). In this study, the impact of adjunct dietary supplementation with ω-3 PUFAs on periodontal therapy outcomes was in the same range as that of adjunct antimicrobial therapy in similar experiments [37]. For example, the change in PD and CAL observed after ω-3 PUFAs was 0.83 mm and 1.32 mm, respectively; however, subgingivally delivered doxycycline hyclate as adjunct therapy improved PD by 1.1 mm [37]. The implications of these findings are not entirely clear; however, the data from this study imply that an inflammatory response was present in the TG as evidenced by the significant changes in the clinical parameters, which is similar to the study using doxycycline [37]. Thus, locally delivered ω-3 PUFAs may give better results than other locally delivered agents.
We analyzed our data further to determine whether changes in the distribution of the CAL were evident throughout the follow-up. The frequency distributions of the CAL between groups were compared before and after treatment; CAL were improved in the TG compared to the CG. At 6 weeks, 0% of the CG versus 20% in the TG had a CAL between 2-3 mm (P=0.43). At 12 weeks, 17.2% of the CG versus 37.9% of the TG had a CAL between 2-3 mm (P<0.001). Therefore, approximately 20% of the CAL sites were improved in the TG compared to the CG.
In addition to evaluating the potential of HMT use in periodontal treatment, we evaluated the efficacy of dietary supplementation as an inexpensive, sustainable public health measure aimed at modifying the course of periodontal disease. In this context, ω-6 PUFAs may provide a measurable benefit in subjects with periodontitis as well as with other inflammatory diseases and conditions.
In addition, the CRP level in moderate and severe periodontitis patients decreased significantly after periodontal therapy (from baseline to 12 weeks) in both the groups. Moreover, the percentage of patients with a CRP level >3 mg/L significantly dropped. This finding is in agreement with other studies [38,39] that reported significant reduction in serum CRP level after treatment.
This study has limitations. First, the duration of the follow-up was short. Second, changes in parameters after drug stoppage could not be examined. Last, patients' daily dietary intake of fatty acids and their body mass index may have affected our results. These parameters were not considered during the design of the study.
In conclusion, among the many methods available to treat periodontitis, host modulation by means of dietary therapy as an adjunct to conventional nonsurgical therapies (SRP) may be important in controlling the potential deteriorative effects of the host response. Observed improvements in the TG could have been attributed to the host modulating phenomenon of ω-3 PUFAs in patients with chronic periodontitis. However, these beneficial results were only observed within the clinical parameters, but not within the biochemical parameter (serum CRP level). Hence, more randomised controlled trials with a larger sample size and longer duration of the follow-up are warranted to validate the usage of ω-3 PUFAs as an adjunctive dietary therapy option to treat chronic periodontitis. If effective, this therapeutic modality may be a cheaper and safer adjunctive therapy option than currently available ones for the prevention and treatment of periodontitis.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993;64(5 Suppl):432–444. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- 2.Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents: a systematic review. Ann Periodontol. 2003;8:12–37. doi: 10.1902/annals.2003.8.1.12. [DOI] [PubMed] [Google Scholar]
- 3.Bhatavadekar NB, Williams RC. New directions in host modulation for the management of periodontal disease. J Clin Periodontol. 2009;36:124–126. doi: 10.1111/j.1600-051X.2008.01354.x. [DOI] [PubMed] [Google Scholar]
- 4.Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. J Clin Periodontol. 2005;32(Suppl 6):108–129. doi: 10.1111/j.1600-051X.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 5.Williams RC. Host modulation for the treatment of periodontal disease. Compend Contin Educ Dent. 2008;29:160–162. 164, 166–168. passim. [PubMed] [Google Scholar]
- 6.Novak MJ, Johns LP, Miller RC, Bradshaw MH. Adjunctive benefits of subantimicrobial dose doxycycline in the management of severe, generalized, chronic periodontitis. J Periodontol. 2002;73:762–769. doi: 10.1902/jop.2002.73.7.762. [DOI] [PubMed] [Google Scholar]
- 7.Ciancio SG. Systemic medications: clinical significance in periodontics. J Clin Periodontol. 2002;29(Suppl 2):17–21. [PubMed] [Google Scholar]
- 8.Preshaw PM, Hefti AF, Jepsen S, Etienne D, Walker C, Bradshaw MH. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis: a review. J Clin Periodontol. 2004;31:697–707. doi: 10.1111/j.1600-051X.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 9.Kunchur R, Goss AN. The oral health status of patients on oral bisphosphonates for osteoporosis. Aust Dent J. 2008;53:354–357. doi: 10.1111/j.1834-7819.2008.00078.x. [DOI] [PubMed] [Google Scholar]
- 10.Barragan-Adjemian C, Lausten L, Ang DB, Johnson M, Katz J, Bonewald LF. Bisphosphonate-related osteonecrosis of the jaw: model and diagnosis with cone beam computerized tomography. Cells Tissues Organs. 2009;189:284–288. doi: 10.1159/000151451. [DOI] [PubMed] [Google Scholar]
- 11.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 17.Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-Edwards JW. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol. 2005;162:1108–1113. doi: 10.1093/aje/kwi323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008;35:277–290. doi: 10.1111/j.1600-051X.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 19.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7 Suppl):1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10:28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Leeb BF, Sautner J, Andel I, Rintelen B. Intravenous application of omega-3 fatty acids in patients with active rheumatoid arthritis. The ORA-1 trial. An open pilot study. Lipids. 2006;41:29–34. doi: 10.1007/11745-006-5066-x. [DOI] [PubMed] [Google Scholar]
- 22.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 23.Moeintaghavi A, Haerian-Ardakani A, Talebi-Ardakani M, Tabatabaie I. Hyperlipidemia in patients with periodontitis. J Contemp Dent Pract. 2005;6:78–85. [PubMed] [Google Scholar]
- 24.Marion-Letellier R, Butler M, Dechelotte P, Playford RJ, Ghosh S. Comparison of cytokine modulation by natural peroxisome proliferator-activated receptor gamma ligands with synthetic ligands in intestinal-like Caco-2 cells and human dendritic cells--potential for dietary modulation of peroxisome proliferator-activated receptor gamma in intestinal inflammation. Am J Clin Nutr. 2008;87:939–948. doi: 10.1093/ajcn/87.4.939. [DOI] [PubMed] [Google Scholar]
- 25.González-Periz A, Horrillo R, Ferre N, Gronert K, Dong B, Moran-Salvador E, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 27.Alam SQ, Bergens BM, Alam BS. Arachidonic acid, prostaglandin E2 and leukotriene C4 levels in gingiva and submandibular salivary glands of rats fed diets containing n-3 fatty acids. Lipids. 1991;26:895–900. doi: 10.1007/BF02535974. [DOI] [PubMed] [Google Scholar]
- 28.Campan P, Planchand PO, Duran D. Pilot study on n-3 polyunsaturated fatty acids in the treatment of human experimental gingivitis. J Clin Periodontol. 1997;24:907–913. doi: 10.1111/j.1600-051x.1997.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 29.Vardar S, Buduneli E, Turkoglu O, Berdeli AH, Baylas H, Baskesen A, et al. Therapeutic versus prophylactic plus therapeutic administration of omega-3 fatty acid on endotoxin-induced periodontitis in rats. J Periodontol. 2004;75:1640–1646. doi: 10.1902/jop.2004.75.12.1640. [DOI] [PubMed] [Google Scholar]
- 30.Elkhouli AM. The efficacy of host response modulation therapy (omega-3 plus low-dose aspirin) as an adjunctive treatment of chronic periodontitis (clinical and biochemical study) J Periodontal Res. 2011;46:261–268. doi: 10.1111/j.1600-0765.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- 31.El-Sharkawy H, Aboelsaad N, Eliwa M, Darweesh M, Alshahat M, Kantarci A, et al. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 Fatty acids and low-dose aspirin. J Periodontol. 2010;81:1635–1643. doi: 10.1902/jop.2010.090628. [DOI] [PubMed] [Google Scholar]
- 32.Vardar-Sengul S, Buduneli N, Buduneli E, Kardesler L, Baylas H, Atilla G, et al. Dietary supplementation of omega-3 fatty acid and circulating levels of interleukin-1beta, osteocalcin, and C-reactive protein in rats. J Periodontol. 2006;77:814–820. doi: 10.1902/jop.2006.050214. [DOI] [PubMed] [Google Scholar]
- 33.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35:772–781. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 34.Madsen T, Christensen JH, Blom M, Schmidt EB. The effect of dietary n-3 fatty acids on serum concentrations of C-reactive protein: a dose-response study. Br J Nutr. 2003;89:517–522. doi: 10.1079/BJN2002815. [DOI] [PubMed] [Google Scholar]
- 35.Geelen A, Brouwer IA, Schouten EG, Kluft C, Katan MB, Zock PL. Intake of n-3 fatty acids from fish does not lower serum concentrations of C-reactive protein in healthy subjects. Eur J Clin Nutr. 2004;58:1440–1442. doi: 10.1038/sj.ejcn.1601986. [DOI] [PubMed] [Google Scholar]
- 36.Rosenstein ED, Kushner LJ, Kramer N, Kazandjian G. Pilot study of dietary fatty acid supplementation in the treatment of adult periodontitis. Prostaglandins Leukot Essent Fatty Acids. 2003;68:213–218. doi: 10.1016/s0952-3278(02)00272-7. [DOI] [PubMed] [Google Scholar]
- 37.Garrett S, Johnson L, Drisko CH, Adams DF, Bandt C, Beiswanger B, et al. Two multi-center studies evaluating locally delivered doxycycline hyclate, placebo control, oral hygiene, and scaling and root planing in the treatment of periodontitis. J Periodontol. 1999;70:490–503. doi: 10.1902/jop.1999.70.5.490. [DOI] [PubMed] [Google Scholar]
- 38.D'Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84:269–273. doi: 10.1177/154405910508400312. [DOI] [PubMed] [Google Scholar]
- 39.Montebugnoli L, Servidio D, Miaton RA, Prati C, Tricoci P, Melloni C, et al. Periodontal health improves systemic inflammatory and haemostatic status in subjects with coronary heart disease. J Clin Periodontol. 2005;32:188–192. doi: 10.1111/j.1600-051X.2005.00641.x. [DOI] [PubMed] [Google Scholar]