Abstract
NFAT5 is a transcription factor that protects the kidney from hypertonic stress and also is activated by hypoxia. We hypothesized that NFAT5 mitigates the extent of renal damage induced by ischemia-reperfusion injury (IRI). Mice were subjected to IRI by unilateral clamping of the left renal pedicle for 30 min followed by reperfusion. After 3 h of reperfusion, the level of NFAT5 mRNA was similar in contralateral and clamped kidneys. However, after 48 h NFAT5 mRNA accumulation increased approximately three-fold in both outer medulla and medullary thick ascending limb (mTAL) tubules. NFAT1 levels were elevated at 3 h but did not increase further at 48 h. Mice were then either pretreated for 72 h with an intrarenal injection of a lentivirus shRNA construct to silence NFAT5 (EGFP-U6-N5-ex8), or a control vector (EGFP-U6) before induction of IRI. NGAL and Kim-1 mRNA levels increased after IRI and further increased after knockdown of NFAT5, suggesting that silencing of NFAT5 exacerbates renal damage during IRI. In contrast, silencing of NFAT1 had no effect on the levels of NGAL or Kim-1 mRNA. H&E staining revealed patchy denudation of renal epithelial cells and tubular dilation when NFAT5 was silenced. The number of TUNEL-positive cells in the outer and inner medulla of the clamped kidney increased nearly 2-fold after knockdown of NFAT5 and was associated with an increase in the number of caspase-3 positive cells. Collectively, the data suggest that NFAT5 is part of a protective mechanism that limits renal damage induced by IRI.
Keywords: NFAT5, Ton/EBP, acute kidney injury, ischemia reperfusion injury
INTRODUCTION
Ischemic injury to the kidney results in tubular cell necrosis and apoptosis in association with infiltration of inflammatory cells into the interstitium. The renal outer medulla is subject to hypertonic and hypoxic stress, and the late proximal tubule (S3 segment) as well as the thick ascending limb (TAL) of Henle’s loop are susceptible to damage in acute kidney injury, a condition associated with high morbidity and mortality1. The induction and attempted resolution of tubular injury follows a course characterized by epithelial cell damage that peaks 2–3 days after injury and, subsequently, a reparative phase that involves tubule regeneration. Mechanisms that limit the extent of renal damage and contribute to the tubular cell-mediated repair phase are associated with the alteration of many genes in response to ischemic injury and are still being defined 2–4. Significant alterations in the transcriptional control of gene expression involving several transcription factors such as c-fos and hypoxia-inducible transcription factors (HIF) are prominent in IRI models 3, 5.
NFAT5/(Ton/EBP), a transcription factor crucial for cellular responses to hypertonic stress 6, 7, is expressed in the renal medulla and contributes to the induction of genes that increase accumulation of organic osmolytes that protect cells against damage in a hypertonic environment 8. Recently, activation and expression of NFAT5 was shown to increase in a rat model of IRI and in response to hypoxia in vitro, and silencing of this transcription factor increased markers of apoptosis 9. The functional consequences of NFAT5 have not yet been established in an in vivo model of IRI.
We previously showed that NFAT5 and NFAT1 are major NFAT isoforms expressed in the TAL 10. In addition, inhibition of apical Cl− entry into mTAL cells is NFAT5-dependent suggesting that NFAT5 is part of a mechanism that attenuates NaCl transport in the mTAL 10. The mTAL is moderately resistant to hypoxia provided that energy consumption related to ion transport activity is appropriately regulated 11. Since NaCl reabsorption in the TAL is an energy-dependent process, and an imbalance between metabolic supply and demand within an ischemic organ favors tissue hypoxia, we hypothesized that NFAT5 attenuates the extent of renal damage in IRI.
METHODS
Animals
Male C57BL/6J mice (8–12 wk; Jackson Laboratory) were maintained on standard diet, given tap water ad libitum and used in accordance with institutional and international guidelines for the welfare of animals (A3362-01).
Antibodies
The anti-NFAT5 antibody (Santa Cruz) was used at a 1:1,000 dilution and anti-EGFP antibody (Abcam) was used at a 1:10,000 dilution.
Plasmid constructs and virus preparation
The NFAT5-dominant negative (NFAT5-DN) expression plasmid was generated as previously described 12. The inhibitory construct for NFAT5 or NFAT1 was designed using a short-hairpin (sh) RNA-expressing construct targeting exon 8 of murine NFAT5 (U6-N5-ex8) or NFAT1 (U6-N1-ex8), as described previously 10, 12. Subcloning of EGFP, U6-N5-ex8 or U6-N1-ex8 into a pLKO.1 vector and cotransfecting HEK293-T cells with pLKO.1 was performed to generate lentivirus encoding EGFP, U6-N5-ex8 or U6-N1-ex8.
Lentivirus preparation and administration
Generation of lentiviral supernatants was performed as previously described using psPAX2, pMD2.G (Addgene) and pLKO.1 or psiLV plasmids 13. In anesthetized mice, a 31G needle was inserted at the lower pole of the both kidneys parallel to the long axis and was carefully pushed toward the upper pole. As the needle was slowly removed, 50 μl filter-purified lentivirus (EGFP, U6-N5-ex8, or U6-N1-ex8 ~3×107 TUs) was injected. Lentiviral-mediated EGFP protein expression in kidney parenchyma was robust after 72 h 13.
Isolation of mTAL tubules and cells
mTAL tubules and cells (90–95% purity) were isolated from mice as previously described 12, and as detailed in the online supplement.
Transient transfection of mTAL cells
mTAL cells were cultured to 70–80% confluence in 6-well plates on membrane inserts (BD Biosciences) and transfected using Lipofectamine 2000 as previously described 12.
Model of renal IRI
The left renal pedicle was clamped for 30 min with microvascular clips (FE 723 K, Aesculap) to induce ischemia, which was verified by the change of renal color. Clamps were not applied in the sham group. Following removal of the clamp, mice were sacrificed at 3 and 48 h after reperfusion.
Isolation of total RNA and amplification of cDNA fragments/qRT-PCR
Total RNA was isolated from medulla, mTAL tubules, and mTAL cells as previously described; see online supplement for qRT-PCR analysis 13.
Western Blot Analysis
Solubilized samples were heated at 60ºC in loading buffer and protein concentration determined with a Bio-Rad protein assay kit. Equal amounts of protein were separated by SDS-PAGE and transferred to nitrocellulose membranes, blocked, and probed at 4ºC overnight with primary antibodies. After incubation with horseradish peroxidase (HP)-conjugated secondary antibodies (Amersham), proteins were detected by enhanced chemiluminescence (ECL; Amersham).
Immunohistochemistry Analysis
Kidneys were perfusion fixed in situ with 4% paraformaldehyde, fixed in 4% neutral-buffered paraformaldehyde overnight, and then embedded in paraffin. Serial sections (3–5 μm) were stained with hematoxylin and eosin or antibodies against EGFP and caspase-3.
TUNEL staining
An ApopTag-fluorescein in situ DNA fragmentation detection kit (Chemicon) was used to visualize TUNEL-positive cells, which were quantitated in different regions of the kidney by laser-scanning cytometry (CompuCyte).
Statistics
All data are presented as mean ± SE. Statistical analysis was performed using a one-way ANOVA followed by Tukey’s multiple comparisons or unpaired t-test as appropriate. Differences with p<0.05 were considered statistically significant.
RESULTS
Construction and validation of a lentivirus construct to silence NFAT5 in vivo
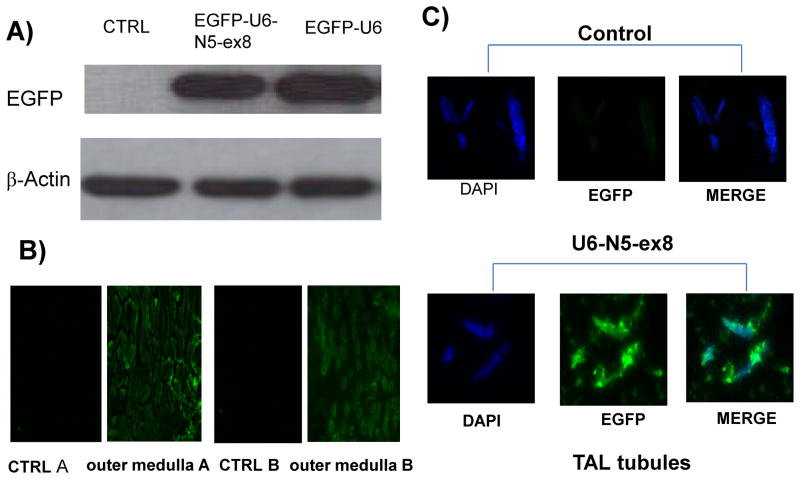
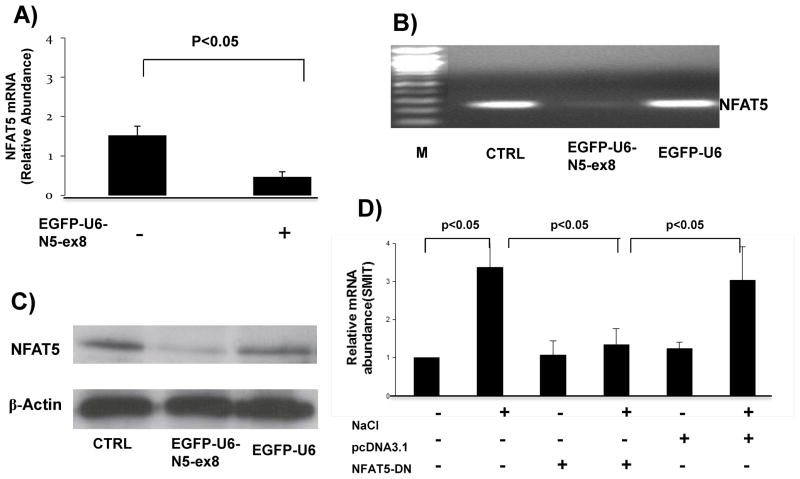
An shRNA construct targeting exon 8 of NFAT5 10 was used to prepare a novel lentivirus construct (EGFP-U6-N5-ex8) to silence NFAT5 expression in vivo. Kidneys were harvested from mice given an intrarenal injection of EGFP-U6 (control) or EGFP-U6-N5-ex8, containing a CMV promoter to drive expression of the EGFP gene. Western blot analysis showed robust expression of EGFP in mTAL tubules obtained from mice 72 h after administration of lentivirus constructs (Figure 1A). EGFP expression also was detected by immunofluorescence microscopy in outer medulla (OM) and TAL tubules from kidneys injected with lentivirus constructs but not PBS (control) (Figures 1B and C, respectively). Furthermore, endogenous NFAT5 mRNA accumulation was reduced by approximately 70% (Figures 2A and B). NFAT5 protein expression also was markedly inhibited in OM isolated from mice injected with EGFP-U6-N5-ex8, but not the control construct (EGFP-U6) (Figure 2C). Additionally, knockdown of NFAT5 prevented the increase in Na+/myo-inositol cotransporter (SMIT), a downstream target of NFAT5 that was induced when mTAL cells were exposed to media made hypertonic by addition of NaCl (400 mosmol/kg H2O) (Figure 2D). Collectively, these data show that the silencing vector blocks NFAT5 transcriptional activity and is effective in vivo.
Figure 1. Validation of control and silencing lentivirus constructs in vivo.
A lentivirus construct targeting exon 8 of NFAT5 (EGFP-U6-N5-ex8) was designed to silence NFAT5 in vivo. The construct was injected directly into the kidney and the EGFP transgene was used as a marker to verify expression 72 h after injection of the construct.
Figure 2. shNA lentivirus specific silencing of NFAT5 expression in vivo.
(A–C) Injection of EGFP-U6-N5-ex8 significantly inhibited the level of NFAT5 mRNA and protein levels in OM; control construct EGFP-U6 had no effect. (D) SMIT mRNA levels decreased when NFAT5 transcriptional activity was inhibited.
Expression of NFAT5 and NFAT1 in outer medulla and mTAL tubules after IRI
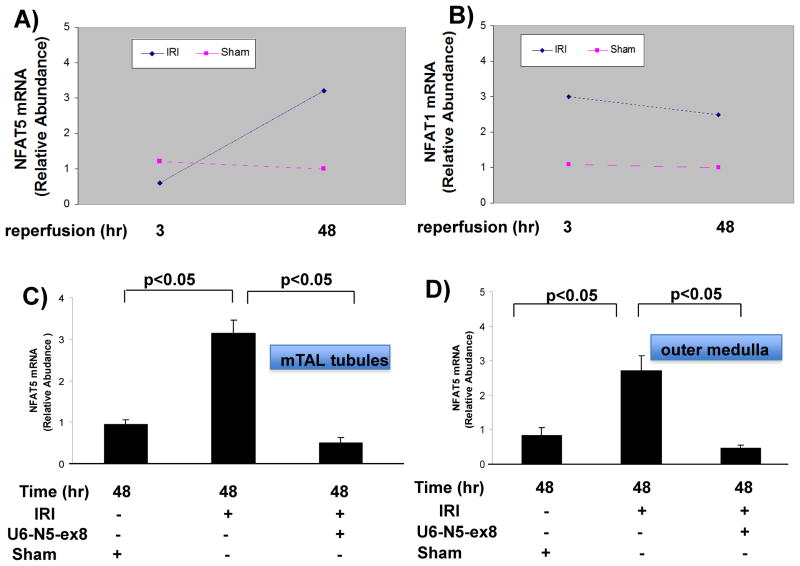
Kidneys were recovered 3 and 48 h after mice were subjected to 30 min of IRI induced by unilateral clamping of the left renal pedicle, and NFAT isoform mRNA levels were determined by qRT-PCR analysis in OM isolated from the clamped kidney or kidney of a sham-operated mouse. After 3 h of reperfusion, the relative abundance of NFAT5 mRNA was similar in OM from sham and ischemic kidneys, while NFAT1 mRNA increased (Figures 3A and B). However, after 48 h of reperfusion NFAT5 mRNA accumulation increased approximately three-fold in both mTAL tubules and OM, while NFAT1 mRNA accumulation did not increase further (Figures 3A–D). The increase in NFAT5 in both mTAL tubules and OM was inhibited in mice that were pretreated with the lentivirus construct, U6-N5-ex8, designed to silence NFAT5 (Figures 3C and D). Together, these data suggest that NFAT5 and NFAT1 are differentially regulated during IRI, and the targeting vector efficiently silences NFAT5 during IRI.
Figure 3. Regulation of NFAT isoforms during IRI.
(A and B) Kidneys were recovered at 3 or 48 h after mice were subjected to IRI induced by unilateral clamping of the renal pedicle for 30 min. NFAT5 and NFAT1 mRNA levels from outer medulla were analyzed by qRT-PCR. (C and D) The efficacy of NFAT5 silencing was shown during IRI and NFAT5 mRNA levels compared in sham and IRI mice.
Effect of NFAT5 silencing on biomarkers of renal damage during IRI
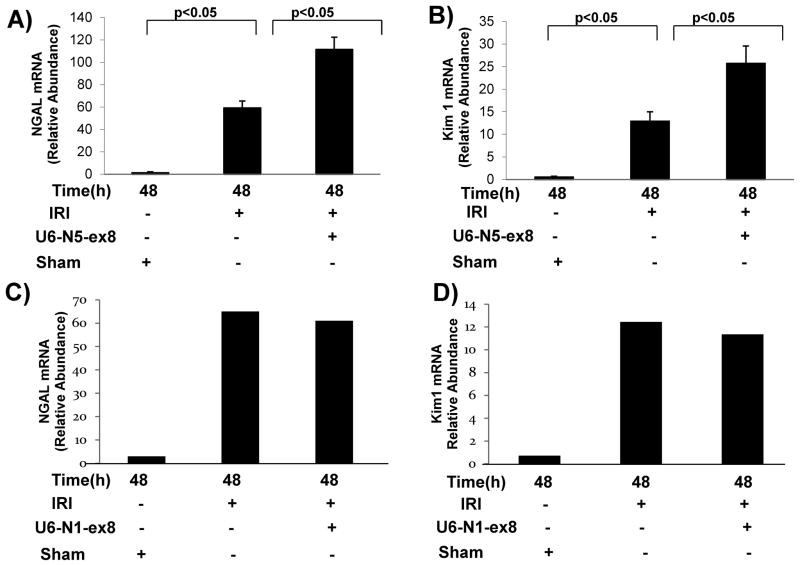
Kidney ischemia molecule (Kim)-1 and neutrophil gelatinase-associated lipocalin (NGAL) in the urine originate from damaged renal tubular epithelial cells and correlate well with their respective mRNA levels in the kidney 4, 14, 15. Since NGAL is a biomarker that anticipates the diagnosis of acute kidney injury and is expressed specifically in distal tubular segments injured by ischemia 14, 15, the effects of NFAT5 silencing on NGAL mRNA levels was determined in OM after 48 h of reperfusion. A marked increase in NGAL and Kim-1 mRNA levels were observed in OM from clipped kidneys compared with kidneys from sham-operated mice (Figures 4A and B). Silencing of NFAT5 in mice pretreated with U6-N5-ex8 further increased the mRNA levels of NGAL and Kim-1 (Figures 4A and B). In contrast, although NFAT1 mRNA accumulation remained elevated 48 h after reperfusion in OM, silencing of NFAT1 (U6-N1-ex8) did not affect NGAL or Kim-1 mRNA levels (Figures 4C and D). The data suggest that inhibition of NFAT5, but not NFAT1, worsens injury to nephron segments after IRI.
Figure 4. Knockdown of NFAT5 increases levels of renal damage biomarkers during IRI.
(A and B) NFAT5 or (C and D) NFAT1 was silenced down in vivo. Three days later IRI was induced and total mRNA was prepared from OM and analyzed by qRT-PCR 48 h after reperfusion.
Silencing of NFAT5 exacerbates renal damage induced by IRI
Intensified damage to both the proximal and distal tubule may have occurred when NFAT5 was silenced before initiation of renal ischemia, and high expression of NFAT5 in inner medullary (IM) collecting ducts may also make this region of the nephron susceptible to damage. Accordingly, we evaluated the effect of silencing NFAT5 on renal damage by evaluating sections from OM and IM that were stained with H&E after 30 min of unilateral IRI and 48 h reperfusion. Silencing of NFAT5 aggravated damage in OM and IM while injection of the control vector had no effect (Figure 5). Both regions of the medulla displayed patchy loss (denudation) of renal tubular cells and flattened tubular epithelium resulting in tubular dilation when NFAT5 was silenced, suggesting that NFAT5 is part of a mechanism that diminishes damage elicited by IRI.
Figure 5. Inhibition of NFAT5 increases renal damage induced by IRI.
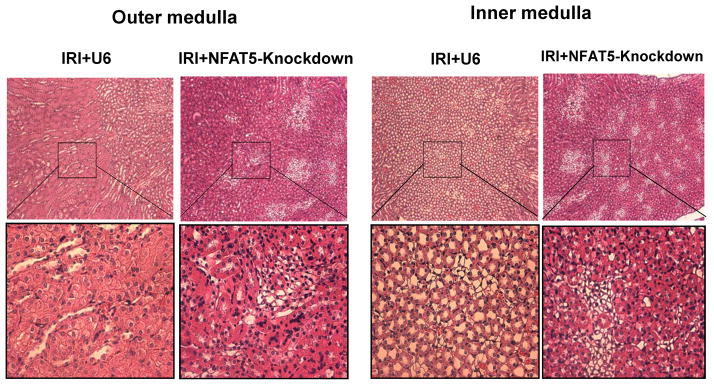
Renal histology was evaluated after 30 min of unilateral IRI and 48 h reperfusion. H&E sections of OM and IM were from mice injected with empty vector (IRI+U6) or silencing vector (NFAT5-knockdown). Low magnification images (top panels); higher magnification of the selected areas are shown in the lower panels.
TUNEL analysis of apoptosis during IRI
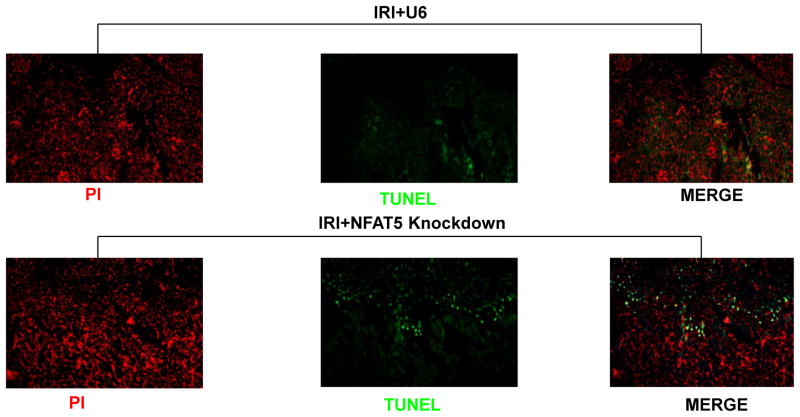
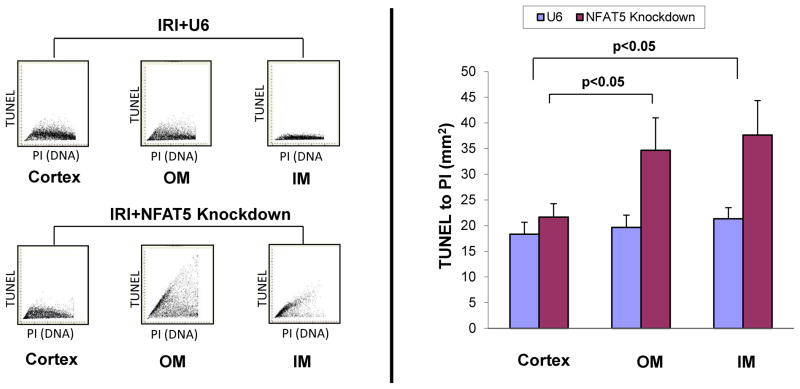
The nature of tubular destruction following silencing of NFAT5 was assessed to identify pathways that may be linked to this transcription factor. Since apoptosis is part of the profile of damage induced by IRI, we examined the effect of silencing NFAT5 on the frequency of TUNEL-positive cells. There were few TUNEL positive cells in OM or IM of IRI mice pretreated with control lentivirus (IRI +U6), in concert with the relatively mild protocol selected for the present study (unilateral 30 min occlusion) (Figure 6). However, silencing of NFAT5 was associated with a distinct increase in the frequency of TUNEL-positive cells in the OM (Figure 6). The renal OM is sensitive to damage due to hypoxia. However, since no information is available regarding the function of NFAT5 in IRI, we also measured apoptosis in the cortex and IM. Analysis by laser-scanning cytometry showed that the frequency of TUNEL-positive cells in OM and IM increased approximately 2- to 3-fold after silencing of NFAT5 (Figure 7). In contrast, silencing of NFAT5 did not result in damage to the cortex. These findings suggest that apoptosis may be part of the mechanism that contributes to IRI-induced renal medullary damage in the absence of NFAT5.
Figure 6. Silencing NFAT5 increases apoptosis in OM during IRI.
Renal sections from mice injected with empty vector (IRI+U6) or silencing vector were evaluated after 30 min of unilateral IRI and 48 h reperfusion. TUNEL-positive (green) and PI counterstained (red) cells were viewed by fluorescence microscopy.
Figure 7. NFAT5 knockdown does not increase apoptosis in the cortex during IRI.
Immunofluorescence analysis was performed using laser-scanning microscopy to evaluate frequency of apoptosis induced by IRI in different regions of the kidney. The data are from mice injected with empty vector (IRI+U6) or silencing vector and panels show the fluorescence intensity for TUNEL staining on the y-axis and propidium iodide (PI) staining on the x-axis. The ratio of the mean TUNEL per PI fluorescence intensity representing the frequency of apoptotic cells within the unit of scanned area is shown in the bar graphs.
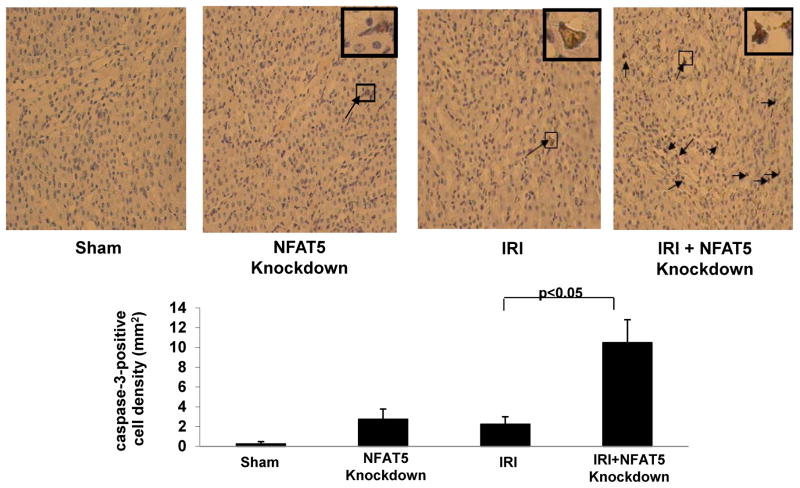
Knockdown of NFAT5 increases caspase-3 expression
As both caspase-dependent and -independent pathways induce apoptosis, the effect of silencing NFAT5 on caspase-3 expression was determined in sections of OM. Few caspase-3-positive cells were observed after IRI and 48 h of reperfusion in mice with an intact NFAT5 response, while silencing of NFAT5 increased the frequency of caspase-3-positive cells (Figure 8). The results suggest that knockdown of NFAT5 predisposes mice subjected to IRI to increased apoptosis by a mechanism involving caspase-3 activation.
Figure 8. NFAT5 knockdown increases caspase-3 expression in OM during IRI.
Light microscopy analysis was performed to evaluate expression of caspase-3 in OM; NFAT5 was silenced in sham mice and mice subjected to IRI. Caspase-3 positive cells (arrows) are stained brown.
DISCUSSION
We demonstrated that silencing of NFAT5 markedly increased renal damage in a model of IRI. NFAT5 mRNA levels increased in response to 30 min of unilateral IRI and 48 h of reperfusion. Silencing of NFAT5 increased NGAL and Kim-1, biomarkers of renal damage in IRI. In contrast, knockdown of NFAT1, which also was elevated 48 h after reperfusion, did not affect NGAL and Kim-1 levels. Extensive damage that included denudation of tubular epithelial cells and tubular dilatation was observed in OM and IM, but not in the cortex, of mice in which NFAT5 was silenced before initiation of IRI. Moreover, silencing NFAT5 increased the number of TUNEL-positive cells in OM and IM but not the cortex and the increase in apoptotic cells was associated with an increase in caspase-3 expression. Collectively, the data suggest that NFAT5 acts as a protective transcription factor in IRI, by diminishing the extent of renal injury via a mechanism that includes suppression of caspase-3-dependent apoptosis.
Recovery from acute kidney injury includes restoration of glomerular filtration rate and tubular repair but may lead to impaired renal hemodynamic and natriuretic responses that contribute to the development of salt-sensitive hypertension and chronic kidney disease. Elucidation of adaptive mechanisms that lessen renal damage induced by ischemic injury will establish a framework that provides options for new therapeutic approaches. The failure to increase NFAT5 during an early (3 h) stage of reperfusion injury coupled with its increase during the reparative phase (48 h) supports the notion that this transcription factor may be part of a protective mechanism that attenuates damage to hypoxic injury. In general, NFAT5 exhibits several functions that are renal protective, including the critical roles it plays as part of the adaptive response to hypertonic stress and during renal development 8, 16. For instance, deletion of NFAT5 in mice causes embryonic and perinatal lethality associated with dramatic effects on the architecture of the renal medulla, while the cortex was relatively normal 16. Increased apoptosis also was primarily observed in the medulla. These findings are consistent with the increased damage and apoptosis observed in OM and IM from mice that lacked an adequate NFAT5 response when subjected to IRI in the present study.
Our data are consistent with studies showing that 30 min of unilateral IRI produces limited damage to S3 segment of the proximal tubules 1. In general, longer and more severe ischemia is required to elicit significant distal nephron injury. The capacity of the distal tubule to use anaerobic glycolysis under conditions of diminishing oxygen availability also contributes to the resistance of the TAL to damage induced by hypoxia, providing that energy expenditure for ion transport is limited. Indeed, transport function is a major determinant of TAL injury in the context of limited oxygen availability, and damage to the TAL is observed when oxygen consumption for ion transport is exaggerated 11, 17, 18. We previously showed that NFAT5 is part of a mechanism in the TAL that increases TNF production, which acts an endogenous inhibitor of NKCC2 activity 19. Given the delicate balance between oxygen supply and demand with respect to cellular viability, it is tempting to speculate that attenuation of ion transport pathways is part of the mechanism by which NFAT5 is protective during IRI. The contribution of hypertonicity to the increasing damage in IRI following NFAT5 knockdown has not yet been addressed directly. However, preliminary experiments showed that silencing of NFAT5 within the time frame of the ischemia-reperfusion protocol used in the present study did not affect water intake or urine volume (unpublished data). In addition, the increase in NFAT5 in response to hypoxia in IMCD was similar under isotonic and hypertonic conditions 9.
Renal ischemic damage may be induced by several different mechanisms. For instance, TAL segment targeting of the von Hippel-Lindau protein (Vhl), which mediates degradation of HIF, limited IRI-induced damage in the proximal tubule as well as the TAL without a corresponding reduction in inflammatory cell infiltration 5. Alternatively, targeted apoptosis in the TAL is sufficient to induce severe acute kidney injury, and cross-talk between S3 segments of the proximal tubule and TAL via Tamm-Horsfall glycoprotein protects the kidney against ischemia by inhibiting inflammatory signaling between these adjacent nephron segments 20, 21. Such mechanisms are consistent with the finding that inflammatory cell recruitment need not be affected to improve renal damage 5. HIF is induced rapidly after IRI and then diminishes by 48 h 22. In contrast, NFAT5 expression was unchanged 3 h after IRI but increased at 48 h. As both these transcription factors lessen renal damage associated with IRI, the temporal distinction suggests that HIF and NFAT5 may contribute to different aspects of renal protection during IRI. In addition, Vhl deletion in the TAL did not affect the number of apoptotic cells 5 unlike silencing of NFAT5, which increased apoptosis in the present study. Thus, it is possible that the detrimental effects of silencing NFAT5 may be unrelated to HIF mechanisms, which supports in vitro data showing that HIF and NFAT5 are regulated independently 9.
Cell death programs including apoptosis, autophagy-associated cell death, and necrosis are initiated in response to IRI and are invoked or suppressed to different degrees by various molecules 23. For instance, although the renal protective effect of Tamm-Horsfall glycoprotein involved suppression of the inflammatory response, there did not appear to be an effect on apoptosis, suggesting that Tamm-Horsfall glycoprotein may inhibit processes related to necrosis 24. There also was no difference in the number of apoptotic cells in wild type and Vhl-deficient mice 20. In contrast, apoptosis was more severe in renalase-deficient mice and in the present study after NFAT5 silencing in mice subjected to IRI 25. Caspases are thought to play an important role in apoptosis as well as necrotic cell death 25, 26,27. However, a recent study showed that cleaved caspase-3 was absent during the course of IRI, and suggested this pathway may be of relatively minor importance in IRI 28. The increase in caspase-3 expression following NFAT5 silencing suggests that an NFAT5-dependent mechanism offsets renal damage induced by apoptosis in renal epithelial cells by suppressing caspase-3-dependent apoptosis.
The precise signals that induce NFAT5 during IRI are not yet defined and may include hypoxia as well as a change in the osmolality of the medulla due to altered expression of renal transporters. Nevertheless, NFAT5 appears to function as an upstream regulator of molecules and signaling pathways that integrate mechanisms directly related to tubular cell survival in the context of IRI.
Perspectives
Acute kidney injury results in rapid loss of renal function in association with death of tubular epithelial cells. This damage occurs despite adaptive changes within the kidney that attempt to minimize injury induced by hypoxia at the cellular level. Hypertension and other conditions such as diabetes may potentiate the ongoing impairment and accelerate the progression to chronic kidney disease. We demonstrated that induction of NFAT5 during the reparative phase of renal IRI is part of a mechanism that limits the extent of kidney injury induced by hypoxia. Moreover, the present study suggests that NFAT5 may function as a molecular switch that directs renal epithelial cell fate toward survival after ischemic injury. Future studies will evaluate the molecular mechanisms that trigger induction of caspase-3-dependent apoptosis observed following silencing of NFAT5.
Supplementary Material
Novelty and Significance.
What is New?
This is the first report to show a protective effect of NFAT5 in an in vivo model of hypoxia-induced renal damage.
When NFAT5 was silenced in vivo, increased damage was observed in renal epithelial cells in the outer and inner medulla.
The protective effects of NFAT5 are related to suppression of caspase-3-dependent apoptosis.
What is Relevant?
Renal functional changes during acute kidney injury include altered tubuloglomerular feedback, as sodium reabsorption in the proximal tubule is decreased thereby causing afferent arteriole constriction and a decrease in glomerular filtration rate—a hallmark feature of acute kidney injury.
Recovery from acute kidney injury includes restoration of glomerular filtration rate and tubular repair but may be associated with impaired renal hemodynamic and natriuretic responses that contribute to the development of salt-sensitive hypertension and chronic kidney disease.
Understanding the mechanisms that limit excessive damage in IRI may reveal novel targets that can be exploited to exert a positive influence on the repair, regeneration, and remodeling of the injured tubule.
Summary
NFAT5 is activated during hypoxic stress to the kidney as part of a protective mechanism that limits apoptotic cell death in the renal medulla.
Subversion of NFAT5-dependent protective mechanisms may promote the transition of acute kidney injury to chronic kidney disease and the development of hypertension.
Acknowledgments
Sources of funding: This work was supported by NIH grant HL34300, American Heart Association grant 12GRNT12060350, Fondecyt 1130741, and PGB 12-2007 CARE UC-SQM.
Footnotes
Disclosures: NONE.
References Cited
- 1.Heyman SN, Rosenberger C, Rosen S. Experimental ischemia-reperfusion: Biases and myths-the proximal vs. Distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77:9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- 2.Safirstein R. Gene expression in nephrotoxic and ischemic acute renal failure. J Am Soc Nephrol. 1994;4:1387–1395. doi: 10.1681/ASN.V471387. [DOI] [PubMed] [Google Scholar]
- 3.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heyman SN, Evans RG, Rosen S, Rosenberger C. Cellular adaptive changes in aki: Mitigating renal hypoxic injury. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27:1721–1728. doi: 10.1093/ndt/gfs100. [DOI] [PubMed] [Google Scholar]
- 5.Schley G, Klanke B, Schodel J, Forstreuter F, Shukla D, Kurtz A, Amann K, Wiesener MS, Rosen S, Eckardt KU, Maxwell PH, Willam C. Hypoxia-inducible transcription factors stabilization in the thick ascending limb protects against ischemic acute kidney injury. J Am Soc Nephrol. 2011;22:2004–2015. doi: 10.1681/ASN.2010121249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Rodriguez C, Aramburu J, Rakeman AS, Rao A. Nfat5, a constitutively nuclear nfat protein that does not cooperate with fos and jun. Proc Natl Acad Sci U S A. 1999;96:7214–7219. doi: 10.1073/pnas.96.13.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci U S A. 1999;96:2538–2542. doi: 10.1073/pnas.96.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87:1441–1474. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva S, Suazo C, Santapau D, Perez F, Quiroz M, Carreno JE, Illanes S, Lavandero S, Michea L, Irarrazabal CE. Nfat5 is activated by hypoxia: Role in ischemia and reperfusion in the rat kidney. PLoS One. 2012;7:e39665. doi: 10.1371/journal.pone.0039665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao S, Zhao H, Darzynkiewicz Z, Battula S, Ferreri NR. Expression and function of nfat5 in medullary thick ascending limb (mtal) cells. Am J Physiol Renal Physiol. 2009;296:F1494–1503. doi: 10.1152/ajprenal.90436.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brezis M, Rosen S. Hypoxia of the renal medulla--its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 12.Hao S, Zhao H, Darzynkiewicz Z, Battula S, Ferreri NR. Differential regulation of nfat5 by nkcc2 isoforms in medullary thick ascending limb (mtal) cells. Am J Physiol Renal Physiol. 2011;300:F966–F975. doi: 10.1152/ajprenal.00408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao S, Bellner L, Ferreri NR. Nkcc2a and nfat5 regulate renal tnf production induced by hypertonic nacl intake. AJP: Renal Physiology. 2013;304:F533–F542. doi: 10.1152/ajprenal.00243.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 15.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D’Agati V, Lin CS, Barasch J. The ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17:216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Rodriguez C, Antos CL, Shelton JM, Richardson JA, Lin F, Novobrantseva TI, Bronson RT, Igarashi P, Rao A, Olson EN. Loss of nfat5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci U S A. 2004;101:2392–2397. doi: 10.1073/pnas.0308703100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brezis M, Rosen S, Silva P. Transport activity modifies thick ascending limb damage in isolated perfused kidney. Kidney Inter. 1984;25:65–72. doi: 10.1038/ki.1984.9. [DOI] [PubMed] [Google Scholar]
- 18.Heyman SN, Brezis M, Epstein FH, Spokes K, Rosen S. Effect of glycine and hypertrophy on renal outer medullary hypoxic injury in ischemia reflow and contrast nephropathy. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1992;19:578–586. doi: 10.1016/s0272-6386(12)80838-9. [DOI] [PubMed] [Google Scholar]
- 19.Battula S, Hao S, Pedraza PL, Stier CT, Ferreri NR. Tumor necrosis factor-alpha is an endogenous inhibitor of the na+-k+-2cl- cotransporter (nkcc2) in the thick ascending limb. Am J Physiol Renal Physiol. 2011;301:F94–F100. doi: 10.1152/ajprenal.00650.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srichai MB, Hao C, Davis L, Golovin A, Zhao M, Moeckel G, Dunn S, Bulus N, Harris RC, Zent R, Breyer MD. Apoptosis of the thick ascending limb results in acute kidney injury. J Am Soc Nephrol. 2008;19:1538–1546. doi: 10.1681/ASN.2007101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Achkar TM, McCracken R, Rauchman M, Heitmeier MR, Al-Aly Z, Dagher PC, Wu XR. Tamm-horsfall protein-deficient thick ascending limbs promote injury to neighboring s3 segments in an mip-2-dependent mechanism. Am J Physiol Renal Physiol. 2011;300:F999–1007. doi: 10.1152/ajprenal.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberger C, Rosen S, Shina A, Frei U, Eckardt KU, Flippin LA, Arend M, Klaus SJ, Heyman SN. Activation of hypoxia-inducible factors ameliorates hypoxic distal tubular injury in the isolated perfused rat kidney. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:3472–3478. doi: 10.1093/ndt/gfn276. [DOI] [PubMed] [Google Scholar]
- 23.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering tlr4 expression. Am J Physiol Renal Physiol. 2008;295:F534–544. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HT, Kim JY, Kim M, Wang P, Tang L, Baroni S, D’Agati VD, Desir GV. Renalase protects against ischemic aki. J Am Soc Nephrol. 2013;24:445–455. doi: 10.1681/ASN.2012090943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nath KA, Croatt AJ, Warner GM, Grande JP. Genetic deficiency of smad3 protects against murine ischemic acute kidney injury. Am J Physiol Renal Physiol. 2011;301:F436–442. doi: 10.1152/ajprenal.00162.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki A. Amyloid beta-protein induces necrotic cell death mediated by ice cascade in pc12 cells. Exp Cell Res. 1997;234:507–511. doi: 10.1006/excr.1997.3639. [DOI] [PubMed] [Google Scholar]
- 28.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.