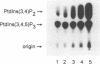
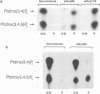
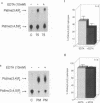
Abstract
Agonist-stimulated production of phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3], is considered the primary output signal of activated phosphoinositide (PI) 3-kinase. The physiological targets of this novel phospholipid and the identity of enzymes involved in its metabolism have not yet been established. We report here the identification of two enzymes which hydrolyze the 5-position phosphate of PtdIns(3,4,5)P3, forming phosphatidylinositol (3,4)-bisphosphate. One of these enzymes is the 75 kDa inositol polyphosphate 5-phosphatase (75 kDa 5-phosphatase), which has previously been demonstrated to metabolize inositol 1,4,5-trisphosphate [Ins(1,4,5)P3], inositol 1,3,4,5-tetrakisphosphate [Ins(1,3,4,5)P4] and phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2]. We have identified a second PtdIns(3,4,5)P3 5-phosphatase in the cytosolic fraction of platelets, which forms a complex with the p85/p110 form of PI 3-kinase. This enzyme is immunologically and chromatographically distinct from the platelet 43 kDa and 75 kDa 5-phosphatases and is unique in that it removes the 5-position phosphate from PtdIns(3,4,5)P3, but does not metabolize PtdIns(4,5)P2, Ins(1,4,5)P3 or Ins(1,3,4,5)P4. These studies demonstrate the existence of multiple PtdIns(3,4,5)P3 5-phosphatases within the cell.
Full text
PDF

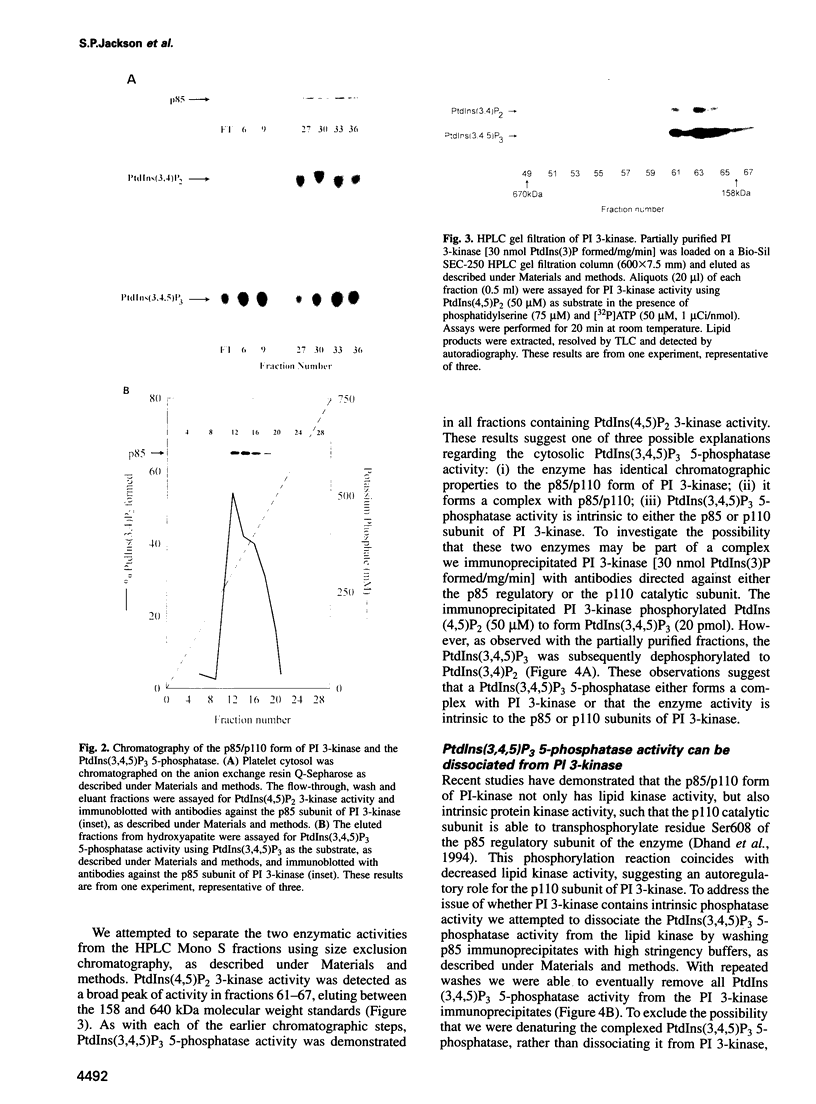



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali N., Craxton A., Shears S. B. Hepatic Ins(1,3,4,5)P4 3-phosphatase is compartmentalized inside endoplasmic reticulum. J Biol Chem. 1993 Mar 25;268(9):6161–6167. [PubMed] [Google Scholar]
- Attree O., Olivos I. M., Okabe I., Bailey L. C., Nelson D. L., Lewis R. A., McInnes R. R., Nussbaum R. L. The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature. 1992 Jul 16;358(6383):239–242. doi: 10.1038/358239a0. [DOI] [PubMed] [Google Scholar]
- Caldwell K. K., Lips D. L., Bansal V. S., Majerus P. W. Isolation and characterization of two 3-phosphatases that hydrolyze both phosphatidylinositol 3-phosphate and inositol 1,3-bisphosphate. J Biol Chem. 1991 Sep 25;266(27):18378–18386. [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Duckworth B. C., Auger K. R., Cohen B., Schaffhausen B. S., Cantley L. C. Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990 Nov 15;265(32):19704–19711. [PubMed] [Google Scholar]
- Carter A. N., Huang R., Sorisky A., Downes C. P., Rittenhouse S. E. Phosphatidylinositol 3,4,5-trisphosphate is formed from phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. Biochem J. 1994 Jul 15;301(Pt 2):415–420. doi: 10.1042/bj3010415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Connolly T. M., Lawing W. J., Jr, Majerus P. W. Protein kinase C phosphorylates human platelet inositol trisphosphate 5'-phosphomonoesterase, increasing the phosphatase activity. Cell. 1986 Sep 12;46(6):951–958. doi: 10.1016/0092-8674(86)90077-2. [DOI] [PubMed] [Google Scholar]
- De Smedt F., Verjans B., Mailleux P., Erneux C. Cloning and expression of human brain type I inositol 1,4,5-trisphosphate 5-phosphatase. High levels of mRNA in cerebellar Purkinje cells. FEBS Lett. 1994 Jun 20;347(1):69–72. doi: 10.1016/0014-5793(94)00509-5. [DOI] [PubMed] [Google Scholar]
- Dhand R., Hiles I., Panayotou G., Roche S., Fry M. J., Gout I., Totty N. F., Truong O., Vicendo P., Yonezawa K. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 1994 Feb 1;13(3):522–533. doi: 10.1002/j.1460-2075.1994.tb06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divecha N., Banfić H., Irvine R. F. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J. 1991 Nov;10(11):3207–3214. doi: 10.1002/j.1460-2075.1991.tb04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Carter A. N. Phosphoinositide 3-kinase: a new effector in signal transduction? Cell Signal. 1991;3(6):501–513. doi: 10.1016/0898-6568(91)90027-r. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erneux C., Lemos M., Verjans B., Vanderhaeghen P., Delvaux A., Dumont J. E. Soluble and particulate Ins(1,4,5)P3/Ins(1,3,4,5)P4 5-phosphatase in bovine brain. Eur J Biochem. 1989 May 1;181(2):317–322. doi: 10.1111/j.1432-1033.1989.tb14726.x. [DOI] [PubMed] [Google Scholar]
- Fry M. J. Structure, regulation and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1994 Jul 18;1226(3):237–268. doi: 10.1016/0925-4439(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Grondin P., Plantavid M., Sultan C., Breton M., Mauco G., Chap H. Interaction of pp60c-src, phospholipase C, inositol-lipid, and diacyglycerol kinases with the cytoskeletons of thrombin-stimulated platelets. J Biol Chem. 1991 Aug 25;266(24):15705–15709. [PubMed] [Google Scholar]
- Hawkins P. T., Jackson T. R., Stephens L. R. Platelet-derived growth factor stimulates synthesis of PtdIns(3,4,5)P3 by activating a PtdIns(4,5)P2 3-OH kinase. Nature. 1992 Jul 9;358(6382):157–159. doi: 10.1038/358157a0. [DOI] [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Hodgkin M., Craxton A., Parry J. B., Hughes P. J., Potter B. V., Michell R. H., Kirk C. J. Bovine testis and human erythrocytes contain different subtypes of membrane-associated Ins(1,4,5)P3/Ins(1,3,4,5)P4 5-phosphomonoesterases. Biochem J. 1994 Feb 1;297(Pt 3):637–645. doi: 10.1042/bj2970637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope H. M., Pike L. J. Purification and characterization of a polyphosphoinositide phosphatase from rat brain. J Biol Chem. 1994 Sep 23;269(38):23648–23654. [PubMed] [Google Scholar]
- Irvine R. Second messengers and Lowe syndrome. Nat Genet. 1992 Aug;1(5):315–316. doi: 10.1038/ng0892-315. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Schoenwaelder S. M., Yuan Y., Rabinowitz I., Salem H. H., Mitchell C. A. Adhesion receptor activation of phosphatidylinositol 3-kinase. von Willebrand factor stimulates the cytoskeletal association and activation of phosphatidylinositol 3-kinase and pp60c-src in human platelets. J Biol Chem. 1994 Oct 28;269(43):27093–27099. [PubMed] [Google Scholar]
- Jefferson A. B., Majerus P. W. Properties of type II inositol polyphosphate 5-phosphatase. J Biol Chem. 1995 Apr 21;270(16):9370–9377. doi: 10.1074/jbc.270.16.9370. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Raptis L., Garcea R. L., Pallas D., Roberts T. M., Cantley L. Phosphatidylinositol metabolism and polyoma-mediated transformation. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3624–3628. doi: 10.1073/pnas.83.11.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan K. M., Chan B. K., Tetaz T., Bird P. I., Mitchell C. A. Characterization of a cDNA encoding the 43-kDa membrane-associated inositol-polyphosphate 5-phosphatase. J Biol Chem. 1994 Jun 24;269(25):17305–17310. [PubMed] [Google Scholar]
- Laxminarayan K. M., Matzaris M., Speed C. J., Mitchell C. A. Purification and characterization of a 43-kDa membrane-associated inositol polyphosphate 5-phosphatase from human placenta. J Biol Chem. 1993 Mar 5;268(7):4968–4974. [PubMed] [Google Scholar]
- Majerus P. W. Inositol phosphate biochemistry. Annu Rev Biochem. 1992;61:225–250. doi: 10.1146/annurev.bi.61.070192.001301. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Ross T. S., Cunningham T. W., Caldwell K. K., Jefferson A. B., Bansal V. S. Recent insights in phosphatidylinositol signaling. Cell. 1990 Nov 2;63(3):459–465. doi: 10.1016/0092-8674(90)90442-h. [DOI] [PubMed] [Google Scholar]
- Matzaris M., Jackson S. P., Laxminarayan K. M., Speed C. J., Mitchell C. A. Identification and characterization of the phosphatidylinositol-(4, 5)-bisphosphate 5-phosphatase in human platelets. J Biol Chem. 1994 Feb 4;269(5):3397–3402. [PubMed] [Google Scholar]
- Mitchell C. A., Connolly T. M., Majerus P. W. Identification and isolation of a 75-kDa inositol polyphosphate-5-phosphatase from human platelets. J Biol Chem. 1989 May 25;264(15):8873–8877. [PubMed] [Google Scholar]
- Norris F. A., Majerus P. W. Hydrolysis of phosphatidylinositol 3,4-bisphosphate by inositol polyphosphate 4-phosphatase isolated by affinity elution chromatography. J Biol Chem. 1994 Mar 25;269(12):8716–8720. [PubMed] [Google Scholar]
- Panayotou G., Waterfield M. D. Phosphatidyl-inositol 3-kinase: a key enzyme in diverse signalling processes. Trends Cell Biol. 1992 Dec;2(12):358–360. doi: 10.1016/0962-8924(92)90042-l. [DOI] [PubMed] [Google Scholar]
- Ross T. S., Jefferson A. B., Mitchell C. A., Majerus P. W. Cloning and expression of human 75-kDa inositol polyphosphate-5-phosphatase. J Biol Chem. 1991 Oct 25;266(30):20283–20289. [PubMed] [Google Scholar]
- Shears S. B. Metabolism of inositol phosphates. Adv Second Messenger Phosphoprotein Res. 1992;26:63–92. [PubMed] [Google Scholar]
- Shibasaki F., Homma Y., Takenawa T. Two types of phosphatidylinositol 3-kinase from bovine thymus. Monomer and heterodimer form. J Biol Chem. 1991 May 5;266(13):8108–8114. [PubMed] [Google Scholar]
- Stephens L. R., Hughes K. T., Irvine R. F. Pathway of phosphatidylinositol(3,4,5)-trisphosphate synthesis in activated neutrophils. Nature. 1991 May 2;351(6321):33–39. doi: 10.1038/351033a0. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Jackson T. R., Hawkins P. T. Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochim Biophys Acta. 1993 Oct 7;1179(1):27–75. doi: 10.1016/0167-4889(93)90072-w. [DOI] [PubMed] [Google Scholar]
- Stephens L., Smrcka A., Cooke F. T., Jackson T. R., Sternweis P. C., Hawkins P. T. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994 Apr 8;77(1):83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Susa M., Keeler M., Varticovski L. Platelet-derived growth factor activates membrane-associated phosphatidylinositol 3-kinase and mediates its translocation from the cytosol. Detection of enzyme activity in detergent-solubilized cell extracts. J Biol Chem. 1992 Nov 15;267(32):22951–22956. [PubMed] [Google Scholar]
- Toker A., Meyer M., Reddy K. K., Falck J. R., Aneja R., Aneja S., Parra A., Burns D. J., Ballas L. M., Cantley L. C. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994 Dec 23;269(51):32358–32367. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verjans B., De Smedt F., Lecocq R., Vanweyenberg V., Moreau C., Erneux C. Cloning and expression in Escherichia coli of a dog thyroid cDNA encoding a novel inositol 1,4,5-trisphosphate 5-phosphatase. Biochem J. 1994 May 15;300(Pt 1):85–90. doi: 10.1042/bj3000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verjans B., Moreau C., Erneux C. The control of intracellular signal molecules at the level of their hydrolysis: the example of inositol 1,4,5-trisphosphate 5-phosphatase. Mol Cell Endocrinol. 1994 Jan;98(2):167–171. doi: 10.1016/0303-7207(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D., Roberts T., Cantley L. Evidence for two distinct phosphatidylinositol kinases in fibroblasts. Implications for cellular regulation. Biochem J. 1987 Oct 1;247(1):165–174. doi: 10.1042/bj2470165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woscholski R., Dhand R., Fry M. J., Waterfield M. D., Parker P. J. Biochemical characterization of the free catalytic p110 alpha and the complexed heterodimeric p110 alpha.p85 alpha forms of the mammalian phosphatidylinositol 3-kinase. J Biol Chem. 1994 Oct 7;269(40):25067–25072. [PubMed] [Google Scholar]
- Zhang J., Fry M. J., Waterfield M. D., Jaken S., Liao L., Fox J. E., Rittenhouse S. E. Activated phosphoinositide 3-kinase associates with membrane skeleton in thrombin-exposed platelets. J Biol Chem. 1992 Mar 5;267(7):4686–4692. [PubMed] [Google Scholar]
- Zhang J., Zhang J., Benovic J. L., Sugai M., Wetzker R., Gout I., Rittenhouse S. E. Sequestration of a G-protein beta gamma subunit or ADP-ribosylation of Rho can inhibit thrombin-induced activation of platelet phosphoinositide 3-kinases. J Biol Chem. 1995 Mar 24;270(12):6589–6594. doi: 10.1074/jbc.270.12.6589. [DOI] [PubMed] [Google Scholar]