Abstract
Rationale
Fifty kilohertz ultrasonic vocalizations (USVs) have been sometimes shown to reflect positive affective-like states in rats. Rewarding events, such as access to palatable food or drugs of abuse, increase the number of anticipatory 50 kHz USVs. However, little is known about the predictability of USVs, subtypes of USVs involved, and underlying neurobiological mechanisms.
Objectives
We examined whether cue-induced anticipatory 50 kHz USVs predict palatable food intake and tested the effects of dopamine D1 and μ-opioid receptor antagonism on anticipatory USVs.
Materials
Food-restricted rats received repeated sessions of a 2 min cue light immediately followed by 5 min access to palatable food. Ultrasonic vocalizations were recorded during cue presentation. After 24 pairing sessions, the rats were pretreated with the D1 receptor antagonist SCH 23390 (5, 10, and 20 μg/kg) and μ-opioid receptor antagonist naltrexone (0.03, 0.06, 0.13, 0.25, 0.5, and 1 mg/kg) in a Latin-square design, and USVs were recorded during cue presentation.
Results
Rats emitted 50 kHz USVs during cue presentation, and the number of USVs increased across sessions with robust and stable interindividual differences. Escalation in USVs was subtype-dependent, with non-trill calls significantly increasing over time. Palatable food intake was positively correlated with anticipatory 50 kHz USVs. Moreover, anticipatory USVs were dose-dependently prevented by antagonism of D1 and μ-opioid receptors.
Conclusions
These findings demonstrate that anticipatory 50 kHz USVs represent a stable phenotype of increased motivation for food, and dopamine and opioid systems appear to mediate anticipatory 50 kHz USVs.
Keywords: ultrasonic vocalization, reward, dopamine, motivation, opiate, anticipation
Introduction
Ultrasonic vocalizations (USVs) are thought to reflect positive and negative affective-like states in rats (Brudzynski 2013; Covington and Miczek 2003; Knutson et al. 2002). Frequency-modulated (FM) 50 kHz USVs, particularly calls with rapid-frequency oscillations (“trills”), have been associated with positive emotional states (Burgdorf et al. 2011). Frequency-modulated calls are elicited by reinforcing, appetitive stimuli, such as play (Burgdorf et al. 2008), social contact (Brudzynski and Pniak 2002), tickling (Burgdorf and Panksepp 2001), and psychostimulant drug reward (Ahrens et al. 2009). However, not all rewarding stimuli elicit FM USVs (Simola et al 2012; Wright et al., 2012), and FM calls have been observed in aversive conditions (Vivian and Miczek 1993, Wöhr et al. 2008). Nevertheless, FM USVs and, more generally, 50 kHz USVs may be an index of motivation during reward anticipation (Ahrens et al. 2009, Ma et al. 2010, Mahler et al., 2013).
Previous reports have also shown that 50 kHz USVs are elicited by cues predictive of rewarding stimuli, such as food (Burgdorf et al. 2000), copulation (Bialy et al. 2000), cocaine (Ma et al. 2010), and rewarding brain stimulation (Burgdorf et al. 2000). However, little is known about the specific call subtypes and the pharmacological mechanisms involved in cue-induced anticipatory USVs.
μ-Opioid receptor antagonism has been suggested to regulate feeding behavior by interfering with the perceived palatability of food, whereas the dopamine system has been suggested to be more involved in the motivation to obtain food (De Tomasi and Juárez, 2010; Barbano et al. 2009). Dopamine D1 receptors, in particular, have been shown to play a role in both anticipatory behavior and food-seeking behavior (Ball et al. 2011; Grimm et al. 2011). We hypothesized that D1 receptor antagonism but not μ-opioid receptor antagonism attenuates anticipatory USVs. Here, we measured anticipatory 50 kHz USVs in food-restricted rats given intermittent (every 24-48 h) and limited (5 min) access to a highly palatable food for 6 weeks. We then tested the effects of the D1 receptor antagonist SCH 23390 and μ-opioid receptor antagonist naltrexone on anticipatory USVs and food intake.
Materials and Methods
Animals
A total of 16 adult male Wistar rats, weighing 270-380 g at the beginning of the experiment, were used. The rats were housed in groups of two to three in plastic cages in a temperature-controlled (21°C) vivarium on a 12 h/12 h light/dark cycle (lights on at 6:00 AM). During behavioral testing, the animals received 15 g chow (7012 Teklad LM-485, Harlan Laboratories) per day, which was provided 10-60 min after testing. With this food restriction procedure, the rats’ body weights were approximately 80% of their free-feeding body weights. Water was provided ad libitum in the home cages. All of the behavioral tests were conducted during the dark cycle between 6:00 PM and 10:00 PM. All of the procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs
Naltrexone and R(+)-SCH 23390 hydrochloride (expressed as salt) were purchased from Sigma (St. Louis, MO, USA). Naltrexone is a preferential nonselective competitive opioid receptor antagonist with higher affinity for μ-opioid receptors compared with κ- and δ-opioid receptors. SCH 23390 is a selective dopamine D1 receptor antagonist. Naltrexone (0.03, 0.06, 0.13, 0.25, 0.5, and 1 mg/kg) and SCH 23390 (5, 10, and 20 μg/kg) were dissolved in saline and injected subcutaneously in a volume of 1 ml/kg using a within-subjects Latin-square design.
Experimental procedures
Cue-food paired
Before testing, the rats (n = 8) received 1 week of daily access to the palatable food (Purina TestDiet 1811443 [5 TUL] AIN-76A Rodent tablet chocolate, 45 mg) to prevent neophobia. To minimize novelty-induced USVs, the rats were habituated to the testing environment for several days before beginning the experiment and were always tested in the same cage, and bedding was not changed between sessions. Four rats were tested at a time in separate 22.5 cm × 45 cm × 20 cm plastic cages with wire lids and bedding. Sound-attenuating barriers were placed between adjacent rats. Twenty-four test sessions were conducted 3-7 days per week over the course of 6 weeks. In each session, the rats were placed in the testing cages in a dark room for a variable interval (15-45 min) to prevent cue prediction. A dim light (20 lux) was then presented for 2 min. Immediately following the light cue, a hopper that contained 20 g of the palatable food pellets was placed in the cage, and the rats were allowed to feed freely for 5 min.
For pharmacological testing sessions, the rats were given saline, SCH 23390 (5-20 μg/kg), or naltrexone (0.03-1 mg/kg) subcutaneously 30 min before recording USVs. The rats were first tested with SCH 23390 (0, 5, 10, and 20 μg/kg) and then tested with naltrexone (0, 0.25, 0.5, and 1 mg/kg). The rats were then allowed chow ad libitum in their home cages for 3 weeks. Because all of the doses of naltrexone decreased the number of USVs, additional lower doses were required to cover the full dose-response. Rats were food restricted for 1 week before the final naltrexone treatments (0, 0.03, 0.06, and 0.13 mg/kg).
Cue only
A separate group of rats (n = 8) was used. All of the procedures were the same as described above, except for the following changes: after the cue light, the rats did not receive palatable food and were returned to their home cages. The rats were tested for 21 sessions, and pharmacological tests were not performed with this group.
Ultrasonic vocalization recording and analysis
Condenser microphones (CM16/CMPA, 10-200 kHz frequency range, Avisoft Bioacoustics, Berlin, Germany) were positioned 5 cm above the wire lids of the testing cages and coupled to an UltraSoundGate 816H data acquisition device (250 kHz sampling rate, 16-bit resolution, Avisoft Bioacoustics). Ultrasonic vocalizations between 10 and 100 kHz were recorded and analyzed using Avisoft SASLab Pro (version 5.1, Avisoft Bioacoustics). Spectrograms were generated with a fast Fourier transform length of 512 points and overlap of 50% (FlatTop window, 100% frame size), providing a frequency resolution of 419 Hz and time resolution of 1.19 ms. An observer blind to experimental condition classified USVs into seven categories adapted from Brudzynski (2013): trill FM, step-trill FM, step FM, other FM, flat, long 22 kHz, and short 22 kHz.
Ultrasonic vocalization classification
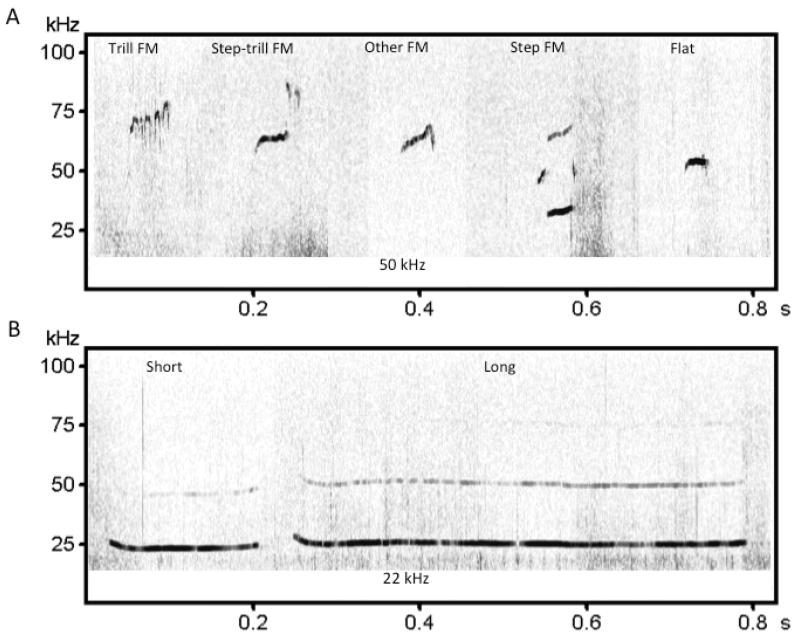
Examples of each USV type are shown in Fig. 1. Calls with a mean peak frequency between 30 and 90 kHz were classified into one of five 50 kHz USV categories: trill FM, step-trill FM, step FM, flat, or other FM (Fig. 1A). Trill FM and step-trill FM calls were defined as calls with a bandwidth of at least 10 kHz, containing at least one cycle of rapid (about 15 ms) frequency modulation in an inverted-U or sinusoidal pattern. Step-trill FM calls contained an additional low bandwidth (≤ 10 kHz) component. Step FM calls contained at least one frequency jump and monotonic components. Flat calls were monotonic calls with a bandwidth no greater than 5 kHz and duration of at least 10 ms. Other FM calls included 50 kHz USVs that did not meet the requirements for the previous four categories. This category included complex, upward ramp, downward ramp, short, multi-step, and inverted-U subtypes, based on Wright et al. (2010). Calls with a mean peak frequency between 18 and 30 kHz and a flat appearance (bandwidth ≤ 10 kHz) were classified into one of two 22 kHz USV categories: long and short (Fig. 1B). Twenty-two kilohertz USVs with a duration greater than 300 ms were considered long calls, whereas 22 kHz USVs with a duration less than 300 ms were considered short calls.
Figure 1.
Classification of 50 and 22 kHz USVs. Representative spectrograms are shown for each call subtype. (A) Frequency-modulated (FM) 50 kHz USVs (trill, step-trill, step, and other) and flat 50 kHz USVs. (B) Long and short 22 kHz USVs.
Statistical analyses
The data are expressed as the mean and standard error of the mean (SEM). Prior to any comparisons, all of the dependent variables were subjected to Shapiro-Wilk’s W-test of normality. Because the Shapiro-Wilk’s W-test of normality was significant for the anticipatory 50 kHz USV counts, the data were square-root-transformed before statistical analysis. Ultrasonic vocalization counts and food intake over 24 sessions were analyzed by one-way repeated-measures analysis of variance (ANOVA), followed by a test for linear trend and the Dunnett post hoc test. Within- and between-subjects coefficients of variation were calculated during the escalation period (sessions 4-24) and compared using Student’s two-tailed t-test. Because data transformation did not successfully normalize USV subtype data, nonparametric Wilcoxon tests were used for statistical comparisons. When appropriate, Pearson’s correlations were used. Statistical analyses were performed with Statistica version 10 (StatSoft, Inc.), except for linear trend tests, which were performed with Prism 5 (GraphPad Software, Inc.). For all of the tests, two-tailed values of p < 0.05 were considered statistically significant.
Results
Escalation of food intake and 50 kHz ultrasonic vocalizations
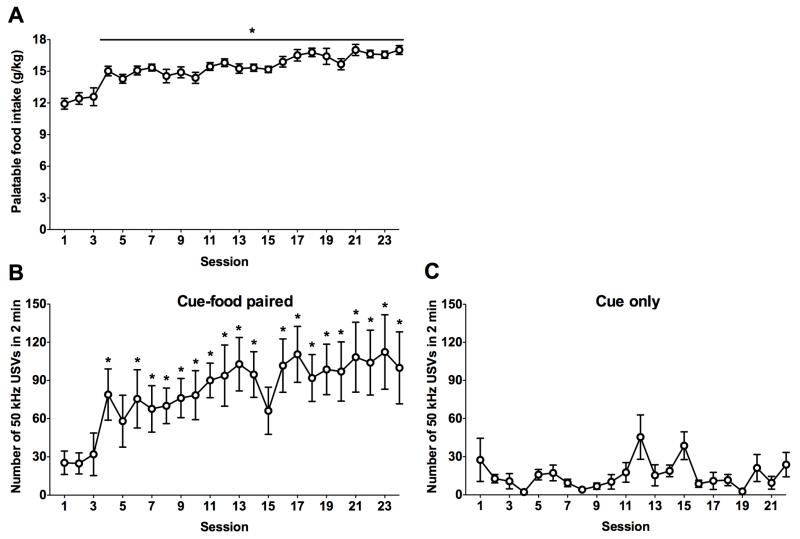
Fig. 2 shows palatable food intake (in g/kg) across 24 sessions (Fig. 2A) and 50 kHz USVs during the anticipation of palatable food per session across 24 sessions (Fig. 2B). A one-way repeated-measures ANOVA showed a significant effect of session on palatable food intake (F23,161 = 13.2, p < 0.001). A linear-trend post hoc test indicated a significant increase in palatable food intake across sessions (slope = 0.085, R2 = 0.38, p < 0.001). Dunnett’s post hoc comparisons indicated significant increases from session 4 onward (p < 0.001) compared with session 1. The ANOVA revealed a significant effect of session on anticipatory USVs (F23,161 = 5.0, p < 0.001). A linear-trend post hoc test indicated a significant increase in USVs across sessions (slope = 0.099, R2 = 0.14, p < 0.001). Dunnett’s post hoc comparisons indicated significant increases in session 4 onward (p < 0.05), with the exception of sessions 5 and 15 compared with session 1. To confirm that the escalation of USVs was not caused by light exposure alone, the rats were exposed to light only (i.e., no food and light pairings). Although a one-way repeated-measures ANOVA showed a significant effect of session on USVs (F21,147 = 2.2, p < 0.01), the linear-trend post hoc test was not statistically significant (slope = 0.009, R2 = 0.002, p > 0.5). Additionally, Dunnett’s post hoc test did not indicate any sessions that were significantly different from session 1 (Fig. 2C).
Figure 2.
(A) Escalation of palatable food intake (g/kg/session) and (B) number of 50 kHz USVs during 2 min food-cue presentation in rats that received cue-food pairings. n = 8. *p < 0.05, different from session 1. (C) Number of 50 kHz USVs during 2 min cue presentation in rats that did not receive cue-food pairings. n = 8.
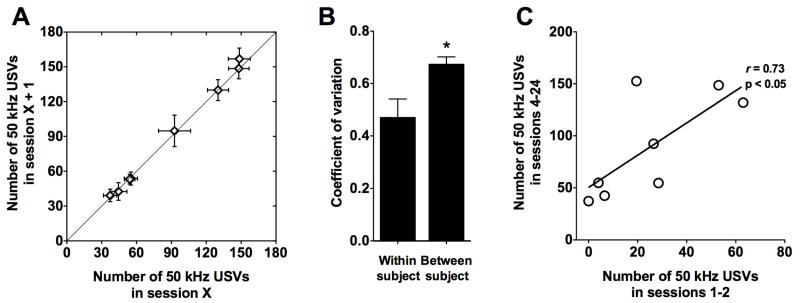
To test whether the number of anticipatory 50 kHz USVs represents a stable phenotype with reliable interindividual differences, we compared the within- and between-subjects coefficients of variation for anticipatory USVs during escalated sessions (sessions 4-24). Fig. 3A shows the number of USVs in each session (session X) vs. the number of USVs in the subsequent session (session X + 1) for each rat. The rats exhibited high interindividual differences, reflected by the higher variability between subjects than within subjects (t27 = −3.2, p < 0.01; Fig. 3B). Moreover, the number of USVs before escalation (sessions 1-2) was significantly correlated (r = 0.73, p < 0.05) with the number of USVs after escalation (sessions 4-24; Fig. 3C).
Figure 3.
(A) Interindividual differences in cue-induced 50 kHz USVs between sessions after the escalation of food intake (sessions 4-24). Each data point represents one subject. The x-axis shows USVs for each session (X), and the y axis shows USVs for each subsequent session (X + 1). (B) Coefficients of variation within and between subjects during sessions 4-24. *p < 0.05, significantly different from within-subject (within-subject, n = 8; between-subject, n = 21). (C) 50 kHz USVs emitted during the first 2 sessions versus 50 kHz USVs emitted after the escalation of food intake (sessions 4-24). Each data point represents one subject.
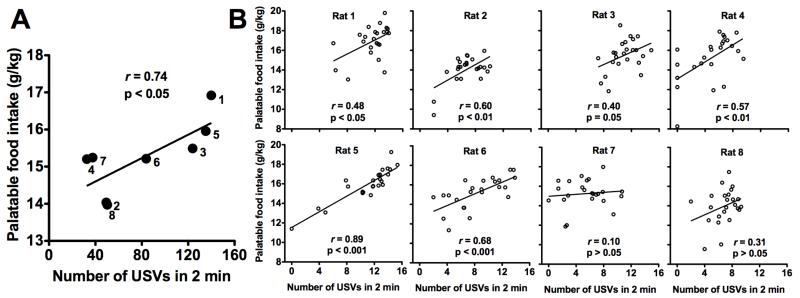
Fig. 4 shows correlations between anticipatory 50 kHz USVs and palatable food intake. Across rats, the averages (sessions 1-24) of 50 kHz USVs were positively correlated with averages (sessions 1-24) of palatable food intake (r = 0.74, p < 0.05, Fig. 4A). For five of the eight rats, within-session 50 kHz USV production was positively correlated with subsequent palatable food intake (rat 1: r = 0.48, p < 0.05; rat 2: r = 0.60, p < 0.01; rat 4: r = 0.57, p < 0.01; rat 5: r = 0.89, p < 0.001; rat 6: r = 0.68, p < 0.001; Fig. 4B). Of the remaining rats, one rat showed a nonsignificant positive trend (rat 3, r = 0.40, p = 0.05), and two rats did not show a relationship (rat 7: r = 0.10, p > 0.05; rat 8: r = 0.31, p > 0.05) between within-session 50 kHz USVs and palatable food intake (Fig. 4B).
Figure 4.
(A) Average number of food cue-induced 50 kHz USVs (2 min) vs. average palatable food intake (g/kg) over all sessions (1-24). Each data point represents a single rat, labeled by subject number. (B) Number of cue-induced 50 kHz USVs (2 min) vs. palatable food intake (g/kg) for each session (n = 24), shown for all rats (1-8).
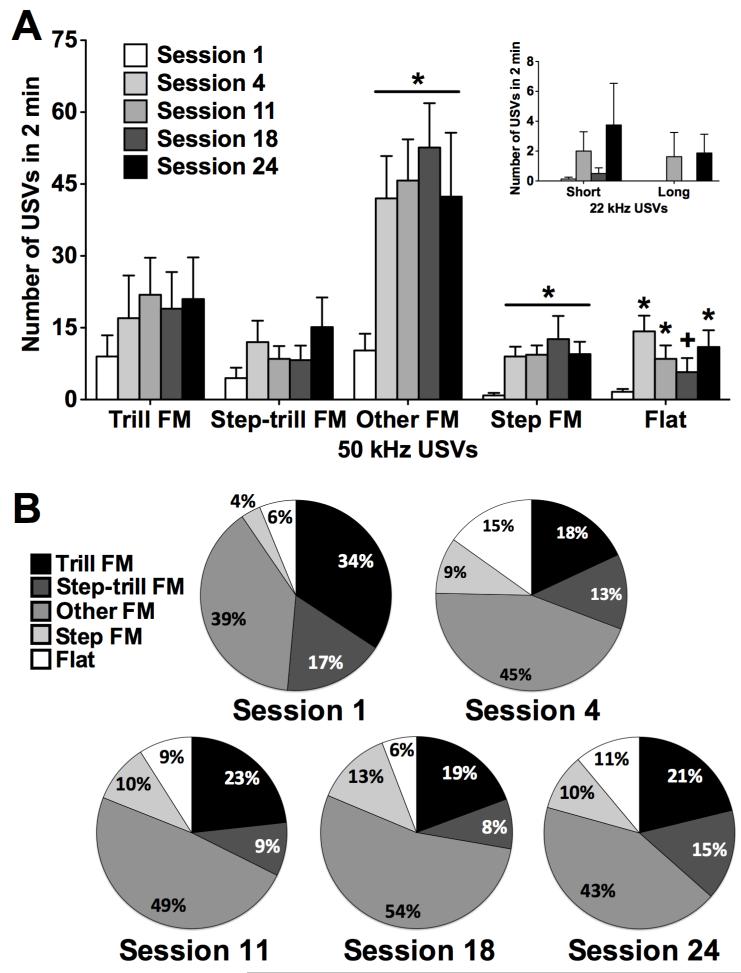
To test whether the escalation of food intake was associated with the escalation of particular subtypes of USVs, anticipatory 50 kHz USVs were classified into five subtypes (trill FM, step-trill FM, step FM, flat, and other FM) for sessions 1, 4, 11, 18, and 24 (Fig. 5). Wilcoxon comparisons indicated that the numbers of both other FM and step FM calls were significantly increased in sessions 4, 11, 18, and 24 compared with session 1 (p < 0.05). Flat calls were significantly increased in sessions 4, 11, and 24 compared with session 1, and flat calls were significantly reduced in session 18 compared with sessions 4 and 24 (p < 0.05). No significant differences were found for trill FM and step-trill FM calls. The 22 kHz USVs were infrequent during the escalation of food intake. Five of the eight animals occasionally emitted short 22 kHz USVs, whereas two rats occasionally emitted long 22 kHz USVs. Wilcoxon comparisons did not show significant differences between sessions 1, 4, 11, 18, and 24 for both short and long 22 kHz USVs, but there was a trend toward an increase in 22 kHz USVs in session 24 (Fig. 5A, inset).
Figure 5.
Subtypes of USVs in sessions 1, 4, 11, 18, and 24. (A) The number of trill FM (frequency modulated), step-trill FM, other FM, step FM, and flat 50 kHz USVs for each of the five sessions. The inset shows the number of short and long 22 kHz USVs emitted during the 5 sessions. *p < 0.05, different from session 1 within each subtype; +p < 0.05, different from sessions 4 and 24 within flat USVs (n = 8). (B) Distribution of percentage of different subtypes of 50 kHz USVs for each of the 5 sessions.
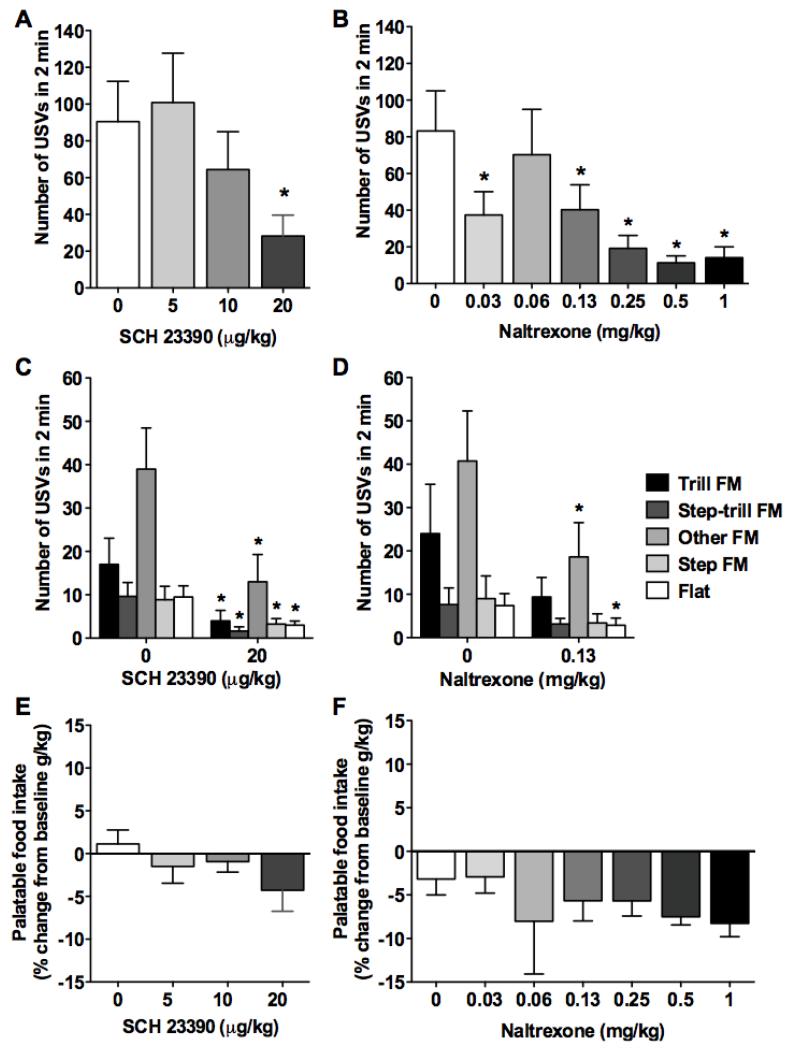
A one-way repeated-measures ANOVA revealed a significant effect of SCH 23390 on the number of 50 kHz USVs (F3,21 = 6.0, p < 0.01, Fig. 6A). Dunnett’s post hoc test indicated that 20 μg/kg SCH 23390 significantly decreased 50 kHz USVs compared with saline (p < 0.01), and this decrease was significant within each 50 kHz subtype (Wilcoxon comparisons, p < 0.05; Fig. 6C). For naltrexone, a repeated-measures ANOVA revealed a significant effect on the number of 50 kHz USVs (F6,42 = 9.1, p < 0.001; Fig. 6B). Dunnett’s post hoc test indicated that naltrexone significantly decreased 50 kHz USVs at all doses tested, with the exception of 0.06 mg/kg, compared with saline (p < 0.05). Considering that these data were obtained using two Latin-square designs, we first ruled out any difference in USVs in the two saline groups. A paired t-test showed no significant difference between USVs for the two saline tests (t7 = 1.0, p > 0.05); therefore, these two values were averaged. We then analyzed the same set of data using separate repeated-measures ANOVAs. The ANOVA of low doses (0.03-0.13 mg/kg) revealed an effect of naltrexone on the number of USVs (F3,21 = 8.1, p < 0.001). Dunnett’s post hoc comparisons indicated that naltrexone significantly decreased 50 kHz USVs at 0.03 and 0.13 mg/kg compared with saline (p < 0.05). Similarly, the ANOVA of high doses (0.25-1 mg/kg) revealed a significant effect of naltrexone on the number of USVs (F3,21 = 13.5, p < 0.001). Dunnett’s post hoc comparisons indicated that naltrexone significantly decreased 50 kHz USVs at all three doses. For 50 kHz USV subtypes, Wilcoxon comparisons between saline and 0.13 mg/kg naltrexone revealed a significant decrease in other FM and flat calls (p < 0.05). Palatable food intake during SCH 23390 and naltrexone testing is presented as the percent change from baseline food intake (i.e., the last session before pharmacological testing began) to take into account any change in bodyweight between tests (Fig. 6E, F). A repeated-measures ANOVA showed no effect of SCH 23390 on the percent change in palatable food intake (F3,21 = 2.1, p > 0.05; Fig. 6E). For naltrexone, a paired t-test showed no significant difference between changes in food intake for the two saline tests (t7 = 0.4, p > 0.05); therefore, these two values were averaged. A repeated-measures ANOVA of the average saline food intake data and food intake data for all six naltrexone doses did not show an effect of naltrexone on palatable food intake (F6,42 = 0.7, p > 0.05). The naltrexone data were also analyzed in separate repeated-measures ANOVAs. No significant effect of naltrexone (0.03-0.13 mg/kg) was found on the percent change in palatable food intake (F3,21 = 0.4, p > 0.05). However, a significant effect of naltrexone (0.25-1 mg/kg) was found on the percent change in food intake (F3,21 = 5.7, p < 0.01). Dunnett’s post hoc tests indicated that naltrexone significantly decreased food intake at 0.5 and 1 mg/kg compared with saline (p < 0.01).
Figure 6.
Effects of D1 and μ-opioid receptor antagonism on cue-induced 50 kHz USVs during the anticipation of palatable food in food-restricted rats. (A) Number of cue-induced 50 kHz USVs (2 min) for each dose (0, 5, 10, and 20 μg/kg, subcutaneous) of the D1 receptor antagonist SCH 23390. (B) Number of cue-induced 50 kHz USVs (2 min) for each dose (0, 0.03, 0.06, 0.25, 0.5, and 1 mg/kg, subcutaneous) of the μ-opioid receptor antagonist naltrexone. (C) Number of trill FM (frequency modulated), step-trill FM, other FM, step FM, and flat 50 kHz USVs for 0 and 20 μg/kg SCH 23390. (D) Number of trill FM, step-trill FM, other FM, step FM, and flat 50 kHz USVs for 0 and 0.13 mg/kg naltrexone. *p < 0.05, significantly different from the respective subtype after vehicle administration. (E) Percent change in palatable food intake compared with baseline palatable food intake (g/kg) for each dose (0, 5, 10, and 20 μg/kg) of the D1 receptor antagonist SCH 23390. (F) Percent change in palatable food intake compared with baseline palatable food intake (g/kg) for each dose (0, 0.03, 0.06, 0.25, 0.5, and 1 mg/kg) of the μ-opioid receptor antagonist naltrexone.
Discussion
The present study found that rats emitted anticipatory 50 kHz USVs during the presentation of a cue predictive of palatable food, with robust and stable interindividual differences. The escalation of palatable food intake was associated with the escalation of anticipatory 50 kHz USVs, particularly flat and non-trill FM USVs. Moreover, dopamine D1 and μ-opioid receptor antagonism dose-dependently reduced anticipatory 50 kHz USVs.
The escalation of cue-induced 50 kHz USVs has been observed during the anticipation of various appetitive stimuli, including social interaction (Brudzynski and Pniak 2002; Willey and Spear 2012), copulation (Bialy et al. 2000), cocaine (Ma et al. 2010), and electrical brain stimulation (Burgdorf et al. 2000). However, these effects can be age-dependent, such that adolescent rats do not show escalation of 50 kHz USVs in anticipation of social interaction (Willey and Spear 2012). The present study showed that adult animals progressively increased the number of 50 kHz USVs emitted during food anticipation over a period of several weeks. Burgdorf et al. (2000) reported similar results, showing escalation of food cue-induced 50 kHz USVs over six sessions. The present study expands these observations by demonstrating that anticipatory 50 kHz USVs have high interindividual differences and low intraindividual variability. Moreover, the initial amount of anticipatory 50 kHz USVs predicted the level of increase in 50 kHz USVs after the escalation of food intake. Furthermore, anticipatory USVs were positively correlated with palatable food intake. Notably, presentation of the cue only (i.e., no food and light pairings) failed to produce escalation of 50 kHz USVs across sessions.
The escalation of food cue-induced 50 kHz USVs was mainly driven by flat, step FM, and other FM calls. All subtypes of 50 kHz USVs tended to increase over time. However, only flat, step FM, and other FM calls reached statistical significance, whereas a nonsignificant trend toward an increase was observed for trill FM and step-trill FM calls. Trill calls have been sometimes linked to appetitive or positive emotional states in rats (Ahrens et al. 2009; Burgdorf et al. 2007, 2008). All of the rats exhibited trill FM and/or step-trill FM calls during the sessions in which USV types were analyzed (sessions 1, 4, 11, 18, and 24). However, we did not observe a significant increase in trill calls over the course of USV escalation. This might have been attributable to a lack of statistical power. Alternatively, the anticipation of food intake may not be linked to positive affect, given that trill calls have been reported not to be exclusively linked to positive affect (Simola et al. 2012; Wright et al. 2012; Vivian and Miczek 1993). Consistent with this hypothesis, five of the eight animals emitted 22 kHz USVs, and a nonsignificant trend toward an increase in the number of 22 kHz USVs was observed across sessions (more in sessions 11, 18, and 24 than in sessions 1 and 4). Twenty-two kHz USVs have been reliably observed in stressful/aversive situations (Covington and Miczek 2003; Litvin et al. 2007; Miczek et al. 1995). Step FM and other FM calls were also increased during food anticipation. However, the acoustic pattern of these calls is heterogeneous, and their biological significance is unclear (Brudzynski 2013; Haney and Miczek 1994; Mahler et al. 2013; Thomas et al. 1983). Altogether, the present findings suggest that cue-induced anticipatory 50 kHz USVs, at least under the present experimental conditions, may be driven by aspects of positive, neutral, or even negative affect.
Dopamine D1 and μ-opioid receptor antagonism dose-dependently reduced anticipatory 50 kHz USVs produced by cue presentation at doses that did not affect food intake. Only the highest doses of naltrexone (0.5 and 1 mg/kg) may have slightly reduced food intake. These results are consistent with previous studies that reported that D1 receptor antagonism decreased both anticipatory behavior and food seeking but not food intake (Barbano and Cador 2006; Barbano et al. 2009; Ball et al. 2011; Grimm et al. 2011), whereas μ-opioid receptor antagonism had mixed results (Barbano and Cador 2006; Barbano et al. 2009). Our results demonstrate that the D1- and μ-opioid systems modulate anticipatory 50 kHz USVs in situations of heightened motivation for palatable food at doses that minimally affect food intake.
Previous studies reported the effects of μ-opioid and dopamine receptors on 50 kHz USVs (Scardochio and Clarke 2013; Wöhr and Schwarting 2009). Naloxone suppressed 50 kHz USVs and reduced approach behavior in response to the playback of 50 kHz USVs (Wöhr and Schwarting 2009). High doses of naloxone reduced 50 kHz USVs and increased 22 kHz USVs (Burgdorf et al. 2001b). Other studies reported a role for opioids in the number or characteristics of 50 kHz USVs (Hamed et al. 2012; Simola et al. 2012; Vivian and Miczek, 1993). Dopamine administration in the nucleus accumbens (Burgdorf et al. 2001a) and indirect dopamine agonism by psychostimulants (Burgdorf et al. 2001a; Brudzynski et al. 2012; Thompson et al. 2006; Williams and Undieh 2010; Wintink and Brudzynski 2001) have been shown to increase 50 kHz USVs. However, both dopamine agonism and antagonism have been found to reduce 50 kHz USVs (Scardocio and Clarke, 2013; Burgdorf et al. 2007; Scardochio and Clarke 2013; Thompson et al. 2006; Williams and Undieh 2010; Wright et al. 2013). Trill calls and FM calls have been shown to be sensitive to dopaminergic modulation, whereas flat calls have been shown to be somewhat resistant to manipulation of this system (Burgdorf et al. 2007; Wright et al. 2013). In contrast, our results showed that D1 receptor antagonism reduced all 50 kHz subtypes, including flat calls. We also found that anticipatory trill FM, step-trill FM, and step FM calls were more resistant to a μ-opioid receptor antagonist than other FM calls and flat calls, suggesting that the opioid systems may be differentially involved in the generation of different subtypes of 50 KHz USVs during food anticipation. However, increasing the power of the study could reveal a significant effect on these subtypes because we observed a nonsignificant trend toward a decrease in trill FM, step-trill FM, and step FM calls after naltrexone.
In summary, rats emitted 50 KHz USVs during the presentation of a cue predictive of palatable food, with robust and stable interindividual differences, and the escalation of palatable food intake was related to the escalation of anticipatory 50 kHz USVs, particularly flat, step FM, and other FM 50 kHz USVs but not trill FM or step-trill FM 50 kHz USVs. Anticipatory 50 kHz USVs were dose-dependently reduced by the administration of dopamine D1 and μ-opioid receptor antagonists, independent of changes in food intake. These results indicate that food cue-induced anticipatory 50 kHz USVs represent a stable phenotype that is mediated by the dopamine and opioid systems.
Acknowledgements
This is publication number 24014 from The Scripps Research Institute. Research was financially supported by National Institutes of Health grants DA004043 and DA004398 from the National Institute on Drug Abuse and AA008459 from the National Institute on Alcohol Abuse and Alcoholism and the Pearson Center for Alcoholism and Addiction Research. The authors would like to thank Michael Arends for proofreading the manuscript.
References
- Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Schallert T. Repeated intravenous amphetamine exposure: rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behav Brain Res. 2009;197:205–209. doi: 10.1016/j.bbr.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KT, Combs TA, Beyer DN. Opposing roles for dopamine D1- and D2-like receptors in discrete cue-induced reinstatement of food seeking. Behav Brain Res. 2011;222:390–393. doi: 10.1016/j.bbr.2011.03.064. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31:1371–1381. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Le Saux M, Cador M. Involvement of dopamine and opioids in the motivation to eat: influence of palatability, homeostatic state, and behavioral paradigms. Psychopharmacology (Berl) 2009;203:475–487. doi: 10.1007/s00213-008-1390-6. [DOI] [PubMed] [Google Scholar]
- De Tomasi EB, Juárez J. Differential effects of chronic naltrexone treatment on food intake patterns and body weight in rats depend on their food deprivation status. Eur J Pharmacol. 2011;650:261–267. doi: 10.1016/j.ejphar.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav Neurosci. 2000;114:983–990. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.01.014. in press. DOI: 10.1016/j.conb.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Komadoski M, St. Pierre J. Quinpirole-induced 50 kHz ultrasonic vocalization in the rat: Role of D2 and D3 dopamine receptors. Behav Brain Res. 2012;226:511–518. doi: 10.1016/j.bbr.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Pniak A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. J Comp Psychol. 2002;116:73–82. doi: 10.1037/0735-7036.116.1.73. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114:320–327. [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci. 2001a;115:940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Shippenberg TS. Evaluation of rat ultrasonic vocalizations as predictors of the conditioned aversive effects of drugs. Psychopharmacology (Berl) 2001b;155:35–42. doi: 10.1007/s002130100685. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122:357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. Tickling induces reward in adolescent rats. Physiol Behav. 2001;72:167–173. doi: 10.1016/s0031-9384(00)00411-x. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Moskal JR. Frequency-modulated 50 kHz ultrasonic vocalizations: a tool for uncovering the molecular substrates of positive affect. Neurosci Biobehav Rev. 2011;35:1831–1836. doi: 10.1016/j.neubiorev.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–283. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Vocalizations during withdrawal from opiates and cocaine: possible expressions of affective distress. Eur J Pharmacol. 2003;467:1–13. doi: 10.1016/s0014-2999(03)01558-9. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Harkness JH, Ratliff C, Barnes J, North K, Collins S. Effects of systemic or nucleus accumbens-directed dopamine D1 receptor antagonism on sucrose seeking in rats. Psychopharmacology (Berl) 2011;216:219–233. doi: 10.1007/s00213-011-2210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed A, Taracha E, Szyndler J, Krza̧ścik P, Lehner M, Maciejak P, Skórzewska A, Płaźnik A. The effects of morphine and morphine conditioned context on 50 kHz ultrasonic vocalisation in rats. Behav Brain Res. 2012;229:447–450. doi: 10.1016/j.bbr.2012.01.053. [DOI] [PubMed] [Google Scholar]
- Haney M, Miczek KA. Ultrasounds emitted by female rats during agonistic interactions: effects of morphine and naltrexone. Psychopharmacology (Berl) 1994;114:441–448. doi: 10.1007/BF02249334. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Litvin Y, Blanchard DC, Blanchard RJ. Rat 22kHz ultrasonic vocalizations as alarm cries. Behav Brain Res. 2007;182:166–172. doi: 10.1016/j.bbr.2006.11.038. [DOI] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;212:109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Feltenstein MW, Cox BM, Ogburn KB, Bachar M, McGonigal JT, Ghee SM, See RE. A rodent “self-report” measure of methamphetamine craving? Rat ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behav Brain Res. 2013;236:78–89. doi: 10.1016/j.bbr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Weerts EM, Vivian JA, Barros HM. Aggression, anxiety and vocalizations in animals: GABAA and 5-HT anxiolytics. Psychopharmacology (Berl) 1995;121:38–56. doi: 10.1007/BF02245590. [DOI] [PubMed] [Google Scholar]
- Scardochio T, Clarke PBS. Inhibition of 50-kHz ultrasonic vocalizations by dopamine receptor subtype-selective agonists and antagonists in adult rats. Psychopharmacology (Berl) 2013;226:589–600. doi: 10.1007/s00213-012-2931-6. [DOI] [PubMed] [Google Scholar]
- Simola N, Fenu S, Costa G, Pinna A, Plumitallo A, Morelli M. Pharmacological characterization of 50-kHz ultrasonic vocalizations in rats: comparison of the effects of different psychoactive drugs and relevance in drug-induced reward. Neuropharmacology. 2012;63:224–234. doi: 10.1016/j.neuropharm.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Takahashi LK, Barfield RJ. Analysis of ultrasonic vocalizations emitted by intruders during aggressive encounters among rats (Rattus norvegicus) J Comp Psychol. 1983;97:201–206. [PubMed] [Google Scholar]
- Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Miczek KA. Morphine attenuates ultrasonic vocalization during agonistic encounters in adult male rats. Psychopharmacology (Berl) 1993;111:367–375. doi: 10.1007/BF02244954. [DOI] [PubMed] [Google Scholar]
- Willey AR, Spear LP. Development of anticipatory 50 kHz USV production to a social stimuli in adolescent and adult male Sprague-Dawley rats. Behav Brain Res. 2012;226:613–618. doi: 10.1016/j.bbr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SN, Undieh AS. Brain-derived neurotrophic factor signaling modulates cocaine induction of reward-associated ultrasonic vocalization in rats. J Pharmacol Exp Ther. 2010;332:463–468. doi: 10.1124/jpet.109.158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintink AJ, Brudzynski SM. The related roles of dopamine and glutamate in the initiation of 50-kHz ultrasonic calls in adult rats. Pharmacol Biochem Behav. 2001;70:317–323. doi: 10.1016/s0091-3057(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav. 2008;93:766–776. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Schwarting RKW. Ultrasonic communication in rats: effects of morphine and naloxone on vocal and behavioral responses to playback of 50-kHz vocalizations. Pharmacol Biochem Behav. 2009;94:285–295. doi: 10.1016/j.pbb.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Wright JM, Deng L, Clarke PB. Failure of rewarding and locomotor stimulant doses of morphine to promote adult rat 50-kHz ultrasonic vocalizations. Psychopharmacology (Berl) 2012;224:477–487. doi: 10.1007/s00213-012-2776-z. [DOI] [PubMed] [Google Scholar]
- Wright JM, Dobosiewicz MRS, Clarke PBS. The role of dopaminergic transmission through D1-like and D2-like receptors in amphetamine-induced rat ultrasonic vocalizations. Psychopharmacology (Berl) 2013;225:853–868. doi: 10.1007/s00213-012-2871-1. [DOI] [PubMed] [Google Scholar]
- Wright JM, Gourdon JC, Clarke PBS. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology (Berl) 2010;211:1–13. doi: 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]