Abstract
Despite high rates of marijuana abuse in schizophrenia, the physiological interactions between tetrahydrocanabinol (THC) and antipsychotic medications are poorly understood. A well-characterized feature of schizophrenia is poor gating of the P50 auditory-evoked potential. This feature has been translationally modeled by the DBA/2 mouse, which exhibits poor suppression of the P20-N40 AEP, the rodent analogue of the human P50. Previous work has demonstrated that this deficit is reversed by the antipsychotic clozapine. It is unknown, however, if this effect is altered by THC administration. Using a conditioning-testing paradigm with paired auditory stimuli, the effects of clozapine and dronabinol (a pharmaceutical THC formulation) on inhibitory P20-N40 AEP processing were assessed from in vivo hippocampal CA3 recordings in anesthetized DBA/2 mice. The effects of clozapine (0.33 mg/kg) and dronabinol (10 mg/kg) were assessed alone and in combination (0.33, 1, 1.83, or 3.33 mg/kg clozapine with 10 mg/kg dronabinol). Improved P20-N40 AEP gating was observed after acute administration of 0.33 mg/kg clozapine. Co-injection of 0.33 mg/kg clozapine and 10 mg/kg THC, however, did not improve gating relative to baseline. This effect was overcome by higher doses of clozapine (1 and 1.83 mg/kg), as these doses improved gating relative to baseline in the presence of 10 mg/kg THC. 10 mg/kg THC alone did not affect gating. In conclusion, THC does not prevent improvement of P20-N40 gating by clozapine.
Keywords: Auditory, Cannabinoid, Clozapine, DBA/2, Dronabinol, Gating, Marinol, P20-N40, P50, Schizophrenia
1. Introduction
A daunting challenge facing clinicians who attempt to treat patients with schizophrenia is the large number of comorbidities that present alongside the illness, including increased rates of diabetes, heart disease, obesity, and substance abuse (Buckley et al. 2009; Mitchell et al. 2013; Volkow 2009). Substance abuse in particular is one of the most prevalent comorbid conditions, with nearly half of patients presenting with a lifetime history of substance abuse disorders (Volkow 2009). One of the most commonly abused drugs in schizophrenia is cannabis, with 50% or more of patients dependent on the substance according to DSM-IV criteria, and up to 80% of patients reporting current or recent use, depending on the study (Volkow 2009). The relationship between cannabis use and schizophrenia is complex and not well-understood. A meta-analysis by Moore et al. (2007) demonstrated higher risk for developing schizophrenia in subjects who used marijuana in the past. Other studies have shown that marijuana use may exacerbate existing symptoms (e.g. Bersani et al. (2002). In contrast, other groups have argued that cannabis use is not causally related to the illness (Costain 2008; Hambrecht and Hafner 2000).
An added concern regarding cannabis abuse in schizophrenia is how tetrahydrocannabinol (THC), the principal psychoactive constituent of marijuana, may neuropharmacologically interact with antipsychotics and alter their therapeutic effectiveness. Cannabis can worsen positive symptoms in medicated patients (Foti et al. 2010; Zammit et al. 2008), suggesting it may dampen antipsychotic effectiveness. However, THC may improve negative symptoms, reduce stress and anxiety, improve social outcomes, and even improve cognition in patients (Potvin et al. 2006; Rabin et al. 2011; Salyers and Mueser 2001; Yucel et al. 2012). The ineffectiveness of antipsychotic medication at treating negative and cognitive symptoms has led some researchers to speculate that patients use marijuana as a form of “self-medication” (Khantzian 1997; Schneier and Siris 1987).
Although the symptomatic correlates of cannabis administration are clinically and therapeutically informative, the underlying heterogeneity of schizophrenia makes it problematic to neuropharmacologically interpret the effects of THC in the illness by measuring symptoms alone. To better understand how THC affects brain function, researchers have used techniques such as scalp electroencephalography (EEG) to measure neurophysiological responses to stimuli. Starting with the observations of Bleuler (1911) as well as McGhie and Chapman (1961), researchers have frequently observed that schizophrenia patients are hyper-responsive to sensory stimulation, most commonly in the auditory domain. Patients are particularly impaired in the ability to “tune out” repetitive auditory stimuli such as a fan blowing or a clock ticking. Physiologically, this phenotype may be associated with reduced “P50” gating in the illness. P50-gating is an electrophysiological phenomenon in which individuals reduce their early (~50 ms post-stimulus) neuronal response to the second of a pair of repeated identical auditory clicks. This suppression is typically quantified by the “P50 ratio”, or ratio of the magnitude of the second response (S2) over the first response (S1), i.e. S2/S1. Healthy subjects, on average, present with P50 ratios less than 0.50, whereas patients with schizophrenia often present with ratios of 0.75 or greater (Adler et al. 1982). This abnormality may be correlated with impaired selective attention (Smucny et al. 2013).
One advantage to studying P50 gating is its translational applicability. By recording directly from the hippocampal CA3 subfields of various mouse strains, gene knockouts, and pharmacologic models, researchers have been able to better characterize the molecular underpinnings that are involved in auditory P50 gating in a P50 analogue called the P20-N40 auditory evoked potential. The DBA/2 mouse, a strain that displays multiple putative symptom analogues of schizophrenia (e.g. cognitive dysfunction) and shows reduced α7 nicotinic receptor expression, shows particularly poor hippocampal P20-N40 gating relative to most other strains (Stevens and Wear 1997). Interestingly, this deficit can be reversed by the atypical antipsychotic clozapine (Simosky et al. 2003). This clozapine effect is blocked by nicotinic receptor (NAChR) antagonists, suggesting that clozapine may improve gating through a cholinergic mechanism (Simosky et al. 2003). Specifically, it is hypothesized that activation of α7 NAChRs facilitates the release of the inhibitory neurotransmitter gamma-aminobutyric acid (Callstrom et al.), enhancing suppression of neuronal response (Miwa et al. 2011; Simosky et al. 2003).
Despite the widespread use of cannabis in schizophrenia, its effects on auditory gating, particularly when co-administered with antipsychotics, are not well understood. The goal of the present study was to better understand the interaction between acute THC and clozapine administration in mice that show poor gating (analogous to schizophrenia patients) at baseline. To that end, this study examined the effects of dronabinol (Marinol), a pharmaceutical formulation of Δ9 tetrahydrocannabinol (THC), on clozapine-induced improvement of P20-N40 gating in DBA/2 mice. We hypothesized that THC would impair the ability of clozapine to improve gating, based on its demonstrated ability to reduce GABA release (Katona et al. 1999) and attenuate pharmacologic modulation of inhibitory neurotransmission.
2. Materials and methods
2.1 Mice
Male DBA/2 mice were purchased from Harlan (Indianapolis, IN) and group housed in shoe-box cages on Aspen chip bedding until recording. Mice were 7–10 weeks old at the time of recording. Food (Purina Rodent Chow) and water was available ad libitum during housing. Animals were maintained under a 12-hour light/dark cycle (lights off at 6 pm). “Principles of Laboratory Animal Care” (NIH Publication No. 85-23, revised 1985) were followed. The Institutional Animal Care and Use Committee of the Denver Veterans Affairs Medical Center and/or the University of Colorado Anschutz Medical Campus approved the experimental protocols.
2.2 In vivo hippocampal P20-N40 recordings
Adult DBA/2 mice (20–25 g; n = 46) were anesthetized with 400 mg/kg chloral hydrate i.p. and 400 mg/kg pyrazole i.p. Mice were then placed in a stereotaxic apparatus, and hollow ear bars with attached earphones were positioned adjacent to the mouse’s ears. Body temperature was maintained at 37 °C with a heating pad. A teflon-coated stainless-steel recording electrode (0.127 mm in diameter) was inserted into the pyramidal layer of hippocampal area CA3 at 1.8 mm posterior to bregma, 2.7 mm lateral to the midline and 1.5–1.7 mm below the surface of the dura (Paxinos and Franklin 2001). Final location was identified by the presence of complex action potentials typical of hippocampal pyramidal neurons (Miller and Freedman 1995). A similar reference electrode was placed on the dura, contralateral to the recording electrode, just anterior to bregma. Tones (3000 Hz, 10 ms, 70 dB SPL) were presented in pairs separated by 500 ms with 10 s between tone pairs. A baseline period of 50 ms preceded each tone pairing. Recordings were segmented from −50 to 350 ms after stimulus onset. Eighteen sets (5 m duration/set) of averaged responses to 16 tone-pairs were recorded per animal. Of these eighteen sets, 6 sets were taken before drug administration as a baseline, and the remaining 12 were taken after drug administration. Thus, responses were measured for up to one hour post injection.
Each averaged response was amplified 1000 times, bandpass filtered between 1–500 Hz, and sent to a computer software program (SciWorks, DataWave, Loveland, CO) for data analysis and storage. The maximum negativity between 20 and 60 ms after each auditory stimulus was selected (i.e. the P20-N40) and measured relative to the preceding positivity. This complex has been shown to have less variability than either component alone (P20 or N40) (Hashimoto et al. 2005).
The number of mice per group were as follows: for 0.33 mg/kg clozapine (only) n = 9, for 0.33 mg/kg clozapine + 10 mg/kg dronabinol n = 9, for 1 mg/kg clozapine + 10 mg/kg dronabinol n = 9, for 1.83 mg/kg clozapine + 10 mg/kg dronabinol n = 9, or 3.33 mg/kg clozapine + 10 mg/kg dronabinol n = 2, and for 10 mg/kg dronabinol (only) n = 8.
2.3 Drugs
Clozapine (0.33, 1, 1.83, or 3.33 mg/kg) was dissolved in saline (pH ~5.5) (80 μl for every 20 g of body weight) and injected i.p. Dronabinol (10 mg/kg) was dissolved in sesame oil (80 μl for every 20 g of body weight) and injected i.p. One drug was injected immediately after the other.
2.4 Statistical Analysis
For these studies, the most relevant components of the P20-N40 AEP response of the DBA/2 mice were the amplitude of the response to the first auditory stimulus (the S1 amplitude) and the amplitude of the response to the second auditory stimulus (the S2 amplitude). The magnitude of inhibitory processing of the S2 response was determined by calculating the S2/S1 ratio, or the S2 amplitude divided by the S1 amplitude. Effective gating is characterized by S2/S1 ratios significantly lower than 1. The S2/S1 ratio is the predominant measure used to assess the efficacy of a drug in normalizing deficit P20-N40 AEP inhibitory processing.
For these experiments, 6 sets of baseline recordings (16 trials/set, with each set 5 minutes apart) were followed by up to 12 identical sets of post-drug administration recordings. The effects of drug(s) administration on the P20-N40 AEP (S1 amplitude, S2 amplitude, S2/S1) were analyzed using repeated measures analyses of variance (ANOVA), with time as a within-subjects factor and dose/drug combination as a between-subjects factor. A priori hypotheses comparing the effects of doses/drugs were tested using the main effect of dose/drug as well as the dose/drug * time interaction. In addition, a priori contrast analyses using Fisher’s LSD post-hoc tests were conducted for each dose to compare the mean of the “baseline” (pre-drug) AEP measures and the mean of the “treatment” (post-drug) AEP measures. Using the a priori contrast analysis maximized the statistical power for detecting expected baseline versus treatment differences (statistical power was preserved because this test was run prior to making multiple comparisons with the other statistical tests used). For each ANOVA, post-hoc tests for each dependent variable were only conducted if the ANOVA demonstrated a main effect of time for that variable.
Baseline (first six time points) S1 vs S2 amplitudes were analyzed using a separate repeated measures ANOVA to examine the main effect of stimulus (S1 or S2).
3. Results
3.1 DBA/2 baseline recordings
Consistent with previous studies, DBA/2 mice failed to suppress S2 amplitudes during baseline (drug-free) recordings as evidenced by no significant main effect of stimulus (S1 or S2) on response amplitude for any experimental group (Supplementary Table 1) and mean S2/S1 ratios of approximately 1 (Figs. 2, 3, and 4, first 6 time points). “Normal” inhibitory processes, in contrast, typically produce S2/S1 ratios of less than 0.5, as evidenced by other strains of mice (e.g. C3H) (Adams et al. 2008).
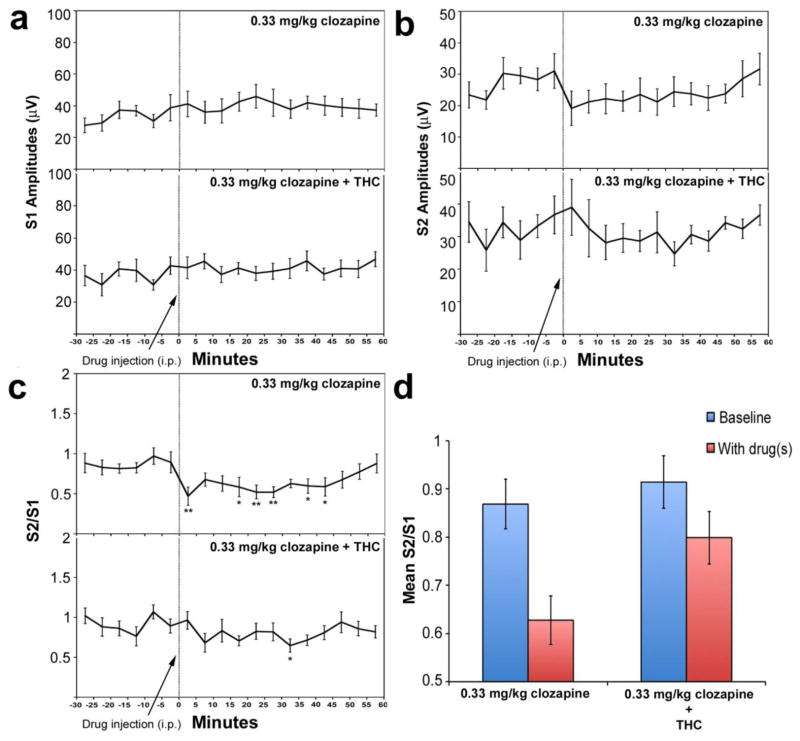
Fig. 2.
Effects of clozapine alone (0.33 mg/kg, i.p.) and the combination of clozapine (0.33 mg/kg, i.p.) and dronabinol (10 mg/kg, i.p.) on S1 response amplitudes (a), S2 response amplitudes (b), and S2/S1 ratios (c, d) in DBA/2 mice. The first six points (−30, −25, etc) refer to the baseline period of recording, prior to administration of drug(s). The last twelve points (0, 5, 10 etc.) refer to the post-drug administration period of recording. Asterisks mark those post-drug time points at which the S1 or S2 amplitude is significantly different from the average of the baseline S1 or S2 amplitudes, as determined using Fisher’s LSD (*P<0.05, **P<0.01). Data are mean +/− SEM, n = 9 per group.
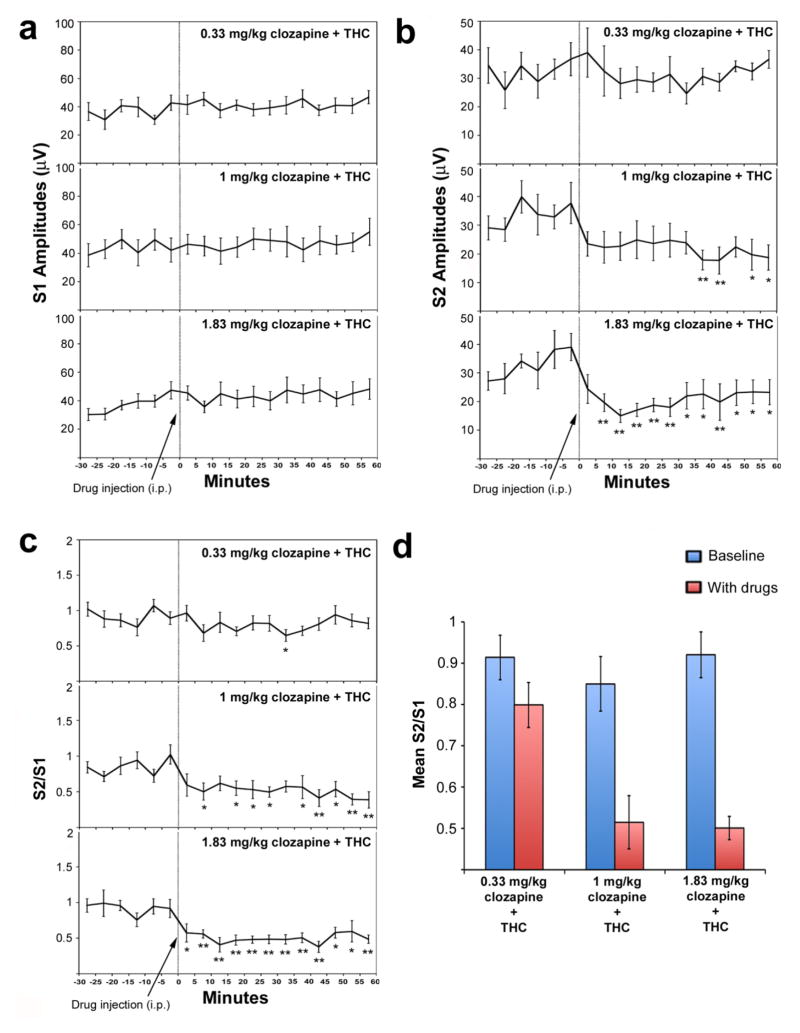
Fig. 3.
Effect of increasing doses of clozapine (0.33, 1, and 1.83 mg/kg, i.p.) on S1 response amplitudes (a), S2 response amplitudes (b), and S2/S1 ratios (c, d) in the presence of dronabinol (10 mg/kg, i.p.) in DBA/2 mice. The first six points (−30, −25, etc) refer to the baseline period of recording, prior to administration of drug(s). The last twelve points (0, 5, 10 etc.) refer to the post-drug administration period of recording. Asterisks mark those post-drug time points at which the S1 or S2 amplitude is significantly different from the average of the baseline S1 or S2 amplitudes, as determined using Fisher’s LSD (*P<0.05, **P<0.01). Data are mean +/− SEM, n = 9 per group.
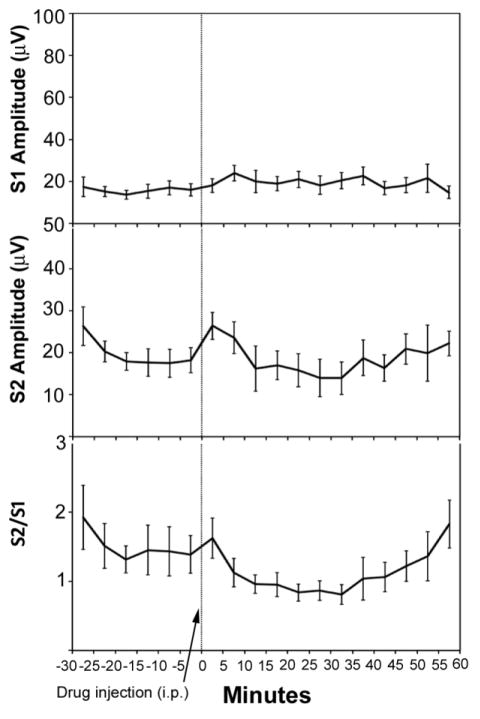
Fig. 4.
Effect of dronabinol on S1 response amplitudes (top), S2 response amplitudes (middle), and S2/S1 ratios (bottom). The first six points (−30, −25, etc) refer to the baseline period of recording, prior to administration of 10 mg/kg dronabinol i.p. The last twelve points (0, 5, 10 etc.) refer to the post-drug administration period of recording. No significant main effects of time were observed on S1 response, S2 response, or S2/S1 ratio. Data are mean +/− SEM, n = 8.
3.2 Effect of clozapine
A previous study by our group found that 0.1, 1, 3.33, and 10 mg/kg of clozapine improved gating in DBA/2 mice (Simosky et al. 2003). In the present study, we decided to first examine the effect of 0.33 mg/kg clozapine on gating in these mice, as this dose was just above the minimally efficacious dose. Representative AEP S1 and S2 response curves after clozapine administration are presented in Fig. 1, and results presented in Fig. 2. Using this dose, no significant effect of time on S1 amplitude (F(17,136) = 1.50, p = 0.10) or S2 amplitude (F(17,136) = 1.65, p = 0.060) was observed (Fig. 2a–b). However, a significant main effect of time was observed on S2/S1 ratio (F(17,136) = 2.79, p = 0.001) (Fig. 2c). Post-hoc tests showed that at several time points, drug administration demonstrated significantly reduced S2/S1 ratios relative to the average baseline S2/S1 ratio (Fig. 2c).
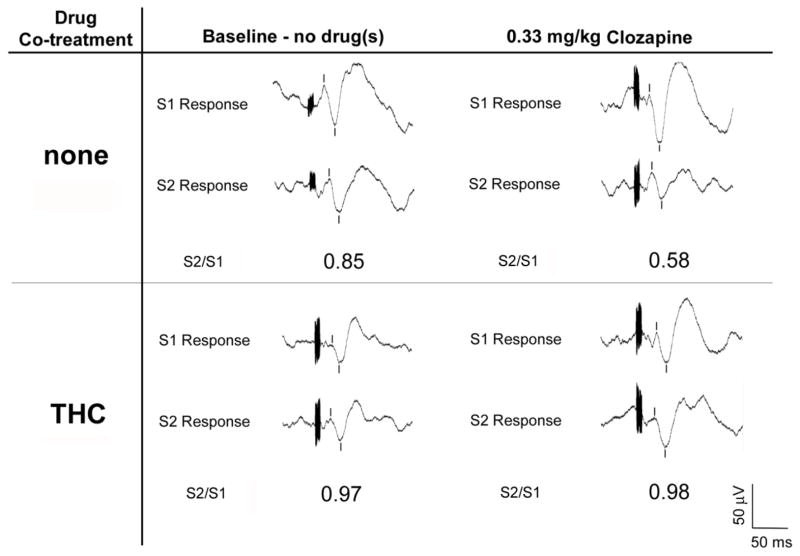
Fig. 1.
Representative waveforms demonstrating differential effects of clozapine alone (0.33 mg/kg, i.p.) vs. the combination of clozapine (0.33 mg/kg, i.p.) and dronabinol (10 mg/kg, i.p.) on P20-N40 gating in DBA/2 mice. Waveforms on the left side represent pre-drug (i.e. baseline) responses to the first (S1) and second (S2) stimuli. Waveforms on the right side represent post-drug responses to the first and second stimuli. Burst response artifacts represent the auditory stimuli, and tic marks denote the P20-N40 auditory evoked potentials. In these representative examples, injection of clozapine alone reduced S2/S1 ratio relative to baseline; however, co-injection of clozapine and dronabinol did not affect S2/S1 ratio relative to baseline.
3.3 Effect of THC and clozapine co-administration
Next, to test the hypothesis that co-administration of THC would reduce the ability to clozapine to improve gating in DBA/2 mice, we administered 10 mg/kg of dronabinol (i.p) in conjunction with 0.33 mg/kg of clozapine (i.p.) and examined P20-N40 AEP gating. The 10 mg/kg dose of THC has previously been shown to decrease prepulse inhibition in the ddY species of mice, an effect that is reversible by antipsychotics (Nagai et al. 2006). Under these pharmacologic conditions, no significant main effect of time was observed for S1 amplitude (F(17,119) = 1.49, p =0.11) or S2 amplitude (F(17,119) = 0.90, p = 0.58). A main effect of time was observed for S2/S1 ratio (F(17,119) = 1.95, p = 0.020). Post-hoc tests revealed that only one time point showed a significant (p < 0.05) decrease in S2/S1 ratio relative to the average baseline S2/S1 ratio (Fig. 2c), in contrast to effect of 0.33 mg/kg clozapine alone.
Comparing effects between drug treatment groups (clozapine alone vs clozapine + THC), no significant time * group interaction (F(17,255) = 0.70, p = 0.81) or main effect of group (F(1,15) = 0.044, p = 0.84) was observed for S1 amplitude. Similarly, no significant time * group interaction (F(17,255) = 0.85, p = 0.63) or main effect of group (F(1,15) = 2.32, p = 0.15) was observed for S2 amplitude. For S2/S1 ratio, no significant time * group interaction was observed (F(17,255) = 1.46, p = 0.11). A significant main effect of group, however, was observed (F(1,15) = 6.20, p = 0.021). The effect was driven by reduced post-drug S2/S1 ratios in mice that received clozapine alone relative to the mice that received clozapine in combination with THC (Fig. 2d).
3.4 Clozapine dose-response curve in the presence of THC
We next examined the effect of co-administration of higher doses of clozapine (1, 1.83, and 3.33 mg/kg, i.p.) and THC on P20-N40 AEP gating. These doses of clozapine are more clinically relevant as they are closer to the doses typically given to schizophrenia patients (see Discussion). For the group of mice that received 1 mg/kg clozapine and THC, no main effect of time was observed on S1 amplitude (F(17,119) = 0.45, p = 0.97). Significant main effects of time, however, were observed on S2 amplitude (F(17,119) = 2.10, p = 0.011) and S2/S1 ratio (F(17,119) = 2.55, p = 0.002). Unlike the 0.33 mg/kg clozapine dose, post-hoc tests showed that at several time points, 1 mg/kg clozapine and THC co-administration significantly reduced S2 amplitudes and S2/S1 ratios relative to average baseline S2 amplitude and S2/S1 ratio, respectively (Fig. 3b–c). For the group of mice that received 1.83 mg/kg clozapine and THC, no main effect of time was observed on S1 amplitude (F(17,119) = 1.51, p = 0.10). Significant main effects of time, however, were observed on S2 amplitude (F(17,119) = 4.28, p < 0.001) and S2/S1 ratio (F(17,119) = 4.49, p < 0.001). Post-hoc tests showed that at all time points, drug administration demonstrated significantly reduced S2 amplitudes and S2/S1 ratios relative to average baseline S2 amplitude and S2/S1 ratio, respectively (Fig. 3b–c).
Comparing effects between groups (0.33, 1, and 1.83 mg/kg clozapine, all co-administered with THC), no significant time x group interaction (F(34,357) = 0.66, p = 0.93) or main effect of drug (F(2,21) = 0.59, p = 0.57) was observed for S1 amplitude. Similarly, no significant time x group interaction (F(34,357) = 1.19, p = 0.22) or main effect of drug (F(2,21) = 1.22, p = 0.31) was observed for S2 amplitude. In contrast, a nearly significant time x group interaction (F(34,357) = 1.43, p = 0.06) and significant main effect of drug (F(2,21) = 9.13, p = 0.001) was observed for S2/S1 ratio. The effect was driven by reduced S2/S1 ratios, relative to baseline, for the 1 and 1.83 mg/kg clozapine doses relative to the 0.33 dose (Fig. 3d).
The combination of 3.33 mg/kg clozapine and 10 mg/kg dronabinol induced fatal seizures in 2 animals. As a result, this dose pairing was not used in additional animals, nor did we examine the effects of pairing higher doses of clozapine with THC.
3.5 Effect of THC
Finally, we examined the effect of the 10 mg/kg dose of THC alone on P20-N40 AEP gating in DBA/2 mice. 10 mg/kg dronabinol (i.p.) produced no significant main effect of time on S1 amplitude (F(17,119) = 1.24, p = 0.24), S2 amplitude (F(17,119) = 1.17, p = 0.30) or S2/S1 ratio (F(17,119) = 1.64, p = 0.065) (Fig. 4), suggesting that this dose of drug did not significantly affect any P20-N40 AEP gating-related measure in DBA/2 mice.
4. Discussion
To our knowledge, this is the first study to examine the modulatory effects of THC alone or on combination with an antipsychotic on sensory gating. Here, we assessed the effects clozapine alone and in combination with a known active dose of THC (Nagai et al. 2006). In agreement with our hypothesis, co-injection of the compound impaired the ability of clozapine to improve gating at a low dose of the antipsychotic. Higher doses of clozapine, however, produced robust improvement of gating in the presence of THC, suggesting that this effect may be overcome with increasing concentrations of the antipsychotic.
To place these findings in a (speculative) conceptual framework, it may be helpful to consider a recent hypothesis regarding the neurobiological mechanism of auditory gating. As described by Miwa et al. (2011), in a paired-stimulus paradigm, the first stimulus activates excitatory pyramidal cells and inhibitory interneurons in the hippocampus. After a delay, interneuronal activity in turn facilitates an inhibitory state in the excitatory cells. As a result, when the second stimulus is presented, these excitatory cells are less able to respond, resulting in a net decrease in response to the second stimulus relative to the first. Pharmacologically, release of GABA can be enhanced by activation of α7 nicotinic acetylcholine receptors expressed on hippocampal interneurons (Buhler and Dunwiddie 2001; Frazier et al. 1998), and reduced by activation of presynaptic cannabinoid 1 receptors (Katona et al. 1999). Thus, activation of nicotinic and cannabinoid receptors may have opposite effects on inhibitory neurotransmission in hippocampal gating-related circuitry.
The described neurochemical circuitry can help explain the interaction between clozapine and THC in the present study. Clozapine increases acetylcholine release fivefold in the rat hippocampus as measured by in vivo microdialysis (Shirazi-Southall et al. 2002) possibly through blockade of 5-HT3 receptors relieving serotonergic inhibition of cholinergic neurons (Adler et al. 1998; Nagamoto et al. 1996). Furthermore, clozapine’s effects on gating are blocked by the α7 receptor antagonist α-bungarotoxin, suggesting that the antipsychotic exerts its gating effects via a cholinergic mechanism (Simosky et al. 2003). A parsimonious explanation of the present findings, therefore, is that clozapine is facilitating release of GABA in the hippocampus through indirect activation of α7 nicotinic receptors on interneurons, and that this effect is opposed by THC through presynaptic inhibition of GABA release on the same or neighboring population(s) of interneurons. The finding that all significant effects on S2/S1 ratios were driven by suppression of the S2 response (as opposed to strengthening of the S1 response) further suggests that pharmacologic gating modulation occurs due to changes in response inhibition to the second stimulus in this study. Nonetheless, we cannot rule out that THC and clozapine may exert opposing effects through contrasting modulation of dopaminergic transmission. Cannabinoids have been shown to increase dopamine release (Cheer et al. 2004), whereas antipsychotic medications (including clozapine) are often potent dopamine receptor antagonists. The pharmacologic interactions between clozapine and THC are likely complex and worthy of further investigation.
Although this study may have clinical implications in that it suggests that clozapine may improve gating in the presence of THC, the dose-specificity of the effect is unclear. On the one hand, comparison of approximate plasma levels at the range of drug doses used in this study suggests that THC may not affect gating at clinically relevant doses of clozapine. The standard patient dose regimen for clozapine is to start treatment at 12.5 mg/day and then to gradually increase the dose in 25–50 mg increments until a steady-state dose of 300–450 mg/day is reached (www.drugs.com/dosage/clozapine.html). This dose results in maximal drug concentrations of 350–420 ng/ml (Savoy et al. 2010). Mice, however, require clozapine doses of well over 3 mg/kg to reach this plasma concentration (Savoy et al. 2010). THC is self-administered in highly variable amounts between individuals, as people often self-titer their own doses, making it difficult to compare the 10 mg/kg dose given to mice in the present study to a “typically” self-administered dose. It is likely, however, that the dose received by the mice in the present study exceeds the average self-administered acute human dose; a recent study found that 3 mg/kg injection of THC resulted in peak plasma levels of 325 ng/ml in mice (Varvel et al. 2005), whereas a high potency marijuana cigarette (19 mg) resulted in a mean plasma concentration of 77 ng/ml in human subjects (Ohlsson et al. 1980). On the other hand, the fact that patients demonstrate highly variable responsiveness to clozapine (with treatment-resistant patients prescribed up to 3-fold higher doses than average) (Meltzer et al. 2008) and sensitivity to cannabis (Goldberger et al. 2010) suggests that any conclusions regarding dose-dependent effects in humans must be considered preliminary. Moreover, the finding that THC in combination with the highest clozapine dose (3.33 mg/kg) induced seizures in DBA/2 mice suggests that the precise physiological effects of co-administration of these drugs should be monitored in patients. Indeed, several studies have demonstrated an association between clozapine and increased seizure risk and epileptiform discharges in schizophrenia across a wide range of doses (Devinsky et al. 1991; Freudenreich et al. 1997; Nielsen et al. 2013), although no seizures were reported in a previous study of DBA/2 mice that administered up to 10 mg/kg of the drug (Simosky et al. 2003).
In the present study, 10 mg/kg dronabinol alone did not impair or improve P20-N40 gating in DBA/2 mice. This result was expected given that DBA/2 mice are already impaired in P20/N40 gating, and that THC does not improve gating in schizophrenia (Rentzsch et al. 2007) or rats that show poor gating at baseline (Dissanayake et al. 2008). Nonetheless, this dose of THC is likely higher than that typically self-administered in humans. The effects of other doses of THC on gating in these animals (as well as their interactions with clozapine and other antipsychotics) should be investigated in future studies.
Although an important step towards understanding the physiological effects of antipsychotic and THC co-administration, this study should be assessed in the context of its limitations. We cannot rule out the possibility that the anesthetic used (chloral hydrate) may influence the observed results. THC has been shown to reduce the sedative effects of chloral hydrate (Sofia and Knobloch 1973). Clozapine, furthermore, may interact with choral hydrate to reduce activity in raphe neurons to a greater extent than either drug alone (Trulson and Trulson 1983). Given that the raphe nucleus mediates serotonin release (and thereby may influence cholinergic transmission), it is possible that chloral hydrate may enhance the pro-gating effects of clozapine. The present findings must therefore be interpreted with caution, particularly in regards to the human equivalents of the dose-dependent effects. Secondly, this study focused on acute co-administration of clozapine and THC; the interactions between the two drugs may differ when used chronically. For example, long-term marijuana use is associated with structural changes in the brain (e.g. reduced hippocampal volume) (Batalla et al. 2013) as well as reduced expression of cortical CB1 receptors (Hirvonen et al. 2012). These long-term changes may potentiate or diminish the acute effect of cannabis administration.
In conclusion, the results of the present study suggest that THC does not prevent improvement of P20-N40 gating by clozapine in DBA/2 mice. To our knowledge, this is the first study to examine the interactions between THC and clozapine on auditory gating in DBA/2 animals. These results suggest that although THC may attenuate clozapine-induced improvement of gating at low doses of the antipsychotic, this effect may be overcome by higher, potentially more clinically relevant doses of clozapine. Future studies in human patient populations may examine dose-dependent interactions of clozapine and THC on sensory gating.
Supplementary Material
Highlights.
THC’s effects on clozapine-induced gating improvement was examined in DBA/2 mice
0.33 mg/kg clozapine alone improved gating
10 mg/kg THC attenuated the effect of 0.33 mg/kg clozapine
10 mg/kg THC did not prevent gating improvement by 1 or 1.83 mg/kg clozapine
Acknowledgments
The authors thank Lijun Zheng, B.S. for technical assistance. This work was supported by the VA Biomedical Laboratory and Clinical Science Research and Development Service, the Brain and Behavior Foundation, the Blowitz-Ridgeway Foundation, and 5P50-MH086383-04.
Footnotes
Ethics and conflicts of interest statement
All experiments were performed within the United States of America and comply with current laws. Author Dr. Stevens is a shareholder of a privately held company investigating the feasibility of central drug administration for the control of seizures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CE, Yonchek JC, Zheng L, Collins AC, Stevens KE. Altered hippocampal circuit function in C3H alpha7 null mutant heterozygous mice. Brain Res. 2008;1194:138–145. doi: 10.1016/j.brainres.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler L, Pachtman E, Franks R, Pecevich M, Waldo M, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998;24:189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [DOI] [PubMed] [Google Scholar]
- Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S, Torrens M, Pujol J, Farre M, Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersani G, Orlandi V, Kotzalidis GD, Pancheri P. Cannabis and schizophrenia: impact on onset, course, psychopathology and outcomes. Eur Arch Psychiatry Clin Neurosci. 2002;252:86–92. doi: 10.1007/s00406-002-0366-5. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox of the group of schizophrenias. International Universities Press; 1911. [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler AV, Dunwiddie TV. Regulation of the activity of hippocampal stratum oriens interneurons by alpha7 nicotinic acetylcholine receptors. Neuroscience. 2001;106:55–67. doi: 10.1016/s0306-4522(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Callstrom MR, York JD, Gaba RC, Gemmete JJ, Gervais DA, Millward SF, Brown DB, Dupuy D, Goldberg SN, Kundu S, Rose SC, Thomas JJ, Cardella JF. Research reporting standards for image-guided ablation of bone and soft tissue tumors. J Vasc Interv Radiol. 2009;20:1527–1540. doi: 10.1016/j.jvir.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costain WF. The effects of cannabis abuse on the symptoms of schizophrenia: patient perspectives. Int J Ment Health Nurs. 2008;17:227–235. doi: 10.1111/j.1447-0349.2008.00538.x. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41:369–371. doi: 10.1212/wnl.41.3.369. [DOI] [PubMed] [Google Scholar]
- Dissanayake DW, Zachariou M, Marsden CA, Mason R. Auditory gating in rat hippocampus and medial prefrontal cortex: effect of the cannabinoid agonist WIN55,212-2. Neuropharmacology. 2008;55:1397–1404. doi: 10.1016/j.neuropharm.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Foti DJ, Kotov R, Guey LT, Bromet EJ. Cannabis use and the course of schizophrenia: 10-year follow-up after first hospitalization. Am J Psychiatry. 2010;167:987–993. doi: 10.1176/appi.ajp.2010.09020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenreich O, Weiner RD, McEvoy JP. Clozapine-induced electroencephalogram changes as a function of clozapine serum levels. Biol Psychiatry. 1997;42:132–137. doi: 10.1016/S0006-3223(96)00298-3. [DOI] [PubMed] [Google Scholar]
- Goldberger C, Dervaux A, Gourion D, Bourdel MC, Loo H, Laqueille X, Krebs MO. Variable individual sensitivity to cannabis in patients with schizophrenia. Int J Neuropsychopharmacol. 2010;13:1145–1154. doi: 10.1017/S1461145710000647. [DOI] [PubMed] [Google Scholar]
- Hambrecht M, Hafner H. Cannabis, vulnerability, and the onset of schizophrenia: an epidemiological perspective. Aust N Z J Psychiatry. 2000;34:468–475. doi: 10.1080/j.1440-1614.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Iyo M, Freedman R, Stevens KE. Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: role of alpha 7 nicotinic acetylcholine receptors. Psychopharmacology (Berl) 2005;183:13–19. doi: 10.1007/s00213-005-0142-0. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Bobo WV, Roy A, Jayathilake K, Chen Y, Ertugrul A, Anil Yagcioglu AE, Small JG. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69:274–285. doi: 10.4088/jcp.v69n0214. [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience. 1995;69:371–381. doi: 10.1016/0306-4522(95)00249-i. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39:306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa JM, Freedman R, Lester HA. Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron. 2011;70:20–33. doi: 10.1016/j.neuron.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Nagai H, Egashira N, Sano K, Ogata A, Mizuki A, Mishima K, Iwasaki K, Shoyama Y, Nishimura R, Fujiwara M. Antipsychotics improve Delta9-tetrahydrocannabinol-induced impairment of the prepulse inhibition of the startle reflex in mice. Pharmacol Biochem Behav. 2006;84:330–336. doi: 10.1016/j.pbb.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R. Gating of auditory P50 in schizophrenics: unique effects of clozapine. Biol Psychiatry. 1996;40:181–188. doi: 10.1016/0006-3223(95)00371-1. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Correll CU, Manu P, Kane JM. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74:603–613. doi: 10.4088/JCP.12r08064. [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28:409–416. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- Potvin S, Sepehry AA, Stip E. A meta-analysis of negative symptoms in dual diagnosis schizophrenia. Psychol Med. 2006;36:431–440. doi: 10.1017/S003329170500574X. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Zakzanis KK, George TP. The effects of cannabis use on neurocognition in schizophrenia: a meta-analysis. Schizophr Res. 2011;128:111–116. doi: 10.1016/j.schres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Rentzsch J, Penzhorn A, Kernbichler K, Plockl D, Gomez-Carrillo de Castro A, Gallinat J, Jockers-Scherubl MC. Differential impact of heavy cannabis use on sensory gating in schizophrenic patients and otherwise healthy controls. Exp Neurol. 2007;205:241–249. doi: 10.1016/j.expneurol.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Salyers MP, Mueser KT. Social functioning, psychopathology, and medication side effects in relation to substance use and abuse in schizophrenia. Schizophr Res. 2001;48:109–123. doi: 10.1016/s0920-9964(00)00063-3. [DOI] [PubMed] [Google Scholar]
- Savoy YE, Ashton MA, Miller MW, Nedza FM, Spracklin DK, Hawthorn MH, Rollema H, Matos FF, Hajos-Korcsok E. Differential effects of various typical and atypical antipsychotics on plasma glucose and insulin levels in the mouse: evidence for the involvement of sympathetic regulation. Schizophr Bull. 2010;36:410–418. doi: 10.1093/schbul/sbn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Siris SG. A review of psychoactive substance use and abuse in schizophrenia. Patterns of drug choice. J Nerv Ment Dis. 1987;175:641–652. doi: 10.1097/00005053-198711000-00001. [DOI] [PubMed] [Google Scholar]
- Shirazi-Southall S, Rodriguez DE, Nomikos GG. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacology. 2002;26:583–594. doi: 10.1016/S0893-133X(01)00400-6. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacology (Berl) 2003;165:386–396. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Eichman LC, Lyons E, Tregellas JR. Early sensory processing deficits predict sensitivity to distraction in schizophrenia. Schizophr Res. 2013;147:196–200. doi: 10.1016/j.schres.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia RD, Knobloch LC. The interaction of 9 -tetrahydrocannabinol pretreatment with various sedative-hypnotic drugs. Psychopharmacologia. 1973;30:185–194. doi: 10.1007/BF00421433. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Wear KD. Normalizing effects of nicotine and a novel nicotinic agonist on hippocampal auditory gating in two animal models. Pharmacol Biochem Behav. 1997;57:869–874. doi: 10.1016/s0091-3057(96)00466-2. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Trulson VM. Chloral hydrate anesthesia alters the responsiveness of dorsal raphe neurons to psychoactive drugs. Life Sci. 1983;32:949–956. doi: 10.1016/0024-3205(83)90924-4. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Delta9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther. 2005;314:329–337. doi: 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Substance use disorders in schizophrenia--clinical implications of comorbidity. Schizophr Bull. 2009;35:469–472. doi: 10.1093/schbul/sbp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, Conus P, Takagi MJ, Fornito A, Wood SJ, McGorry PD, Pantelis C. The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr Bull. 2012;38:316–330. doi: 10.1093/schbul/sbq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Moore TH, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry. 2008;193:357–363. doi: 10.1192/bjp.bp.107.046375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.