Sex differences in the prevalence and progression of numerous cardiovascular diseases are well-documented, with males typically exhibiting greater severity and progression of disease compared to age-matched females. In contrast, females are more likely to develop autoimmune disease compared to age-matched males. Nevertheless, there is growing evidence that certain autoimmune diseases, including systemic lupus erythematous (SLE), are associated with an increased risk of developing cardiovascular disease 1. Based on the expanding literature linking the immune system with cardiovascular disease, the question arises if females lose their “cardiovascular protection” with immune dysfunction and what the molecular mechanisms driving cardiovascular disease in SLE.
Historically, female sex hormones have been thought to contribute to the lower incidence of cardiovascular disease in pre-menopausal women. However, despite numerous studies designed to investigate the role of female sex hormones in cardiovascular disease, data from both animal and clinical research remains controversial. Therefore, it was with great interest that we read the study by Gilbert et al 2 in the current issue of Hypertension, which was designed to determine whether estrogen has a causal role in the development of hypertension in adulthood in SLE, an autoimmune disease with high prevalence of hypertension and cardiovascular disease. Using an established mouse model of SLE (female NZBWF1), Gilbert et al have directly confirmed a protective role for 17β-estradiol against SLE mediated hypertension and proteinuria in adult female mice, in part, by reducing TNF-α. In this study, animals were ovariectomized at 30 weeks of age and studied at 34 weeks of age, and the results are in contrast to those obtained where estrogens potentiate the onset of SLE when mice are ovariectomized at <6–8 weeks of age 3. Together, these studies suggest that female sex hormones may act as a double-edged sword in the development and progression of hypertension in SLE, with timing being the critical determinant of the inflammatory and cardiovascular impact of estrogen. The authors suggest that there are temporal effects of estrogens in SLE, with estrogens promoting humoral immunity during subclinical disease, but protecting against inflammation and disease progression in adulthood.
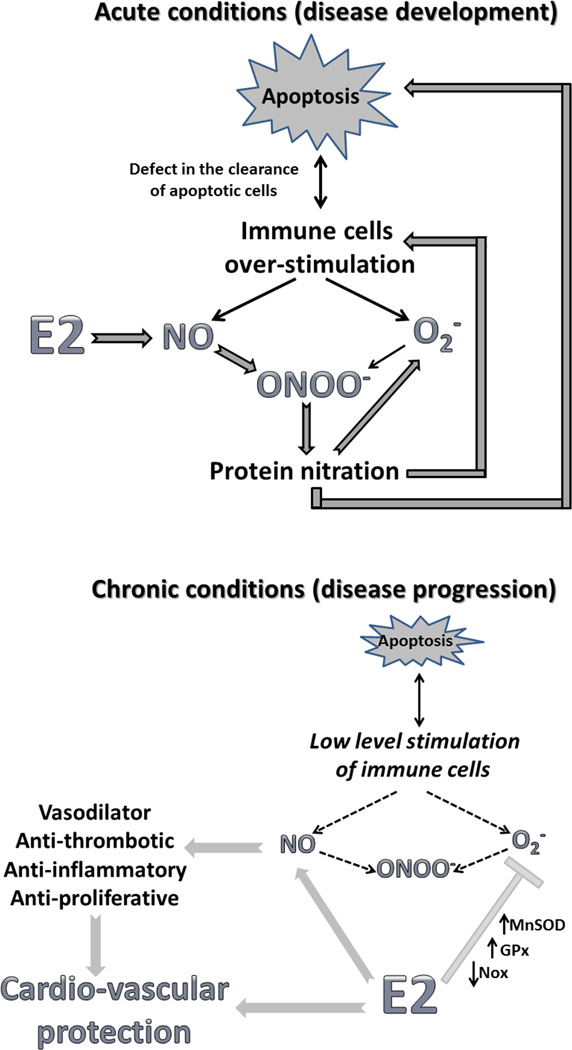
It is well established that 17β-estradiol binds to its cognate receptors (i.e., ERα and ERβ) to initiate extra-nuclear cytosolic signaling pathways and nuclear expression of specific genes by associating with transcriptional cofactors. Non-genomic mechanisms trigger downstream signaling events though rapid post-translation modifications of numerous membrane and cytosolic signaling molecules, including mitogen-activated protein kinase (MAPK), phosphatidylinositide 3 (PI3) kinase, Akt, and endothelial nitric oxide synthase (eNOS; NOS3), which are critical in initiating an immediate response. One result of this concerted signaling cascade is an elevation of nitric oxide (NO) bioavailability, and NO has been suggested to be a primary mechanism for the protection of females against cardiovascular diseases and may contribute to the better resistance against infection among females.
However, during conditions of oxidative stress even an increase in NO could do a disservice through the formation of deleterious peroxynitrite and protein nitration, which contributes to additional cellular damage. There is evidence supporting an NO deficiency in SLE 4, therefore, any additional decrease in NO may potentiate disease progression in SLE, as well as many other cardiovascular diseases. Importantly, these detrimental effects will be primarily evident in acute pathological events, when the antioxidant properties of estrogens have a limited time to impact antioxidant potential compared to the robust increase in free radical production. SLE is known to be associated with increases in oxidative stress, apoptosis and cellular injury 5. Indeed, the formation of autoantibodies against epitopes from apoptotic cells are known to be among the earliest symptoms and a hallmark of SLE 6. Moreover, elevated levels of anti-nitrotyrosine antibodies in serum of patients with SLE have been suggested to be responsible for the breakdown or bypass the self-tolerance 7. Therefore, under certain conditions, estrogen could actually promote increases in oxidative damage and disease progression. Alternatively, since decreases in NO bioavailability promote inflammation, and early loss of estrogen in may SLE promote disease progression, it could be speculated that the early loss of NO promotes SLE-induced cardiovascular disease.
In contrast, chronic low level inflammation in established SLE may provide an adequate amount of time to reveal the protective properties of estrogen, as evidenced in the study by Gilbert et al, where mice were allowed to fully mature and were well into adulthood before sex hormones were removed. NO not only acts as a potent vasodilator, but also possesses antithrombotic, anti-inflammatory and anti-proliferative properties 8, 9. Estrogen also elicits genomic mechanisms mediating relatively slow changes in gene expression, including antioxidant proteins. Therefore, long-term exposure to estrogens has the potential to induce multiple lines of defense against the development of cardiovascular disease. Our group recently reported that treatment with the non-selective NO synthase inhibitor L-NAME resulted in greater increases in blood pressure in female spontaneously hypertensive rats (SHR) than males 10. Moreover, this increase was associated with greater increases in renal adhesion molecule expression and Th17 cell infiltration, which have been linked to the development of autoimmune disease. Our data suggest that NO is a critical regulator of blood pressure and the immune cell profile, particularly in females. Hence, an increase in NO bioavailability induced by estrogen, while damaging during acute oxidative stress, could postpone the development of cardiovascular complications, as demonstrated by Gilbert et al.
In conclusion, the present study provides a new paradigm for a dual role of estrogen in the initiation and progression of SLE, as well as offering protection against the progression of disease. Future investigations that directly confirm the particular molecular mechanisms behind female sex hormone dualism will allow for more defined control of estrogenic activity to achieve beneficial effects regardless of the stage of immune disease.
Supplementary Material
Figure.
Schematic diagram of the potential impact of estrogen to either promote disease development under acute, rapid conditions or protect against disease progression under chronic conditions.
Acknowledgments
Sources of funding: The authors acknowledge funding from the National Institutes of Health (1R01 HL-093271-01A1 to JCS).
Footnotes
Disclosures: NONE
REFERENCES
- 1.Aranow C, Ginzler EM. Epidemiology of cardiovascular disease in systemic lupus erythematosus. Lupus. 2000;9:166–169. doi: 10.1191/096120300678828208. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert ELMK, Ryan MJ. 17β-estradiol protects against the progression of hypertesion during adulthood in a mouse model of systemic lupus erythematosus. Hypertesion. 2013 doi: 10.1161/HYPERTENSIONAHA.113.02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venegas-Pont M, Ryan MJ. Can estrogens promote hypertension during systemic lupus erythematosus? Steroids. 2010;75:766–771. doi: 10.1016/j.steroids.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan MJ, McLemore GR., Jr Hypertension and impaired vascular function in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R736–R742. doi: 10.1152/ajpregu.00168.2006. [DOI] [PubMed] [Google Scholar]
- 5.Mathis KW, Venegas-Pont M, Masterson CW, Stewart NJ, Wasson KL, Ryan MJ. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension. 2012;59:673–679. doi: 10.1161/HYPERTENSIONAHA.111.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieker JW, van der Vlag J, Berden JH. Deranged removal of apoptotic cells: Its role in the genesis of lupus. Nephrol Dial Transplant. 2004;19:282–285. doi: 10.1093/ndt/gfg485. [DOI] [PubMed] [Google Scholar]
- 7.Khan F, Ali R. Antibodies against nitric oxide damaged poly l-tyrosine and 3-nitrotyrosine levels in systemic lupus erythematosus. J Biochem Mol Biol. 2006;39:189–196. doi: 10.5483/bmbrep.2006.39.2.189. [DOI] [PubMed] [Google Scholar]
- 8.Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- 9.Cannon RO., 3rd Role of nitric oxide in cardiovascular disease: Focus on the endothelium. Clin Chem. 1998;44:1809–1819. [PubMed] [Google Scholar]
- 10.Brinson KN, Elmarakby AA, Tipton AJ, Crislip GR, Yamamoto T, Baban B, Sullivan JC. Female shr have greater blood pressure sensitivity and renal t cell infiltration following chronic nos inhibition than males. Am J Physiol Regul Integr Comp Physiol. 2013;305:R701–R710. doi: 10.1152/ajpregu.00226.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.