Abstract
Study Objective
This study was designed to prospectively evaluate the in vivo activities of drug transporters, metabolizing enzymes and pharmacokinetics in patients with chronic kidney diseases (CKD) caused by glomerulonephritis and non-glomerular etiologies.
Design
A prospective study design
Participants
Eighteen adults with CKD
Setting
General Clinical Research Center at the University of North Carolina and University of Pittsburgh
Measurement and Main Results
Blood and urine were collected and assayed for fexofenadine (transporter function) as well as flurbiprofen and 4-hydroxyflurbiprofen (CYP2C9 function). CYP3A4 activity was assessed by the erythromycin breath test. In patients with glomerulonephritis, the apparent oral clearance of fexofenadine (representing transporter activity) was 58.8±34.4L/h, documenting a 40% reduction compared with previous data in healthy controls. The CYP2C9 pathway (4-hydroxyflurbiprofen formation clearance), was similar in all the patients with CKD and was concordant with previous reports of patients with end-stage renal disease (ESRD) and healthy controls. For flurbiprofen, patients with glomerulonephritis had higher oral clearance than those with nonglomeruler CKD, suggesting higher unbound fraction and enhanced metabolism through other (non-CYP2C9) routes. No statistically significant differences in CYP3A4 activity were observed in either group of patients, or when compared with results from previous studies of patients with ESRD or healthy controls.
Conclusions
The current study suggests no statistically significant differences in the in vivo activity of CYP2C9 and CYP3A4 in patients with either glomerulonephritis or non-glomerular CKD. However, there are clinical differences in transporter function as defined by at least a 25% reduction in activity in glomerulonephritis as opposed to healthy controls. A similarity in the in vivo function between patients with glomerulonephritis and ESRD, and between patients with glomerulonephritis and non-glomerular CKD was present despite significant differences in kidney function. Further in vivo and in vitro studies are necessary to fully understand the physiologic and disease-specific nuances that contribute to alterations in drug disposition in patients with kidney diseases.
Keywords: glomerulonephritis, CYP3A4, CYP2C9, P-glycoprotein, single-dose pharmacokinetics, lupus nephritis, small vessel vasculitis
Introduction
Chronic kidney disease (CKD) affects more than 26 million people in the United.States. 1 According to the National Institutes of Health, the three leading etiologies for end-stage renal disease (ESRD) are diabetes mellitus (39%), hypertension (29%), and glomerulonephritis (5%). 2 While it is well established that CKD can result in decreased elimination of drugs via the kidneys, the effects of the disease on nonrenal clearance processes such as specific metabolic or transport routes are less well described. Experimental models of CKD have reported reductions in protein and mRNA expression of hepatic cytochrome P450 (CYP) enzymes including CYP3A1, CYP3A2, CYP2C11, and N-acetyltransferases. 3–5 Various alterations in activity of drug transporters in the kidney have been reported in a CKD model in rats. 6 Reductions in nonrenal clearance in patients with CKD range from 30% to 67% for substrates of the CYP3A4, CYP2D6, CYP2B6, and CYP2C9 enzymes and transporter pathways. 7 In order to fully understand the significance of alterations in protein levels and activity of metabolic and transport pathways in patients with CKD, it is important to evaluate the effects that specific forms of the disease, such as glomerulonephritis, have on these pathways and whether these alterations influence drug disposition in a clinically relevant manner.
Numerous studies have reported various probe drugs or probe drug combinations (e.g., cocktails) as a direct approach to evaluate the in vivo function of drug-metabolizing enzymes and transporters. 8–10 However, only a few studies have been conducted in patients with CKD. 11–14 Thus, applicability of the results from most published studies beyond the healthy control population remains to be established. As patients with CKD are commonly prescribed 10–12 different medications on a daily basis15, alterations in one or more pathway important for drug disposition could have profound effects on prescribed therapies. Hence, studies that evaluate the activity of various drug metabolism pathways should address requirements for modifications in drug regimens and the interaction potential with other commonly prescribed drugs used in the targeted disease populations. There is a particular interest in the CYP3A4 and CYP2C9 enzymes in patients with glomerulonephritis, as these enzymes are important in the metabolic activation of the majority of drugs commonly prescribed to patients with CKD, including cyclophosphamide.
The primary purpose of the current study was to prospectively evaluate the activity of drug transporter and the CYP3A4 and CYP2C9 metabolizing enzyme pathways and pharmacokinetics in patients with CKD caused by glomerulonephritis. In performing these assessments, we investigated the clinical pharmacokinetics of flubiprofen, a pharmacologic probe of CYP2C9 metabolism; fexofenadine, a nonspecific probe of drug transport; and erythromycin, a probe of CYP3A4 metabolism in vivo. Previous results by our group indicated reductions in CYP2B6 activity (assessed by bupropion) in patients with glomerulonephritis compared with other subject groups. 16 A secondary objective of the study was to compare the activity of CYP3A4 and CYP2C9 and drug transport in glomerulonephritis compared with nonglomerular CKD and with previous published reports in healthy controls and in patients with ESRD.
Methods
Patients
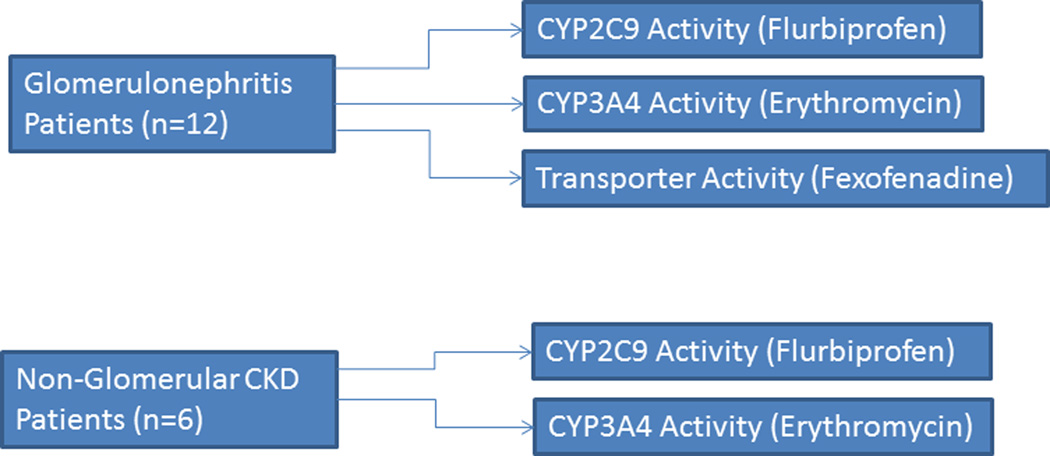
Patients with CKD caused by biopsy-confirmed glomerulonephritis due to either systemic lupus erythematosus (SLE) nephritis or small vessel vasculitis (SVV) consented to participate in an IRB-approved study to assess the in vivo activity of drug transport and drug metabolism (CYP2C9 and CYP3A4) pathways.(Figure 1) A small comparator group of patients with nonglomerular CKD were also evaluated.(Figure 1) At the 72-hour study visit, all of the patients with glomerulonephritis received intravenous cyclophosphamide treatment as part of standard care and an antiemetic regimen, which consisted of oral dexamethasone and ondansetron. Concomitant therapy with other immunosuppressants was allowed. Patients who were unable to abstain from ingestion of alcohol, orange juice, and grapefruit juice for at least 48 hours prior to and during the study were excluded from study participation. Eligibility criteria included the following: normal liver function, stable kidney function, and a negative urine pregnancy test result for women of child-bearing potential. Patients taking drugs (and doses) known to interfere with the metabolism of any of the probe substrates, and those with a known allergy or sensitivity to any of the probe substrates, were also excluded. All patients were required to undergo an overnight fast prior to study initiation and were provided with a CYP450 diet (exclusion of cruciferous vegetables, spinach, garlic, grapefruit, char-grilled meats, and smoked meats) throughout the study. 17 Clinical data, including serum creatinine concentration, estimated creatinine clearance (Clcr) 18, urinary protein to creatinine ratio (UP:Cr), and serum albumin concentration were measured at the time of the study or abstracted from the medical record within 30 days of the study, when available. The study and consent form were approved by the Biomedical Review Boards at both the University of North Carolina at Chapel Hill and the University of Pittsburgh.
Figure 1.
Study Design
Pharmacokinetic Study
For the pharmacokinetic study, patients with glomerulonephritis were admitted to the General Clinical Research Center (GCRC) at the University of North Carolina for a 72-hour inpatient stay. Prior to the start of the cyclophosphamide infusion, these patients received one tablet each concomitantly of fexofenadine (60 mg) to assess transporter function and flurbiprofen (50 mg) to assess CYP2C9 activity, followed by 8 ounces of water. Several hours prior to the cyclophosphamide infusion, patients received intravenous 14C-erythromycin (3 µCi) to assess the activity of CYP3A4. The intravenous 14C-erythromycin (3 µCi) was diluted in 0.9% sodium chloride and administered over 60 seconds. Breath samples were collected into foil balloons at baseline and at 20 minutes to determine CYP3A4 activity. Patients with nonglomerular CKD were studied at the GCRC at the University of Pittsburgh and received only flurbiprofen and 14C-erythromycin. To serve as a comparison with results obtained from the glomerulonephritis patients, we identified previous historical studies reporting fexofenadine, flurbiprofen, and erythromycin phenotyping in ESRD and healthy controls.9,13,19,20 Serial blood samples (0.5, 1, 2, 3, 4, 6, 8, 12, 18, 24, 36, 48, 72 h) were drawn into heparinized vacutainers (7.5mL) for assessment of fexofenadine and/or flurbiprofen. Heparinized blood samples were centrifuged immediately for 10 minutes at 4°C, and plasma was transferred to plastic screw top tubes and stored at −80°C until assay. Urine volume for each collection period was recorded, and 2 mL aliquots were stored at −80°C until analysis.
Analytical Methods
Plasma fexofenadine concentrations were determined by validated methods employing liquid chromatography tandem mass spectrometry as recently described.21 The assay was linear over the concentration range of 1.0 to 500 ng/ml; the precision and accuracy (intra- and interrun) were within 4.3% and 8.0%, respectively.
Flurbiprofen plasma concentrations were determined by liquid chromatography tandem mass spectrometry. Briefly, plasma samples were processed by protein precipitation with acetonitrile with flurbiprofen-d3 used as the internal standard. An aliquot of the processed sample was injected onto a Supelco Discovery HS F5 analytical column (2.1 mm × 50 mm, 3 µM). Binary gradient elution was achieved with high-performance liquid chromatography mobile phases comprising 1 mM acetic acid in water and 1 mM acetic acid in acetonitrile with a flow rate of 0.2 ml/min. The LC/MS/MS system consisted of a Surveyor HPLC autosampler, Surveyor MS quaternary pump and a TSQ Quantum Discovery triple quadrupole mass spectrometer (Thermo, San Jose, CA). The TSQ Quantum mass spectrometer was equipped with an electrospray (ESI) source and operated in the negative ion mode. The acquisition parameters were: spray voltage 3.0 kV, source collision-induced dissociation 10 V and heated capillary temperature of 350°C. The collision energy was 10 eV for all analytes. The selected reaction monitoring scheme followed transitions of the [M+H]+ precursor to selected product ions with the following values: m/z 243.1 > 199.0 for flurbiprofen and m/z 246.1 > 202.0 for flurbiprofen-d3. The standard concentrations ranged from 0.025 mg/mL to 10 mg/mL. The intra- and interday precision (coefficient of variation) and accuracy (relative error) values were within 11% for the low, medium and high flurbiprofen quality control concentrations.
The amount of 14CO2 exhaled in the collected breath samples was quantified by liquid scintillation counting (Packard 1600, Hewlett Packard, Palo Alto, CA).
Pharmacokinetic Analysis
Noncompartmental pharmacokinetic analyses of fexofenadine and flurbiprofen were conducted using WinNonlin v4.1 (Pharsight, Mountain View CA) with the linear up-log down method for area under the curve (AUC) determination. The following parameters for fexofenadine and flurbiprofen were reported: observed concentration maximum (Cmax), time to maximum concentration (Tmax), half-life (T1/2), area under the plasma concentration vs time curve from 0-∞ hours (AUC 0-∞), and apparent oral clearance (Cl/F). For flurbiprofen, the amount of drug and metabolite in the urine (Ae) was calculated by multiplying the measured concentration by the total urine volume for each collection period (0–6, 6–12, 12–18, 18–24, 24–36, 36–48, 48–72 hours). The Ae over the study period was computed by adding the Ae for the relevant collection intervals. Renal clearance (Clr) for flubiprofen was calculated by Ae 0-end of last collection/ AUC 0-last blood sample. The formation clearance of 4-OH flurbiprofen was calculated as the amount of 4-OH flurbiprofen in urine (0-end of last collection) / flurbiprofen AUC0-last blood sample (e.g. 72 hours), based on the assumption that all of the 4-OH flubiprofen that is formed is immediately excreted unchanged in the urine. The metabolism rate of the administered 14C erythromycin dose (3 µCi) was expressed as the percent of administered dose exhaled per hour.
Statistics
Descriptive analyses for pharmacokinetic parameters, demographic variables, and clinical laboratories for the glomerulonephritis cohort included mean values and standard deviations, as appropriate. Analysis of Variance (ANOVA), with Tukey’s post-hoc test, was used to assess the statistical significance of differences in the pharmacokinetic parameters for fexofenadine, flurbiprofen, and 14C-erythromycin activity between patient groups. Mean ±standard deviation pharmacokinetics data from published studies of healthy controls and ESRD patients were used for comparison with our experimental CKD data.
A post-hoc sample size analysis was conducted based on the primary comparison analysis for the secondary study measure - the differences in apparent oral clearance of the probe drugs between glomerulonephritis and healthy controls. For fexofenadine, seven patients per group with expected standard deviations of 30 L/h would have 80% power. For flurbiprofen and 14C erythromycin, 12 patients per group with expected standard deviations of 1 L/h and 1% of dose metabolized per hour, respectively, would have 80% power.
All data are presented as mean ± standard deviation and were analyzed by Instat v3.0 (GraphPad, Inc, La Jolla, CA); p values <0.05 were considered statistically significant.
Results
Patient Demographics
A total of 12 patients with biopsy-confirmed glomerulonephritis were enrolled (SLE =5; SVV =7). Patients with non-glomerular CKD (n=6) were also enrolled. The etiologies for CKD in this later group were diabetes mellitus (n=2), hypertension (n=1), other (n=1), unknown (n=2). The patient demographics are presented in Table 1. The non-Caucasian patients with glomerulonephritis included five African Americans, one Hispanic and one “other” patient. A total of eight patients with glomerulonephritis were receiving glucocorticoids 24±19 mg per day (range 15mg to 60mg daily). The patients with non-glomerular CKD were older (64±17 vs 41±17), and all four of the non-Caucasians were African Americans. The demographics of the healthy patients with ESRD and the healthy controls in the published studies were similar to the experimental CKD study groups.
Table 1.
Demographics of Patients with Glomerulonephritis and Secondary Chronic Kidney Disease
| Glomerulonephritis (n=12) |
Secondary CKD (n=6) |
|
|---|---|---|
| Gender (F/M) | 9/3 | 1/5 |
| Race (Caucasian/NonCaucasian) | 5/7 | 2/4 |
| Age (years) | 41±17 | 64±17 |
| Weight (kg) | 91.2±17.5 | 85.8±24.2 |
| Serum Creatinine (mg/dL) | 1.3±0.7 | 3.1±2.0 |
| Creatinine Clearance (mL/min) | 102±43 | 29±18 |
| Urinary Protein:Urinary Creatinine | 1.6±1.5 | 0.2±0.2 |
| Serum Albumin (g/dL) | 2.0±0.2 | 3.9±0.3 |
Creatinine clearance calculated by Cockcroft and Gault 18
CKD – chronic kidney disease
In Vivo Phenotyping for Transporter, CYP2C9, and CYP3A4 Activity
The mean (±standard deviation) pharmacokinetic parameters for fexofenadine and flurbiprofen in all study and referenced control groups are provided in Tables 2 and 3, respectively. Significant differences in fexofenadine pharmacokinetic parameters were noted across patient groups. Tukey post-hoc tests were used to evaluate specific differences between comparison groups. Patients with glomerulonephritis had significantly longer fexofenadine half-life values (11.5±5.6 hours) than for patients with ESRD (4.6±1.3 hours; p<0.001) or healthy controls (3.4±0.9 hours; p<0.001). Patients with glomerulonephritis patients had lower dose-corrected fexofenadine Cmax values (140±83 ng/mL) than healthy controls (232±97 ng/mL; p<0.05). Significant differences in flurbiprofen pharmacokinetic parameters were noted across patient groups for Cmax, Tmax, half-life, AUC and Cl/F (Table 3). 9,20 Patients with glomerulonephritis had significantly lower flurbiprofen Cmax values (3.1±1.3 µg/mL) than both non-glomerular CKD patients (6.6±1.5 µg/mL; p<0.01) and healthy controls (9.4±2.5 µg/mL;p<0.001. 9 Flurbiprofen Tmax values were greater in glomerulonephritis patients (4.8±2.1 hours) as compared to non-glomerular CKD (1.7±0.8 h; p<0.001), ESRD (2.3±1.2; p<0.01), and healthy controls (2.3±1.0 h; p<0.01).9,20 Higher flurbiprofen apparent oral clearance values (1.9±0.5 L/h) were demonstrated in patients with glomerulonephritis compared with patients who had non-glomerular CKD (0.9±0.3 L/h; p<0.001) and the healthy controls (1.1±0.4 L/h; p<0.001. 9 The 4-OH flurbiprofen formation clearance was similar across the experimental CKD populations and healthy controls.
Table 2.
Pharmacokinetics of fexofenadine in three different populations. Data presented as mean ±standard deviation.
| Glomerulonephritis (n=12) |
ESRD (n=10)19 | Healthy Controls (n=10) 19 |
Overall P Value b |
|
|---|---|---|---|---|
| Cmax, ng/mL |
140±83c | 232±97a,d | 134 ± 65a | 0.0200 |
| Tmax, h | 4.6±3.0 | 2.0 | 2.0 | ND |
| T1/2, h | 11.5±5.6e,f | 4.6±1.3 | 3.4 ± 0.9 | <0.0001 |
| AUC0-∞, ng h/mL |
1351±723 | 1963±922a, g | 690 ± 337a | 0.0016 |
| Cl/F, L/h | 58.8±34.4h | 37.9±19.5g | 103 ± 38 | 0.0001 |
dose adjusted to 60 mg
Overall p value by ANOVA
Results of Tukey’s post-hoc analysis:
glomerulonephritis vs. ESRD; p<0.05
ESRD vs. healthy controls; p<0.05
glomerulonephritis vs ESRD; p<0.001
glomerulonephritis vs healthy controls; p<0.001
ESRD vs healthy controls; p<0.01
glomerulonephritis vs healthy controls; p<0.01
Data presented as means or mean±standard deviation
ESRD – end stage renal disease
ND – not done
NR – not reported
Table 3.
Pharmacokinetics of Flurbiprofen. Data presented as mean ±standard deviation.
| Glomerulonephritis (n=12) |
Non-glomerular CKD (n=6) |
ESRD (n=8)20 | Healthy Controls (n=12) 9 |
Overall P Value b |
|
|---|---|---|---|---|---|
| Cmax, µg/mL | 3.1±1.3c,d | 6.6±1.5a,e,f | 2.3±0.8a,g | 9.4 ± 2.5 | <0.0001 |
| Tmax, h | 4.8±2.1h,i,j | 1.7±0.8 | 2.3±1.2 | 2.3 ± 1.0 | 0.0001 |
| T1/2, h | 6.3±3.5 | 9.1±5.3f | 3.0 | 4.3 ± 1.6 | 0.0268 |
| AUC0-∞, µg h/L | 28.0±9.1c,k | 63.0±31.0a | 11.1±5.5a | 50.6 ± 17.6a | 0.0014 |
| CL/F L/h | 1.9 ± 0.5d,l | 0.9 ± 0.3 | 1.3 | 1.1 ± 0.4 | <0.0001 |
| CLformation, L/h | 0.6±0.2 | 0.5 ± 0.3 | NR | 0.4 ± 0.2 | 0.1065 |
dose adjusted to 50 mg
Overall p value via ANOVA
Results of Tukey’s post-hoc analysis:
glomerulonephritis vs. CKD; p<0.01
glomerulonephritis vs. healthy controls; p<0.001
CKD vs ESRD; p<0.001
CKD vs healthy controls; p<0.05
ESRD vs healthy controls; p<0.001
glomerulonephritis vs CKD; p<0.001
glomerulonephritis vs ESRD; p<0.01
glomerulonephritis vs healthy controls; p<0.01
glomerulonephritis vs healthy controls; p<0.05
glomerulonephritis vs CKD; p<0.001
Data presented as means or mean±standard deviation
CKD – chronic kidney disease
CLformation – calculated as amount 4-hydroxyflurbiprofen in urine/flurbiprofen plasma AUC
CLr – renal clearance (of flurbiprofen)
ESRD – end stage renal disease
The erythromycin breath test results (CYP3A4 activity) were reported as percentage (%) dose metabolized per hour: glomerulonephritis 2.0±0.8% (n=12), ESRD 1.9±0.7% (n=12) 13, non-glomerular CKD 2.2±0.6% (n=6), and healthy controls 2.7±1.0% (n=12) 13. No significant differences (p=0.0996) were observed between groups.
Discussion
This is the first study to evaluate the influence of glomerulonephritis on the in vivo activity of transport proteins (as reflected by fexofenadine disposition) and drug metabolizing enzymes (CYP2C9 and CYP3A4). It is important to elucidate drug metabolism and transport activity in patients with glomerulonephritis to inform clinicians about possible dosing changes for drugs that are substrates for the affected routes. Typical clinical manifestations of glomerulonephritis include combined reductions in kidney function [e.g. glomerular filtration rate (GFR)], and structural abnormalities [e.g. urinary protein excretion often in the nephrotic range (≥3.5 g/day)]. Patients with severe proteinuria may exhibit enhanced filtration of large molecular weight substances including plasma proteins 22, which can result in disruption of elimination characteristics for small molecule drugs. 23 In addition, reduced concentrations of serum albumin may alter the disposition of drugs that are significantly bound to albumin. 23–25
A composite measure of drug transport activity was provided by an assessment of fexofenadine apparent oral clearance. Fexofenadine is a P-glycoprotein substrate that is eliminated unchanged in the feces (80%) and urine (10%), and is 5% bound to plasma proteins. Previous research has identified fexofenadine as an in vivo P-glycoprotein phenotyping probe. 26–28 Additionally, the International Transporter Consortium included fexofenadine as an in vivo P-glycoprotein probe. 29 However, some data published after the current study commenced indicate that in addition to P-glycoprotein, the organic anion transporting polypeptides (OATPs) 30,31 and multidrug resistance associated proteins (MRPs) 32,33, are also involved in fexofenadine disposition. Thus, the specificity of fexofenadine as a P-glycoprotein probe may be somewhat limited.
In order to provide comparisons of drug pharmacokinetics between subjects with glomerulonephritis, non-glomerular CKD, ESRD, and healthy controls, the experimental and published pharmacokinetics data were evaluated. While there are limitations to this approach, such as differences in assay specificity and limits of quantification, the information gained from this approach can be of considerable value when exploring differences, justifying future studies, and informing clinical decision making. The apparent oral clearance of fexofenadine in the glomerulonephritis population was lower than in healthy controls and higher than in ESRD patients, although only the comparison with healthy controls was statistically significant. There appear to be no currently published fexofenadine pharmacokinetics data from patients with non-glomerular CKD for comparison. Significantly longer half-life and lower Cmax values were observed in patients with glomerulonephritis as compared with published values in ESRD patients. 19 Additionally, patients with glomerulonephritis had significantly longer half-life values of fexofenadine than the healthy control population.19 The longer fexofenadine half-life in glomerulonephritis patients as compared with ESRD patients and healthy controls could be due to an increase in the volume of distribution. Decreased apparent oral clearance could also be contributing to the long half-life in patients with glomerulonephritis.
A number of mechanisms could be responsible for the 40% decrease in apparent oral clearance of fexofenadine including: 1) decreased intestinal efflux due to P-glycoprotein, resulting in an increase in systemic exposure, 2) increased intestinal OATP1A2-mediated uptake, resulting in an increase in systemic exposure, 3) decreased hepatic OATP1B1 or OATP1B3 uptake, 4) decreased hepatic and renal P-glycoprotein-mediated excretion, or 5) increased (non-P-glycoprotein) renal or hepatic efflux by transporters on the basolateral membrane of proximal tubular cells. Common P-glycoprotein inhibitors prescribed for the glomerulonephritis population include proton pump inhibitors (e.g. omeprazole, pantoprazole, lansoprazole), simvastatin, and fluoxetine. Combined use of these drugs could amplify the effects of glomerulonephritis on transporter function. However, none of the glomerulonephritis patients included in the study had received these agents.
The activity of the CYP2C9 drug metabolism pathway was assessed by flurbiprofen, a low extraction ratio drug that is highly protein bound (99%) to albumin and eliminated via the kidneys as metabolites, primarily as 4-hydroxyflurbiprofen. The CYP2C9 pathway accounts for the metabolism of approximately 20% of marketed drugs, 34 including drug classes such as nonsteroidal anti-inflammatory drugs (NSAIDs), angiotensin II receptor blockers, sulfonylureas, and warfarin. Several CYP2C9 inhibitors, e.g. fluvastatin, lovastatin, sertraline, and sulfamethoxazole, are commonly used in patients with glomerulonephritis. The current study found that flurbiprofen pharmacokinetics were similar in patients with glomerulonephritis and those with ESRD and were different from patients with non-glomerular CKD and normal controls. 20 Kidney function assessments, as defined by creatinine clearance, demonstrated that patients with glomerulonephritis had essentially normal kidney function (102±43 mL/min), but had flurbiprofen pharmacokinetics that were similar to patients with ESRD. Patients with glomerulonephritis had lower flurbiprofen Cmax values than patients with non-glomerular CKD patients or the healthy controls. Possible explanations for the lower flurbiprofen Cmax values include: 1) decreased bioavailability, 2) increased volume of distribution, or 3) increased clearance. 20 We did not assess bioavailability or volume of distribution, but higher apparent oral clearance values were noted in the patients with glomerulonephritis as compared to data in patients with non-glomerular CKD and healthy controls. Increased apparent oral clearance was not due to increased formation clearance of 4-hydroxyflurbiprofen, as indicated by the nonsignificant differences between comparator groups.
Laboratory data from the current study revealed serum albumin concentrations that were two-fold less in patients with glomerulonephritis (2.0±0.2 g/dL) as compared with patients with non-glomerular CKD (3.9±0.3 g/dL). This is a factor that could contribute to increased total drug clearance and volume of distribution, and lower total plasma concentrations (e.g. Cmax). End stage renal disease is reported to alter the clearance of highly bound drugs through uremia-induced changes in albumin binding. 35,36 Higher apparent oral clearance values in glomerulonephritis and ESRD patients could be a reflection of a higher unbound fraction of flurbiprofen. The increase in apparent oral clearance most likely occurs through metabolism pathways other than 4-OH flurbiprofen, given the similar formation clearance values.
CYP3A4 is the predominant metabolic pathway for pharmaceutical products. Several CYP3A4 probes including erythromycin (via the erythromycin breath test) 37, midazolam 38, and 6-β-hydroxycortisol/cortisol ratio 39, have been used to assess the in vivo activity of this important drug-metabolizing enzyme. In the current study, the erythromycin breath test was selected to assess CYP3A4 in vivo activity over the other common probes because of its safety profile and the presence of glucocorticoid therapy in a proportion of the glomerulonephritis population. Glucocorticoids can induce some metabolism enzymes, including CYP3A4; however, this did not likely affect the study analysis given that CYP3A4 activity in glomerulonephritis was actually reduced, to a similar extent as previously reported in patients with ESRD. These results suggest that factors in glomerulonephritis other than reductions in GFR could be reducing the activity of the CYP3A4 metabolism pathway. Systemic lupus erythematosis (SLE) and SVV are inflammatory conditions and there are data to support reductions in CYP enzyme activity under conditions of inflammation. 40,41 A recent publication reported that OATP uptake transporters and the P-glycoprotein efflux transporter influence the disposition of erythromycin in humans 42, suggesting reduced applicability of the erythromycin breath test results to other CYP3A4 substrates that induce or inhibit these transport proteins.
Conclusions
The current study provides data demonstrating the activity of transport and drug metabolism (CYP3A4 and CYP2C9) pathways in patients with CKD due to glomerulonephritis. There were no statistically significant differences in the in vivo activity for CYP3A4 and CYP2C9 between patient groups. However, there were clearly clinically relevant differences between patients with glomerulonephritis and healthy controls, as defined by a change of 25% for the two pathways. Additionally, several significant pharmacokinetic differences were noted between patients with glomerulonephritis and healthy controls, as well as with other comparison groups. An interesting finding was the similarity between the in vivo function across drug metabolism and transport routes between the glomerulonephritis, nonglomerular CKD, and ESRD patients, despite having significant differences in kidney function. Further in vivo and in vitro studies are necessary to fully understand the physiologic and disease-specific nuances that contribute to alterations in drug disposition between patients with various forms of kidney diseases.
Acknowledgment
The authors would like to thank Gary R. Matzke for his engagement concerning the secondary CKD population data.
This research was funded by the National Institutes of Health K23DK64888 (MSJ), General Clinical Research Centers program of the Division of Research Resources, National Institutes of Health RR00046 (MSJ), Clinical and Translational Science Award U54RR024383 (MSJ), and American College of Clinical Pharmacy Research Institute’s Frontier’s Award (MSJ)
Footnotes
Financial Disclosures: None
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.System, USRD. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 3.Guevin C, Michaud J, Naud J, Leblond FA, Pichette V. Down-regulation of hepatic cytochrome p450 in chronic renal failure: role of uremic mediators. Br J Pharmacol. 2002;137:1039–1046. doi: 10.1038/sj.bjp.0704951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naud J, Michaud J, Leblond FA, Lefrancois S, Bonnardeaux A, Pichette V. Effects of chronic renal failure on liver drug transporters. Drug Metab Dispos. 2008;36:124–128. doi: 10.1124/dmd.107.018192. [DOI] [PubMed] [Google Scholar]
- 5.Simard E, Naud J, Michaud J, Leblond FA, Bonnardeaux A, Guillemette C, Sim E, Pichette V. Downregulation of hepatic acetylation of drugs in chronic renal failure. J Am Soc Nephrol. 2008;19:1352–1359. doi: 10.1681/ASN.2007090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun H, Frassetto L, Benet LZ. Effects of renal failure on drug transport and metabolism. Pharmacol Ther. 2006;109:1–11. doi: 10.1016/j.pharmthera.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Nolin TD, Naud J, Leblond FA, Pichette V. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther. 2008;83:898–903. doi: 10.1038/clpt.2008.59. [DOI] [PubMed] [Google Scholar]
- 8.Chainuvati S, Nafziger AN, Leeder JS, Gaedigk A, Kearns GL, Sellers E, Zhang Y, Kashuba AD, Rowland E, Bertino JS., Jr. Combined phenotypic assessment of cytochrome p450 1A2, 2C9, 2C19, 2D6, and 3A, N-acetyltransferase-2, and xanthine oxidase activities with the "Cooperstown 5+1 cocktail". Clin Pharmacol Ther. 2003;74:437–447. doi: 10.1016/S0009-9236(03)00229-7. [DOI] [PubMed] [Google Scholar]
- 9.Zgheib NK, Frye RF, Tracy TS, Romkes M, Branch RA. Validation of incorporating flurbiprofen into the Pittsburgh cocktail. Clin Pharmacol Ther. 2006;80:257–263. doi: 10.1016/j.clpt.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Frye RF, Matzke GR, Adedoyin A, Porter JA, Branch RA. Validation of the five-drug "Pittsburgh cocktail" approach for assessment of selective regulation of drug-metabolizing enzymes. Clin Pharmacol Ther. 1997;62:365–376. doi: 10.1016/S0009-9236(97)90114-4. [DOI] [PubMed] [Google Scholar]
- 11.Nolin TD, Appiah K, Kendrick SA, Le P, McMonagle E, Himmelfarb J. Hemodialysis acutely improves hepatic CYP3A4 metabolic activity. J Am Soc Nephrol. 2006;17:2363–2367. doi: 10.1681/ASN.2006060610. [DOI] [PubMed] [Google Scholar]
- 12.Dreisbach AW, Japa S, Gebrekal AB, Mowry SE, Lertora JJ, Kamath BL, Rettie AE. Cytochrome P4502C9 activity in end-stage renal disease. Clin Pharmacol Ther. 2003;73:475–477. doi: 10.1016/s0009-9236(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 13.Dowling TC, Briglia AE, Fink JC, Hanes DS, Light PD, Stackiewicz L, Karyekar CS, Eddington ND, Weir MR, Henrich WL. Characterization of hepatic cytochrome p4503A activity in patients with end-stage renal disease. Clin Pharmacol Ther. 2003;73:427–434. doi: 10.1016/s0009-9236(03)00056-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim YG, Shin JG, Shin SG, Jang IJ, Kim S, Lee JS, Han JS, Cha YN. Decreased acetylation of isoniazid in chronic renal failure. Clin Pharmacol Ther. 1993;54:612–620. doi: 10.1038/clpt.1993.198. [DOI] [PubMed] [Google Scholar]
- 15.Manley HJ, Garvin CG, Drayer DK, Reid GM, Bender WL, Neufeld TK, Hebbar S, Muther RS. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant. 2004;19:1842–1848. doi: 10.1093/ndt/gfh280. [DOI] [PubMed] [Google Scholar]
- 16.Joy MS, Frye RF, Stubbert K, Brouwer KR, Falk RJ, Kharasch ED. Use of enantiomeric bupropion and hydroxybupropion to assess CYP2B6 activity in glomerular kidney diseases. J Clin Pharmacol. 2010;50:714–720. doi: 10.1177/0091270009353031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita K. Food-drug interactions via human cytochrome P450 3A (CYP3A) Drug Metabol Drug Interact. 2004;20:195–217. doi: 10.1515/dmdi.2004.20.4.195. [DOI] [PubMed] [Google Scholar]
- 18.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 19.Nolin TD, Frye RF, Le P, Sadr H, Naud J, Leblond FA, Pichette V, Himmelfarb J. ESRD impairs nonrenal clearance of fexofenadine but not midazolam. J Am Soc Nephrol. 2009;20:2269–2276. doi: 10.1681/ASN.2009010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cefali EA, Poynor WJ, Sica D, Cox S. Pharmacokinetic comparison of flurbiprofen in end-stage renal disease subjects and subjects with normal renal function. J Clin Pharmacol. 1991;31:808–814. doi: 10.1002/j.1552-4604.1991.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 21.Stanton ML, Joy MS, Frye RF. Validation and application of a liquid chromatography-tandem mass spectrometric method for quantification of the drug transport probe fexofenadine in human plasma using 96-well filter plates. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:497–501. doi: 10.1016/j.jchromb.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis D, Buffone GJ. Protein clearances and selectivity determinations in childhood nephrosis: a reappraisal. Clin Chem. 1981;27:1397–1400. [PubMed] [Google Scholar]
- 23.Joy MS, Hilliard T, Hu Y, Hogan SL, Dooley MA, Falk RJ, Smith PC. Pharmacokinetics of mycophenolic acid in patients with lupus nephritis. Pharmacotherapy. 2009;29:7–16. doi: 10.1592/phco.29.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joy MS, Gipson DS, Dike M, Powell L, Thompson A, Vento S, Eddy A, Fogo AB, Kopp JB, Cattran D, Trachtman H. Phase I trial of rosiglitazone in FSGS: I. Report of the FONT Study Group. Clin J Am Soc Nephrol. 2009;4:39–47. doi: 10.2215/CJN.02310508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joy MS, Hilliard T, Hu Y, Hogan SL, Wang J, Falk RJ, Smith PC. Influence of clinical and demographic variables on mycophenolic acid pharmacokinetics in antineutrophil cytoplasmic antibody-associated vasculitis. Ann Pharmacother. 2009;43:1020–1027. doi: 10.1345/aph.1L699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemma GL, Wang Z, Hamman MA, Zaheer NA, Gorski JC, Hall SD. The effect of short- and long-term administration of verapamil on the disposition of cytochrome P450 3A and P-glycoprotein substrates. Clin Pharmacol Ther. 2006;79:218–230. doi: 10.1016/j.clpt.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Tahara H, Kusuhara H, Fuse E, Sugiyama Y. P-glycoprotein plays a major role in the efflux of fexofenadine in the small intestine and blood-brain barrier, but only a limited role in its biliary excretion. Drug Metab Dispos. 2005;33:963–968. doi: 10.1124/dmd.105.004192. [DOI] [PubMed] [Google Scholar]
- 28.Xie R, Tan LH, Polasek EC, Hong C, Teillol-Foo M, Gordi T, Sharma A, Nickens DJ, Arakawa T, Knuth DW, Antal EJ. CYP3A and P-glycoprotein activity induction with St. John's Wort in healthy volunteers from 6 ethnic populations. J Clin Pharmacol. 2005;45:352–356. doi: 10.1177/0091270004273320. [DOI] [PubMed] [Google Scholar]
- 29.Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushima S, Maeda K, Ishiguro N, Igarashi T, Sugiyama Y. Investigation of the inhibitory effects of various drugs on the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2008;36:663–669. doi: 10.1124/dmd.107.017814. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, Sugiyama Y. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005;33:1477–1481. doi: 10.1124/dmd.105.004622. [DOI] [PubMed] [Google Scholar]
- 32.Tian X, Swift B, Zamek-Gliszczynski MJ, Belinsky MG, Kruh GD, Brouwer KL. Impact of basolateral multidrug resistance-associated protein (Mrp) 3 and Mrp4 on the hepatobiliary disposition of fexofenadine in perfused mouse livers. Drug Metab Dispos. 2008;36:911–915. doi: 10.1124/dmd.107.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian X, Zamek-Gliszczynski MJ, Li J, Bridges AS, Nezasa K, Patel NJ, Raub TJ, Brouwer KL. Multidrug resistance-associated protein 2 is primarily responsible for the biliary excretion of fexofenadine in mice. Drug Metab Dispos. 2008;36:61–64. doi: 10.1124/dmd.107.017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol. 1998;45:525–538. doi: 10.1046/j.1365-2125.1998.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hooper WD, Bochner F, Eadie MJ, Tyrer JH. Plasma protein binding of diphenylhydantoin. Effects of sex hormones, renal and hepatic disease. Clin Pharmacol Ther. 1974;15:276–282. doi: 10.1002/cpt1974153276. [DOI] [PubMed] [Google Scholar]
- 36.Reidenberg MM, Drayer DE. Alteration of drug-protein binding in renal disease. Clin Pharmacokinet. 1984;9(Suppl 1):18–26. doi: 10.2165/00003088-198400091-00003. [DOI] [PubMed] [Google Scholar]
- 37.Lown KS, Thummel KE, Benedict PE, Shen DD, Turgeon DK, Berent S, Watkins PB. The erythromycin breath test predicts the clearance of midazolam. Clin Pharmacol Ther. 1995;57:16–24. doi: 10.1016/0009-9236(95)90261-9. [DOI] [PubMed] [Google Scholar]
- 38.Lee JI, Chaves-Gnecco D, Amico JA, Kroboth PD, Wilson JW, Frye RF. Application of semisimultaneous midazolam administration for hepatic and intestinal cytochrome P450 3A phenotyping. Clin Pharmacol Ther. 2002;72:718–728. doi: 10.1067/mcp.2002.129068. [DOI] [PubMed] [Google Scholar]
- 39.Burstein AH, Reiss WG, Kantor E, Anderson GD. Cytochrome P450 3A4 activity in premenopausal and postmenopausal women, based on 6-beta-hydroxycortisol:cortisol ratios. Pharmacotherapy. 1998;18:1271–1276. [PubMed] [Google Scholar]
- 40.Aitken AE, Richardson TA, Morgan ET. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol. 2006;46:123–149. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- 41.Morgan ET. Regulation of cytochrome p450 by inflammatory mediators: why and how? Drug Metab Dispos. 2001;29:207–212. [PubMed] [Google Scholar]
- 42.Frassetto LA, Poon S, Tsourounis C, Valera C, Benet LZ. Effects of uptake and efflux transporter inhibition on erythromycin breath test results. Clin Pharmacol Ther. 2007;81:828–832. doi: 10.1038/sj.clpt.6100148. [DOI] [PubMed] [Google Scholar]