Abstract
Objective
Natural Killer (NK) cells are important in innate immune responses to bacterial as well as viral pathogens. HIV-1 infection is associated with opportunistic bacterial infections and with microbial translocation, but the nature of the NK cell response to bacteria during HIV infection has not been studied extensively. The objective of this study was to compare NK cell responses to bacteria in HIV-infected versus uninfected individuals.
Methods
Multi-color flow cytometry was used to evaluate the ability of blood NK cell subsets (CD56+CD16-, CD56+CD16+, and CD56-CD16+) from treated, virally suppressed and untreated viremic subjects with chronic HIV-1 infection, as well as uninfected controls, to secrete IFN-γ in response to in vitro stimulation of peripheral blood mononuclear cells with heat-killed commensal Escherichia coli or pathogenic Salmonella typhimurium.
Results
All three NK cell subsets produced IFN-γ in response to bacteria, but CD56-CD16+ NK cells were least responsive. Untreated HIV-1 infected donors had increased frequencies of CD56-CD16+ NK cells and lower overall frequencies of IFN-γ-producing NK cells responding to E. coli and S. typhimurium than NK cells from uninfected donors. These NK cell defects were not fully restored in ART-treated donors. Monocytes were necessary for NK cells to respond to bacteria, but the HIV-associated defect was intrinsic to NK cells since addition of normal monocytes did not restore IFN-γ production in response to bacteria.
Conclusions
Functional defects and numeric alterations of NK cell subsets lead to decreased frequencies of bacteria-reactive, IFN-γ-producing NK cells in HIV-1 infected subjects, even those on ART.
Keywords: Natural Killer Cells, HIV-1, bacteria, monocytes, cytokine production
Introduction
Natural killer (NK) cells are innate immune cells found throughout the body in both lymphoid and non-lymphoid compartments that contribute to the first lines of defense against invading pathogens.1,2 NK cell responses are driven by finely tuned interactions between NK cell-associated inhibitory and activating receptors and include a potent, fast-acting cytotoxic capability to directly kill infected cells but not healthy normal cells. In addition, NK cells can be activated by cytokines including IL-12 in combination with IL-15 or IL-18, to produce pro-inflammatory cytokines such as TNF-α and IFN-γ and thereby also impact antigen-presenting cell (APC) function and induction of adaptive immune responses.1,2
Human blood NK cell subsets are identified based on differential expression of the surface markers CD56 and CD16 with the vast majority (>90%) of blood NK cell subset defined as CD56+/dimCD16+.2,3 Traditionally, this population of NK cells is considered to be the predominant cytotoxic subset,2,4 but recent studies have indicated that CD56dim NK cells are also capable of cytokine production.5-7 A smaller fraction within the blood, approximately 10% of total NK cells, express high levels of CD56 (CD56bright), but lack expression of CD16 and produce cytokines in response to stimulation by cytokines.2-4,6 A third, typically minor NK cell subset lacks expression of CD56, but maintains CD16. Expansion of this particular NK cell subset has been observed in a number of chronic viral infections, including HIV-1 and HCV 8-10 and is less functional compared to the other NK cell subsets.11
The anti-tumor and anti-viral properties of NK cells have long been known, but NK cells also play a prominent role in anti-bacterial immune responses through an ability to directly lyse infected cells as well as providing early sources of various pro-inflammatory cytokines.12 The importance of these innate immune cells for controlling bacterial infections in humans is uniquely demonstrated by the increased susceptibility of humans with NK cell deficiencies to multiple types of bacterial infections.13 Intrinsic and extrinsic factors contribute to activation of NK cells in response to bacterial challenge. Early studies demonstrated an ability of human NK cells to lyse bacteria-infected Hela cells14 as well as Legionella pneumophilia-15 and Mycobacterium avium-infected monocytes.16 Bacteria-induced IFN-γ production by NK cells has been demonstrated in response to a number of pathogenic strains of bacteria including Staphylococcus aureus,17,18 Helicobacter pylori,19,20 Escherichia coli20 and M. tuberculosis.21-23 Moreover, NK cells can also respond to non-pathogenic bacteria including non-pathogenic E. coli and strains of lactobacillus by upregulating activation markers, producing IFN-γ, and increasing cytolytic activity.17,24-27 Direct activation of NK cells by bacterial products occurs through expression of specific bacterial Toll-like Receptors (TLRs) including TLR2, TLR4 and TLR528-34 whereas indirect activation occurs via accessory cells, such as dendritic cells (DC) or monocytes, typically in response to cytokines produced by the APC themselves such as IL-12 in conjunction with IL-15 or IL-18.28,30,35-38
Much of the work addressing NK cell function during HIV-1 infection has focused on the role of NK cells in anti-viral immunity, and it is not known whether the ability of NK cells to respond to bacteria is compromised during chronic HIV-1 infection. This question is important as dysfunctional anti-bacterial NK cell responses may, in part, contribute to the increased prevalence of bacteria-associated opportunistic infections39 or the high incidence of co-infection with M. tuberculosis in immune-compromised, HIV-1-infected individuals.40 The anti-bacterial response of NK cells may also be impacted by the increase in HIV-associated microbial translocation41 either by inducing NK cells to produce pro-inflammatory cytokines in vivo and thus contributing to a state of chronic immune activation or, conversely, by leading to defective bacteria-associated NK cell responses through overstimulation or exhaustion. To address these possibilities, we investigated the cytokine responses of peripheral blood NK cells to commensal and pathogenic whole bacteria in antiretroviral therapy (ART)-treated and untreated subjects with chronic HIV-1 infection.
Materials and Methods
Study Participants
Blood samples were obtained from 40 HIV-1 infected subjects who were receiving care at the University of Colorado Infectious Disease Group Practice, University of Colorado Hospital (Aurora, CO). Blood samples were also obtained from 24 healthy adults, self-identifying as HIV-1 uninfected, who served as normal controls. HIV-1 infected subjects were either untreated with plasma viremia (ART-naïve or had not been on ART for at least one year at the time of screening; “untreated”; n=23) or were receiving ART for more than 2 years with suppression of plasma viral load to <48 copies HIV-1 RNA/ml at the time of screening (“treated”, n=17). All untreated HIV-1 infected patients were chronically infected and showed no signs of acute illness at the time of enrollment into the study. The clinical characteristics of the cohorts are detailed in Table 1. All study subjects participated voluntarily and gave written, informed consent. This study was approved by the Colorado Multiple Institutional Review Board (COMIRB) at the University of Colorado Anschutz Medical Campus.
Table 1. Subject Characteristics.
| Uninfected (n=24) | Viremic, HIV-infected (n=23) | ART-treated, HIV-infected (n=17) | |
|---|---|---|---|
| Age (yrs) | 31.5 (22-64) | 36 (24-58) | 42 (24-64) |
| Gender (Male, Female) | 11, 13 | 12, 11 | 10, 7 |
| CD4 T cell count (cells/μl) | n/d | 360 (132-792)a | 684 (180-1400)b |
| Plasma viral load (HIV-1 RNA/ml) | n/a | 38.500 (11,000-834,000) | <48 |
Values are shown as median (range). ART: anti-retroviral therapy. All treated subjects have been receiving ART for longer than 2 years. n/d: not determined, n/a: not applicable.
13% of the untreated subjects had CD4 counts below 200 cells/μl;
P=0.001.
Isolation of human PBMC
Peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood using standard Ficoll-Hypaque (Amersham Biosciences, Piscataway, NJ) density gradient centrifugation and were cryopreserved and stored in liquid nitrogen as detailed elsewhere.42,43
Whole bacterial preparations
E. coli (no. 25922; ATCC, Manassas, VA) and Salmonella typhimurium (no. 35986, ATCC), were grown, heat-inactivated and stored as previously described.43,44
Surface and intracellular flow cytometry (IFC) staining assays, acquisition and analysis
Standard flow cytometry staining protocols for surface markers and intracellular IFN-γ are detailed elsewhere.44-46 NK cells were identified within CD3- lymphocytes (PE-Texas Red CD3, ECD; Beckman Coulter, Fullerton, CA) using V450 or PE-Cy5 CD56 and APC-H7 or AF700 CD16 (both BD Biosciences, San Jose, CA). AF700 IFN-γ (BD Biosciences) was used to evaluate frequencies of IFN-γ+ cells following in vitro stimulation. Monocytes were evaluated using V450 CD14 and mDC evaluated using FITC Lineage (CD3, CD14, CD16, CD19, CD20, CD56), APC-Cy7 HLA-DR, PE-Cy5 CD11c (all BD Biosciences) and APC CD123 (Miltenyi Biotec, Auburn, CA) as previously described.42,43,47 All flow cytometry data was acquired on an LSRII Flow Cytometer (BD Biosciences) and analyzed using BD FACSDiva software version 6.1.2 (BD Biosciences).
NK cell subsets were identified by expression of CD56 and CD16. In our initial studies, we noted a reduction in the fraction of CD56brightCD16- NK cells and a corresponding increase in CD56dimCD16- NK cells in culture relative to pre-culture frequencies (Figure S1, A and B, Supplemental Digital Content). Overall CD56 expression levels on CD56+CD16- NK cells were also reduced following both culture and stimulation (Figure S1, C, Supplemental Digital Content). Thus, going forward we utilized a previously published gating strategy that included all CD56+CD16- cells48 rather than gating only on CD56bright NK cells in order to avoid exclusion of CD56bright NK cells that subsequently decreased CD56 expression during the in vitro culture period. A representative example of the gating strategy used is shown in Figure S1, D, Supplemental Digital Content.
In vitro stimulation of PBMC
PBMC were thawed and either assessed for baseline percentages of NK cells by flow cytometry or cultured in RPMI (Invitrogen, Carlsbad, CA) + 10% human AB serum (Gemini Bioproducts, West Sacramento, CA) + 1% penicillin-streptomycin-L-glutamine (Sigma-Aldrich, St Louis, MO) (complete media; CM) with or without heat-inactivated bacteria (6 bacteria: 1 PBMC) for 4 hours. To accommodate early production of IFN-γ as reported by others,5 Brefeldin A (1μg/ml; BD Biosciences) was then added for the remainder of the culture (12-18 hrs).
In vitro stimulation of purified NK cells and mDC or monocyte-depleted cultures
NK cells were isolated from PBMC by negative selection using a magnetic bead kit (NK cell Isolation Kit; Miltenyi Biotec) as per the manufacturer's instructions. NK cells accounted for 90.8-97.5% (n=3) of the isolated cells.
PBMC were depleted of BDCA-1+ and BDCA-3+ mDC or CD14+ monocytes using positive magnetic bead selection protocols (Anti-biotin MicroBeads or CD14 MicroBeads respectively; both from Miltenyi Biotec) as previously described.43 PBMC were depleted of mDC (defined as Lineage-HLA-DR+CD123loCD11c+)47 by 86.8-97.0% (n=6) and depleted of monocytes by 87.9-98.3% (n=6).
Contact-dependence assays
Monocytes were separated from total PBMC using CD14 Microbeads and accounted for 92.9-98.4% of isolated cells. Monocyte-depleted PBMC were plated the bottom wells of a 24-well Costar transwell plate (Corning Inc, Corning, NY) and then monocytes added onto membrane inserts (0.4μm pore size) placed into wells. To account for any effect of attached microbeads on CD14+ monocyte function, the same number of isolated monocytes were mixed back with the monocyte-depleted PBMC and used as control PBMC (final CD14+ percentage within control PBMC: 10.8-22.9%, n=5). E. coli was added either directly to the monocytes in the inserts or to the wells containing control PBMC for 4hrs prior to the addition of Brefeldin A. After overnight culture, cells were collected from the bottom wells and frequencies of IFN-γ+ NK cells evaluated by IFC assay.
Allogeneic Monocyte–NK cell co-cultures
In order to have the same monocytes used for stimulation of NK cells from multiple donors, monocytes were isolated from one uninfected donor using CD14+ Microbeads (final monocyte purity: 99.3%), cryopreserved and stored in liquid nitrogen. NK cells were isolated from uninfected (n=4) and HIV-1 infected (n=4) donors as described above. Monocytes were then thawed and cultured 1:1 with purified NK cells in CM with or without E. coli (6:1) for 19-22hrs and culture supernatants collected and stored at -20°C. IFN-γ production within culture supernatants was evaluated by ELISA (eBioscience, San Diego, CA).
Statistical Analysis
For non-parametric analysis, comparisons between independent groups were made using the Mann-Whitney t test and the Friedman test with a Multiple Dunn's Comparison test for matched-paired comparisons across multiple groups. To determine differences between groups of paired data, the Wilcoxon matched-pairs signed rank test was performed. Correlations between variables were assessed using the Spearman test. For small sample sizes (n<7), comparisons between independent groups were made using the Unpaired t test. The Paired t-test was used for analysis of matched paired groups and the Repeated Measures ANOVA with a Dunnett's Multiple Comparison test used for matched-paired comparisons across multiple groups. In all analyses, a P value <0.05 was considered significant. All statistical analyses were performed using GraphPad Prism Version 6 for Windows (GraphPad Software, San Diego, CA).
Results
NK cells that produce IFN-γ in response to commensal bacteria are reduced in HIV-1 infected individuals
Frequencies of total NK cells (CD56±CD16±) producing IFN-γ were determined within PBMC with and without stimulation with heat-killed E. coli. In the absence of exogenous stimulation, low frequencies of IFN-γ+ NK cells were observed in all study groups without statistical differences observed between them (data not shown). However, frequencies of IFN-γ+ NK cells in response to stimulation of PBMC with E. coli were significantly reduced when the cells were obtained from HIV-infected donor groups as opposed to when the PBMC were from uninfected subjects (Fig. 1A).
Fig. 1. Percentages of IFN-γ-expressing NK cells are reduced in HIV-1 infected subjects following in vitro stimulation of PBMC with commensal E. coli.

(A) Percentages of IFN-γ+ NK cells as a fraction of total NK cells following in vitro culture of PBMC from uninfected donors (n=20), untreated HIV-1 infected (n=20) and ART-treated (n=15) donors with commensal E. coli. (B) Percentages of IFN-γ+ NK cells within each NK cell subset following in vitro culture of PBMC from uninfected donors (n=20) with commensal E. coli. (C) Percentages of IFN-γ+ NK cells within each NK cell subset following in vitro culture of PBMC from uninfected donors (n=20), untreated HIV-1 infected (n=20) and ART-treated (n=15) donors with commensal E. coli. For all cytokine frequency analysis, values are expressed as percentages of IFN-γ+ cells following stimulation with E. coli minus background IFN-γ+ percentages detected in unstimulated cultures. For all graphs, lines represent median values. Comparisons between multiple groups were performed using the Kruskall-Wallis test with comparisons conducted between the cohorts when P<0.05 using the Dunn's Multiple Comparison test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
For all three NK cell subsets, in vitro stimulation of uninfected donor PBMC with E. coli resulted in IFN-γ+production (Fig. 1B). The CD56+CD16- NK cells and CD56+CD16+ NK cells had similarly high percentages of IFN-γ+ cells in response to E. coli with most IFN-γ production coming from the CD56dim populations, regardless of CD16 expression, as has been previously reported.5-7 Fractions of CD56+CD16- and CD56+CD16+ NK cells producing IFN-γ were significantly greater than those of the CD56-CD16+ NK cell subset (Fig. 1B).
NK subset IFN-γ responses to E. coli were next compared between untreated and ART-treated HIV-1-infected donors and uninfected control subjects (Fig. 1C). In PBMC from untreated donors, fewer NK cells in all three subsets produced IFN-γ following stimulation with E. coli than did NK cells from control donors. Among treated donors, the percentage of IFN-γ+ CD56+CD16- NK cells did not statistically differ from uninfected controls. In contrast, the percentage of IFN-γ+ NK cells within the CD56+CD16+ and CD56-CD16+ NK cell subsets in treated donors were statistically lower than in controls and were not significantly different to the untreated donors, suggesting that these responses had not normalized despite viral suppression on ART.
Previous studies have shown that the frequencies of blood NK cell subsets are altered during HIV infection.11,49-51 To determine whether HIV-1-associated decreases in E. coli-induced IFN-γ+ NK cell responses were simply a reflection of altered blood NK cell frequencies, we measured NK cell subset frequencies directly ex vivo (prior to stimulation). Percentages of CD56+CD16- NK cells were similar across all cohorts (Figure S2, Supplemental Digital Content). CD56+CD16+ NK cells were reduced and CD56-CD56+ NK cells increased in untreated subjects compared to uninfected controls whereas frequencies of those NK cell subsets in treated subjects did not differ statistically from those of controls. Despite the significant increase in frequency of CD56-CD16+ NK cells in untreated subjects (Figure S2, Supplemental Digital Content), there was no concurrent increase in the fraction of CD56-CD16+ NK cells producing IFN-γ (Fig. 1C) suggesting the expansion of this subset during untreated HIV-1 infection was dominated by CD56-CD16+ NK cells that failed to produce IFN-γ in response to E. coli. Moreover, despite the normalization of CD56+CD16+ and CD56-CD16+ NK cell numbers in treated subjects, the frequency of bacteria-reactive IFN-γ+ NK cells among these subsets was reduced (Fig. 1C). Taken together, these observations suggest that altered baseline frequencies of NK cells do not fully account for the reduced frequencies of bacteria-reactive IFN-γ+ NK cells in HIV infection.
NK cell IFN-γ responses to pathogenic bacteria are also reduced within HIV-1 infected individuals
NK cell responses within PBMC to pathogenic S. typhimurium were evaluated using cells from uninfected and HIV-1 infected donors. In uninfected subjects, S. typhimurium induced statistically higher frequencies of IFN-γ+ NK cells than did stimulation with E. coli (Fig. 2A). Reduced percentages of IFN-γ-producing NK cells were detected in all three NK cell subsets from untreated subjects following stimulation with S. typhimurium relative to NK cell subsets within S. typhimurium stimulated PBMC from uninfected donors (Fig. 2B). Among treated donors, the percentage of IFN-γ+ CD56+CD16+ and CD56-CD16+ NK cells in response to S. typhimurium were also lower than for the same NK cell subset from uninfected subjects (Fig. 2B).
Fig. 2. Percentages of IFN-γ-expressing NK cells are reduced in HIV-1 infected subjects following in vitro stimulation of PBMC with pathogenic bacteria.
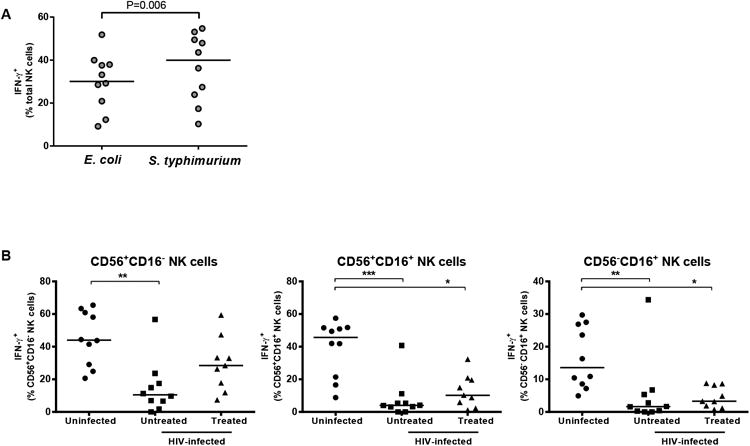
(A) Percentages of IFN-γ+ NK cells following in vitro culture of PBMC from uninfected donors (n=10) with E. coli or S. typhimurium. B) Percentages of IFN-γ+ NK cells within each NK cell subset following in vitro culture of PBMC from uninfected donors (n=10), untreated HIV-1 infected (n=10) and ART-treated (n=9) donors with S. typhimurium. For all graphs, values are expressed as percentages of IFN-γ+ cells following stimulation with E. coli or S. typhimurium minus background IFN-γ+ percentages detected in unstimulated cultures. Lines represent median values. Comparisons between two related groups were performed using the Wilcoxon matched-pairs signed rank test and between multiple groups using the Kruskall-Wallis test with comparisons conducted between the cohorts when P<0.05 using the Dunn's Multiple Comparison test. *P<0.05, **P<0.01, ***P<0.001.
NK cell IFN-γ production in response to bacteria requires monocytes in a contact-dependent manner
Since NK cells produced IFN-γ following stimulation with bacteria we wanted to determine whether the bacteria acted directly on the NK cells or through the bacteria action on accessory cells. Stimulation of purified normal donor NK cells with E. coli failed to induce significant IFN-γ production relative to NK cells stimulated within PBMC (Fig. 3A). This finding indicates that the ability of bacteria to stimulate NK cell production of IFN-γ was not due to direct interaction of the bacteria with NK cells. Since the bacteria did not stimulate the NK cells directly we wanted to determine if mDC or monocytes were required for bacteria-induced NK cell production of IFN-γ, PBMC were depleted of either mDC or monocytes prior to adding the bacteria. The percent of NK cells producing IFN-γ within the accessory cell-depleted PBMC and total PBMC was compared (Fig. 3B). Removal of monocytes from PBMC resulted in a significant decrease in the frequency of IFN-γ-producing NK cells following stimulation with E. coli, whereas minimal differences were observed in mDC-depleted PBMC cultures (Fig. 3B). Following monocyte depletion, the percent of IFN-γ+ cells within the CD56+CD16-, CD56+CD16+ and CD56-CD16+ NK cell subsets was decreased, on average, by 41.1% ± 18.4%, 86.2% ± 6.2%, and 79.2% ± 12.5% respectively.
Fig. 3. Monocytes are required to induce NK cell responses to commensal E. coli, but monocytes from an uninfected donor do not normalize IFN-γ production by NK cells from HIV-1 infected donors.

(A) Percentages of IFN-γ+ NK cells following in vitro culture of either PBMC or purified NK cells from uninfected donors (n=3) with commensal E. coli. Responses within total PBMC and purified NK cells are shown for each donor with the overall decrease in the fraction IFN-γ+ within each condition (total PBMC versus purified NK cells) highlighted. (B) Percentages of IFN-γ+ NK cells following in vitro culture of either total PBMC, PBMC depleted of mDC or PBMC depleted of monocytes, from uninfected donors (n=6) with commensal E. coli. (C) Percentages of IFN-γ+ NK cells following in vitro culture of total PBMC or PBMC with monocytes separated from the monocyte-depleted PBMC using transwell inserts (n=5). (D) IFN-γ levels within culture supernatants when purified NK cells from either uninfected donors (n=4) or untreated, HIV-1 infected donors (n=4) were cultured 1:1 with purified monocytes from one allogeneic, uninfected donor and stimulated with or without E. coli (6 E. coli: 1 NK + monocyte). Values are expressed as percentages of IFN-γ+ NK cells (A-C) or amounts of IFN-γ within culture supernatants (D) following stimulation with E. coli minus background IFN-γ detected in unstimulated cultures with bar graphs displaying mean ± SEM values (B-D). Statistical analysis was performed for (B) using Repeated Measures ANOVA test with comparisons to Total PBMC conducted when P<0.05 using the Dunnett's Multiple Comparison Test (*P<0.05), for (C) using the Paired t test and for (D) using the Unpaired t test.
To determine whether contact between monocytes and NK cells was required, a transwell system was used to separate bacteria-stimulated monocytes from NK cells in monocyte-depleted PBMC. Fewer IFN-γ+ NK cells were detected in response to E. coli stimulation when the stimulated monocytes were separated than when they were in contact with NK cells during stimulation (Fig. 3C). Separation of bacteria-stimulated monocytes resulted in an 82.2% ± 2.9% decrease in the percent of IFN-γ+ cells within the CD56+CD16- NK cell subset and a decrease of 92.8% ± 2.5% and 92.0% ± 3.1% within the CD56+CD16+ and CD56-CD16+ NK cell subsets respectively. These results show that contact between monocytes and NK cells is required for IFN-γ production by NK cells in response to bacteria.
IFN-γ production by NK cells from HIV-1 infected donors is not restored by exposure to uninfected donor monocytes
To determine whether the defect in NK cell IFN-γ production observed in HIV-1 infected individuals was due to dysfunction of monocytes or NK cells, monocytes were isolated from an allogeneic, uninfected donor and cultured with purified NK cells from either uninfected or untreated, HIV-1 infected donors in the presence of E. coli, and IFN-γ+levels measured in culture supernatant. E. coli stimulation of purified monocytes did not induce IFN-γ production (data not shown). Normal donor monocytes stimulated normal donor NK cells to produce IFN-γ (Fig. 3D). However, in the presence of normal donor monocytes, levels of IFN-γ that were detected in culture supernatants of NK cells from HIV-1 infected donors were reduced relative to culture supernatants from uninfected donor NK cells (Fig. 3D).
Discussion
Numeric and functional NK cell defects have been observed during both acute and chronic HIV-1 infection and may contribute to HIV-1 pathogenesis.11,49-52 Specifically, HIV-1-associated changes in NK cell phenotype, including altered expression of activating and inhibitory receptors, have been associated with impaired cytotoxicity against NK-sensitive cell lines, reduced cytokine production in response to known NK cell activation-inducing cytokines, as well as defective ADCC responses.9,11,53-59 Impaired ability of NK cells to kill HIV-infected cells is likely mediated through HIV-induced selective alteration of MHC Class I expression in conjunction with modulating ligands important in triggering NK cell cytotoxic responses.60-62 Long term ART (greater than 2 years) typically results in restoration of NK cell phenotype and function.11,54,63,64. However, some studies have demonstrated a persistent impairment in IFN-γ production in treated subjects despite normalization of phenotype and cytotoxic function.65,66 In addition, a recent study demonstrated that NK cells remained in an activated state, defined by co-expression of HLA-DR and CD38, despite subjects having received ART for a median duration of 11.5yrs.67 Moreover, NK cell inhibitory or activation receptors generally do not return to normal levels when viremia is suppressed by ART, although in some cases normalization occurs after prolonged viral suppression.59,68
In this study, we utilized an in vitro assay to evaluate production of IFN-γ by NK cells in response to bacterial stimulation of PBMC. In agreement with previous studies where isolated CD56dim NK cells produced IFN-γ in response to receptor-mediated and cytokine-mediated activation,5-7 the majority of bacteria-reactive IFN-γ-producing NK cells were found within CD56dim NK cells, irrespective of CD16 expression. We further demonstrate that chronic, untreated HIV-1 infection results in impairment of NK cells to produce IFN-γ in response to both commensal and pathogenic bacteria. Furthermore, limited functional improvement was observed in NK cells from subjects on long-term ART, despite evidence of effective viral suppression and improved CD4 counts. Although we observed relative changes in the frequency of blood NK cell subsets within HIV-infected subjects prior to stimulation, these changes could only partly account for the reduced numbers of IFN-γ+ NK cells. Induction of IFN-γ was dependent on contact with monocytes, yet HIV-associated NK cell function was not restored by exposure to normal monocytes, suggesting that at least a component of the dysfunction is an intrinsic NK cell defect. We believe that our study is the first to show that anti-bacterial NK cell responses are impacted by chronic HIV-1 infection with limited restoration in function following ART.
An important finding of our study is that cell-to-cell contact is required between NK cells and monocytes to induce NK cell-associated IFN-γ production in response to bacterial stimulation. Crosstalk between DC and NK cells is well described,69 but an understanding of the interactions between NK cells and monocytes/macrophages is only beginning to emerge.70 In one study, interactions between NK cell 2B4 and CD48 expressed by low dose LPS-stimulated human macrophages were shown to be necessary to induce NK cell proliferation and IFN-γ production.71 Increased NK cell IFN-γ secretion has also been observed following NKp80 activation via the myeloid-specific AICL expressed by LPS-treated monocytes.72 Further, LPS stimulation of human monocytes induced upregulation of MICA, the ligand for NKG2D, resulting in IFN-γ production by NK cells37 suggesting that NKG2D may also be an important activating receptor permitting the induction of bacteria-induced IFN-γ by NK cells. While some studies observed only minimal differences in NKG2D expression by NK cells in HIV-1-infected individuals,11 a recent study found increased levels of serum MICA in subjects with chronic HIV infection and associated this with reduced NKG2D expression on NK cells and aberrant NKG2D-mediated recognition of target cells.52 This latter study raises the possibility that increased levels of serum MICA, potentially secreted by bacteria-stimulated monocytes, and altered NKG2D expression may also result in reduced bacteria-associated NK cell activation and IFN-γ production.
Decreased anti-bacterial responses by NK cells in HIV-infected individuals may also result from NK cell exhaustion due to over stimulation following exposure to opportunistic viral and bacterial pathogens as well as exposure to translocated bacteria and bacterial products as has been shown to occur during HIV infection.41 Other studies have shown increased PD-1 expression on blood NK cells from both viremic and aviremic HIV-1 infected donors.73 Given that increased PD-1 expression on T cells during chronic HIV-1 infection has been implicated in T cell exhaustion,74,75 elevated expression of PD-1 on NK cells may indicate a similar functional phenotype and contribute to reduced bacteria-responsiveness. To expand on our current pilot study, investigations are now underway to further address these potential mechanisms behind the HIV-associated defective bacterial NK cell responses.
Understanding the impact of in vivo HIV-1 infection on the anti-bacterial responses has clinical implications. It was recognized early in the HIV-1 epidemic that those infected with HIV-1 had a higher prevalence of bacterial infections.39,76 Although rates of bacterial infections have declined with the advent of ART, they remain elevated in areas with a high incidence of HIV-1 infection,39,77,78 and treatments of bacterial infections are now potentially complicated by the emergence of multi-drug resistant bacteria.79,80 Moreover, the role of NK cells in anti-bacterial immunity may take on more importance in bacterial diseases that are predominantly controlled through T cell mediated immunity, responses likely compromised in HIV-1 infected individuals. Indeed, NK cells from individuals with HIV-1 and pulmonary tuberculosis failed to produce IFN-γ when stimulated in vitro with live M. tuberculosis.81 Thus, understanding the mechanisms underlying HIV-1-associated NK cell dysfunction could aid in the development of therapies designed to enhance or restore innate immune responses.
Acknowledgments
We would like to thank the physicians, staff and patients in the Infectious Diseases Group Practice at the University of Colorado Hospital and the uninfected donors for their participation in our study. We would also like to thank Zachary Dong, Kirsten Miller, Zahra Kahn, Christina Briegleb, Spenser Hansen and Lydia Hostetler for assistance with recruiting study subjects. We thank Jennifer Manuzak, Lisa Rogers and Caleb Kelly for technical assistance.
Source of Funding: The work was supported by grants from the National Institute of Health (R01 DK088663, K24 AI074343).
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008 Aug 1;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends in immunology. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 3.Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs R, Hintzen G, Kemper A, et al. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. European journal of immunology. 2001;31:3121–3127. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.De Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56(dim)CD16+ NK cells as rapid producers of abundant IFN-gamma on activation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:728–732. doi: 10.1073/pnas.1012356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–2176. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juelke K, Killig M, Luetke-Eversloh M, et al. CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood. 2010;116:1299–1307. doi: 10.1182/blood-2009-11-253286. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez VD, Falconer K, Bjorkstrom NK, et al. Expansion of functionally skewed CD56-negative NK cells in chronic hepatitis C virus infection: correlation with outcome of pegylated IFN-alpha and ribavirin treatment. J Immunol. 2009;183:6612–6618. doi: 10.4049/jimmunol.0901437. [DOI] [PubMed] [Google Scholar]
- 9.Mavilio D, Lombardo G, Benjamin J, et al. Characterization of CD56-/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier UC, Owen RE, Taylor E, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. Journal of virology. 2005;79:12365–12374. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mavilio D, Benjamin J, Daucher M, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souza-Fonseca-Guimaraes F, Adib-Conquy M, Cavaillon JM. Natural killer (NK) cells in antibacterial innate immunity: angels or devils? Mol Med. 2012;18:270–285. doi: 10.2119/molmed.2011.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes and infection/Institut Pasteur. 2002;4:1545–1558. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 14.Klimpel GR, Niesel DW, Asuncion M, Klimpel KD. Natural killer cell activation and interferon production by peripheral blood lymphocytes after exposure to bacteria. Infection and immunity. 1988;56:1436–1441. doi: 10.1128/iai.56.6.1436-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchard DK, Stewart WE, 2nd, Klein TW, Friedman H, Djeu JY. Cytolytic activity of human peripheral blood leukocytes against Legionella pneumophila-infected monocytes: characterization of the effector cell and augmentation by interleukin 2. J Immunol. 1987;139:551–556. [PubMed] [Google Scholar]
- 16.Katz P, Yeager H, Jr, Whalen G, Evans M, Swartz RP, Roecklein J. Natural killer cell-mediated lysis of Mycobacterium-avium complex-infected monocytes. Journal of clinical immunology. 1990;10:71–77. doi: 10.1007/BF00917500. [DOI] [PubMed] [Google Scholar]
- 17.Haller D, Serrant P, Granato D, Schiffrin EJ, Blum S. Activation of human NK cells by staphylococci and lactobacilli requires cell contact-dependent costimulation by autologous monocytes. Clinical and diagnostic laboratory immunology. 2002;9:649–657. doi: 10.1128/CDLI.9.3.649-657.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshihara R, Shiozawa S, Fujita T, Chihara K. Gamma interferon is produced by human natural killer cells but not T cells during Staphylococcus aureus stimulation. Infection and immunity. 1993;61:3117–3122. doi: 10.1128/iai.61.8.3117-3122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafsi N, Voland P, Schwendy S, et al. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J Immunol. 2004;173:1249–1257. doi: 10.4049/jimmunol.173.2.1249. [DOI] [PubMed] [Google Scholar]
- 20.Yun CH, Lundgren A, Azem J, et al. Natural killer cells and Helicobacter pylori infection: bacterial antigens and interleukin-12 act synergistically to induce gamma interferon production. Infection and immunity. 2005;73:1482–1490. doi: 10.1128/IAI.73.3.1482-1490.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vankayalapati R, Garg A, Porgador A, et al. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2005;175:4611–4617. doi: 10.4049/jimmunol.175.7.4611. [DOI] [PubMed] [Google Scholar]
- 22.Vankayalapati R, Wizel B, Weis SE, et al. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol. 2002;168:3451–3457. doi: 10.4049/jimmunol.168.7.3451. [DOI] [PubMed] [Google Scholar]
- 23.Yoneda T, Ellner JJ. CD4(+) T cell and natural killer cell-dependent killing of Mycobacterium tuberculosis by human monocytes. American journal of respiratory and critical care medicine. 1998;158:395–403. doi: 10.1164/ajrccm.158.2.9707102. [DOI] [PubMed] [Google Scholar]
- 24.Cheon S, Lee KW, Kim KE, et al. Heat-killed Lactobacillus acidophilus La205 enhances NK cell cytotoxicity through increased granule exocytosis. Immunology letters. 2011;136:171–176. doi: 10.1016/j.imlet.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Fink LN, Zeuthen LH, Christensen HR, Morandi B, Frokiaer H, Ferlazzo G. Distinct gut-derived lactic acid bacteria elicit divergent dendritic cell-mediated NK cell responses. International immunology. 2007;19:1319–1327. doi: 10.1093/intimm/dxm103. [DOI] [PubMed] [Google Scholar]
- 26.Haller D, Blum S, Bode C, Hammes WP, Schiffrin EJ. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infection and immunity. 2000;68:752–759. doi: 10.1128/iai.68.2.752-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda K, Suzuki T, Shimada SI, Shida K, Nanno M, Okumura K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clinical and experimental immunology. 2006;146:109–115. doi: 10.1111/j.1365-2249.2006.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalifour A, Jeannin P, Gauchat JF, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104:1778–1783. doi: 10.1182/blood-2003-08-2820. [DOI] [PubMed] [Google Scholar]
- 29.Lauzon NM, Mian F, MacKenzie R, Ashkar AA. The direct effects of Toll-like receptor ligands on human NK cell cytokine production and cytotoxicity. Cellular immunology. 2006;241:102–112. doi: 10.1016/j.cellimm.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Marcenaro E, Ferranti B, Falco M, Moretta L, Moretta A. Human NK cells directly recognize Mycobacterium bovis via TLR2 and acquire the ability to kill monocyte-derived DC. International immunology. 2008;20:1155–1167. doi: 10.1093/intimm/dxn073. [DOI] [PubMed] [Google Scholar]
- 31.Mian MF, Lauzon NM, Andrews DW, Lichty BD, Ashkar AA. FimH can directly activate human and murine natural killer cells via TLR4. Molecular therapy: the journal of the American Society of Gene Therapy. 2010;18:1379–1388. doi: 10.1038/mt.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pisegna S, Pirozzi G, Piccoli M, Frati L, Santoni A, Palmieri G. p38 MAPK activation controls the TLR3-mediated up-regulation of cytotoxicity and cytokine production in human NK cells. Blood. 2004;104:4157–4164. doi: 10.1182/blood-2004-05-1860. [DOI] [PubMed] [Google Scholar]
- 33.Qiu F, Maniar A, Diaz MQ, Chapoval AI, Medvedev AE. Activation of cytokine-producing and antitumor activities of natural killer cells and macrophages by engagement of Toll-like and NOD-like receptors. Innate immunity. 2011;17:375–387. doi: 10.1177/1753425910372000. [DOI] [PubMed] [Google Scholar]
- 34.Sivori S, Falco M, Della Chiesa M, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Athie-Morales V, O'Connor GM, Gardiner CM. Activation of human NK cells by the bacterial pathogen-associated molecular pattern muramyl dipeptide. J Immunol. 2008;180:4082–4089. doi: 10.4049/jimmunol.180.6.4082. [DOI] [PubMed] [Google Scholar]
- 36.Goodier MR, Londei M. Lipopolysaccharide stimulates the proliferation of human CD56+CD3- NK cells: a regulatory role of monocytes and IL-10. J Immunol. 2000;165:139–147. doi: 10.4049/jimmunol.165.1.139. [DOI] [PubMed] [Google Scholar]
- 37.Kloss M, Decker P, Baltz KM, et al. Interaction of monocytes with NK cells upon Toll-like receptor-induced expression of the NKG2D ligand MICA. J Immunol. 2008;181:6711–6719. doi: 10.4049/jimmunol.181.10.6711. [DOI] [PubMed] [Google Scholar]
- 38.Lindgren A, Pavlovic V, Flach CF, Sjoling A, Lundin S. Interferon-gamma secretion is induced in IL-12 stimulated human NK cells by recognition of Helicobacter pylori or TLR2 ligands. Innate immunity. 2011;17:191–203. doi: 10.1177/1753425909357970. [DOI] [PubMed] [Google Scholar]
- 39.Nagappan V, Kazanjian P. Bacterial infections in adult HIV-infected patients. HIV clinical trials. 2005;6:213–228. doi: 10.1310/a3q4-uqqn-x9en-y4he. [DOI] [PubMed] [Google Scholar]
- 40.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;50(3):S201–207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 41.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clinical microbiology reviews. 2013;26:2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dillon SM, Friedlander LJ, Rogers LM, et al. Blood myeloid dendritic cells from HIV-1-infected individuals display a proapoptotic profile characterized by decreased Bcl-2 levels and by caspase-3+ frequencies that are associated with levels of plasma viremia and T cell activation in an exploratory study. Journal of virology. 2011;85:397–409. doi: 10.1128/JVI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manuzak J, Dillon S, Wilson C. Differential interleukin-10 (IL-10) and IL-23 production by human blood monocytes and dendritic cells in response to commensal enteric bacteria. Clinical and vaccine immunology: CVI. 2012;19:1207–1217. doi: 10.1128/CVI.00282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dillon SM, Manuzak JA, Leone AK, et al. HIV-1 infection of human intestinal lamina propria CD4+ T cells in vitro is enhanced by exposure to commensal Escherichia coli. J Immunol. 2012;189:885–896. doi: 10.4049/jimmunol.1200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dillon SM, Rogers LM, Howe R, et al. Human intestinal lamina propria CD1c+ dendritic cells display an activated phenotype at steady state and produce IL-23 in response to TLR7/8 stimulation. J Immunol. 2010;184:6612–6621. doi: 10.4049/jimmunol.1000041. [DOI] [PubMed] [Google Scholar]
- 46.Howe R, Dillon S, Rogers L, et al. Evidence for dendritic cell-dependent CD4(+) T helper-1 type responses to commensal bacteria in normal human intestinal lamina propria. Clin Immunol. 2009;131:317–332. doi: 10.1016/j.clim.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dillon SM, Robertson KB, Pan SC, et al. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunetta E, Fogli M, Varchetta S, et al. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood. 2009;114:3822–3830. doi: 10.1182/blood-2009-06-226332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 50.Hu PF, Hultin LE, Hultin P, et al. Natural killer cell immunodeficiency in HIV disease is manifest by profoundly decreased numbers of CD16+CD56+ cells and expansion of a population of CD16dimCD56- cells with low lytic activity. Journal of acquired immune deficiency syndromes and human retrovirology: official publication of the International Retrovirology Association. 1995;10:331–340. [PubMed] [Google Scholar]
- 51.Tarazona R, Casado JG, Delarosa O, et al. Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. Journal of clinical immunology. 2002;22:176–183. doi: 10.1023/a:1015476114409. [DOI] [PubMed] [Google Scholar]
- 52.Nolting A, Dugast AS, Rihn S, et al. MHC class I chain-related protein A shedding in chronic HIV-1 infection is associated with profound NK cell dysfunction. Virology. 2010;406:12–20. doi: 10.1016/j.virol.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ballan WM, Vu BA, Long BR, et al. Natural killer cells in perinatally HIV-1-infected children exhibit less degranulation compared to HIV-1-exposed uninfected children and their expression of KIR2DL3, NKG2C, and NKp46 correlates with disease severity. J Immunol. 2007;179:3362–3370. doi: 10.4049/jimmunol.179.5.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barker E, Martinson J, Brooks C, Landay A, Deeks S. Dysfunctional natural killer cells, in vivo, are governed by HIV viremia regardless of whether the infected individual is on antiretroviral therapy. AIDS. 2007;21:2363–2365. doi: 10.1097/QAD.0b013e3282f1d658. [DOI] [PubMed] [Google Scholar]
- 55.De Maria A, Fogli M, Costa P, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) European journal of immunology. 2003;33:2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 56.Fogli M, Costa P, Murdaca G, et al. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. European journal of immunology. 2004;34:2313–2321. doi: 10.1002/eji.200425251. [DOI] [PubMed] [Google Scholar]
- 57.Goodier MR, Mela CM, Steel A, Gazzard B, Bower M, Gotch F. NKG2C+ NK cells are enriched in AIDS patients with advanced-stage Kaposi's sarcoma. Journal of virology. 2007;81:430–433. doi: 10.1128/JVI.01567-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gregson JN, Steel A, Bower M, Gazzard BG, Gotch FM, Goodier MR. Elevated plasma lipopolysaccharide is not sufficient to drive natural killer cell activation in HIV-1-infected individuals. AIDS. 2009;23:29–34. doi: 10.1097/QAD.0b013e3283199780. [DOI] [PubMed] [Google Scholar]
- 59.Mela CM, Burton CT, Imami N, et al. Switch from inhibitory to activating NKG2 receptor expression in HIV-1 infection: lack of reversion with highly active antiretroviral therapy. AIDS. 2005;19:1761–1769. doi: 10.1097/01.aids.0000183632.12418.33. [DOI] [PubMed] [Google Scholar]
- 60.Bonaparte MI, Barker E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood. 2004;104:2087–2094. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 61.Jost S, Altfeld M. Evasion from NK cell-mediated immune responses by HIV-1. Microbes and infection/Institut Pasteur. 2012;14:904–915. doi: 10.1016/j.micinf.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ward J, Bonaparte M, Sacks J, et al. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–1214. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nature reviews Immunology. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 64.Parato KG, Kumar A, Badley AD, et al. Normalization of natural killer cell function and phenotype with effective anti-HIV therapy and the role of IL-10. AIDS. 2002;16:1251–1256. doi: 10.1097/00002030-200206140-00007. [DOI] [PubMed] [Google Scholar]
- 65.Azzoni L, Papasavvas E, Chehimi J, et al. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J Immunol. 2002;168:5764–5770. doi: 10.4049/jimmunol.168.11.5764. [DOI] [PubMed] [Google Scholar]
- 66.Goodier MR, Imami N, Moyle G, Gazzard B, Gotch F. Loss of the CD56hiCD16- NK cell subset and NK cell interferon-gamma production during antiretroviral therapy for HIV-1: partial recovery by human growth hormone. Clinical and experimental immunology. 2003;134:470–476. doi: 10.1111/j.1365-2249.2003.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lichtfuss GF, Cheng WJ, Farsakoglu Y, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol. 2012;189:1491–1499. doi: 10.4049/jimmunol.1200458. [DOI] [PubMed] [Google Scholar]
- 68.Brunetta E, Fogli M, Varchetta S, et al. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. AIDS. 2010;24:27–34. doi: 10.1097/QAD.0b013e3283328d1f. [DOI] [PubMed] [Google Scholar]
- 69.Wehner R, Dietze K, Bachmann M, Schmitz M. The bidirectional crosstalk between human dendritic cells and natural killer cells. Journal of innate immunity. 2011;3:258–263. doi: 10.1159/000323923. [DOI] [PubMed] [Google Scholar]
- 70.Michel T, Hentges F, Zimmer J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Frontiers in immunology. 2012;3:403. doi: 10.3389/fimmu.2012.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nedvetzki S, Sowinski S, Eagle RA, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–3785. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 72.Welte S, Kuttruff S, Waldhauer I, Steinle A. Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nature immunology. 2006;7:1334–1342. doi: 10.1038/ni1402. [DOI] [PubMed] [Google Scholar]
- 73.Norris S, Coleman A, Kuri-Cervantes L, Bower M, Nelson M, Goodier MR. PD-1 expression on natural killer cells and CD8(+) T cells during chronic HIV-1 infection. Viral immunology. 2012;25:329–332. doi: 10.1089/vim.2011.0096. [DOI] [PubMed] [Google Scholar]
- 74.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 75.D'Souza M, Fontenot AP, Mack DG, et al. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 76.Gilks CF. Acute bacterial infections and HIV disease. British medical bulletin. 1998;54:383–393. doi: 10.1093/oxfordjournals.bmb.a011695. [DOI] [PubMed] [Google Scholar]
- 77.Nga TV, Parry CM, Le T, et al. The decline of typhoid and the rise of non-typhoid salmonellae and fungal infections in a changing HIV landscape: bloodstream infection trends over 15 years in southern Vietnam. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2012;106:26–34. doi: 10.1016/j.trstmh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 78.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. The Lancet infectious diseases. 2010;10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Byarugaba DK. A view on antimicrobial resistance in developing countries and responsible risk factors. International journal of antimicrobial agents. 2004;24:105–110. doi: 10.1016/j.ijantimicag.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 80.Tosh PK, McDonald LC. Infection control in the multidrug-resistant era: tending the human microbiome. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54:707–713. doi: 10.1093/cid/cir899. [DOI] [PubMed] [Google Scholar]
- 81.Ramana Rao PV, Rajasekaran S, Raja A. Natural killer cell-mediated cytokine response among HIV-positive south Indians with pulmonary tuberculosis. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2010;30:33–42. doi: 10.1089/jir.2009.0018. [DOI] [PubMed] [Google Scholar]


