Abstract
Rationale
Psychological processes such as expectancy, attention, and affect directly influence clinical outcomes. These factors are grouped together as “nonspecific” factors, or placebo effects, in the medical literature, and their individual contributions are rarely considered. The pain-reducing effects of analgesic treatments may reflect changes in these psychological factors, rather than pure drug effects on pain. Furthermore, drug effects may not be isolated by drug vs. placebo comparisons if drugs interact with relevant psychological processes.
Objectives
To determine whether the analgesic effects of opioid and placebo treatment are mediated by changes in attention, expectancy, or affect.
Methods
We crossed intravenous administration of a potent opioid analgesic, remifentanil, with information about drug delivery (treatment expectancy, or placebo) using a balanced placebo design. We measured drug and treatment expectancy effects on pain, attention, and responses to emotional images. We also examined interactions with cue-based expectations about noxious stimulation, or stimulus expectancy.
Results
Pain was additively influenced by treatment expectancy, stimulus expectancy, and drug concentration. Attention performance showed a small but significant interaction between drug and treatment expectancy. Finally, remifentanil enhanced responses to both positive and negative emotional images.
Conclusions
The pain-relieving effects of opioid drugs are unlikely to be mediated by changes in threat or affective processing. Standard open-label opioid administration influences multiple clinically relevant cognitive and emotional processes. Psychological factors can combine with drug effects to influence multiple outcomes in distinct ways. The influence of specific psychological factors should be considered when developing and testing pharmacological treatments.
Keywords: placebo, opioids, analgesia, pain, attention, psychology, expectancy, emotion, remifentanil
Introduction
Responses to drug treatments depend on pharmacological factors along with a host of psychological factors that may influence treatment outcomes. These potential intervening processes are often referred to as “non-specific factors,” and include such factors as expectations about treatment, emotions induced by the treatment context, and social effects of the patient-doctor relationship (Gracely et al, 1983; Atlas and Wager, 2012; Benedetti et al, 2011; Finniss et al, 2010; Gracely et al, 1985; Kaptachuk, 2002). The standard randomized clinical trial (RCT) assumes that these cognitive, emotional, and psychosocial processes will be identical whether a patient receives active treatment or a placebo. The effects in the placebo group are attributed to these common psychological factors, and are subtracted from the effects in an active treatment group. Differences between groups are then attributed to the pharmacological properties of the drug intervention (Colloca and Benedetti, 2005).
Few studies have formally tested the assumptions underlying this approach. In particular, studies have yet to directly measure the effects of both placebo and drug treatments, and their interaction, on these “non-specific factors” themselves. Understanding how drugs and expectancies, in combination, influence low-level processing is critical because these factors may mediate treatment outcomes. For example, it is possible that part of the analgesic efficacy of opioid treatments may depend on changes in attention to pain, general affective shifts, or differential responses to cognitive and affective cues or stimuli (Gospic et al, 2008). Opioid receptors are distributed throughout the cerebral cortex and subcortical structures involved in cognitive and affective processing (Arvidsson et al, 1995; Frost, 1985; Jones et al, 1991) and recent work implicates opioid binding in attention to pain (Sprenger et al), responses to emotional stimuli (Liberzon et al, 2002), and mood states (Zubieta et al, 2003).
Previous studies have used RCTs to assess opioid drug effects on cognitive and emotional outcomes (Gospic et al, 2008; Zacny, 1995), or have compared placebo and control conditions to isolate placebo effects on these processes (Buhle et al, 2012; Flaten et al, 2006; Petrovic et al, 2005; Zhang and Luo, 2009). These studies support the notion that both treatment expectancy and opioid drugs influence emotion processing, while effects on cognitive factors have been mixed. However, drug and placebo effects have been considered principally in isolation.
In a recent study (Atlas et al, 2012), we manipulated both treatment expectancy and opioid administration to assess how each influences measures of cognitive and emotional processing, and to test for interactions between these factors. In two separate studies, we showed that expectancy and remifentanil, an opioid analgesic, have dissociable effects on pain and on brain responses during pain processing. Pain was reduced with overt remifentanil administration, but this reduction was additive with drug effects. Remifentanil effects on pain-related brain responses were the same whether or not participants believed they were receiving the drug (Atlas et al, 2012; Wager et al, 2013), and instructions about drug delivery were accompanied by changes in brain regions outside of traditional pain-processing pathways. This provides evidence for separate, additive effects of remifentanil and expectancy on pain. However, it does not speak to whether each factor influences basic attention and emotional processes, which may mediate effects on pain (Mueller et al, 2012) or have other effects on performance and wellbeing. The purpose of the present experiment was to investigate these potential mediators.
Study 1 of Atlas et al. (2012) included three additional tasks that bear on the broader psychological effects of opioid and expectancy manipulations. We administered remifentanil in a balanced placebo design (Rohsenow and Marlatt, 1981; Ross et al, 1962), which crosses drug treatment with treatment expectancy in a 2 × 2 design (see Figure 1). In each condition, participants performed a demanding visual attention task (Johnston et al, 2012; Shih and Sperling, 2002), and also judged emotional responses to viewing a standard set of affective images (Lang et al, 1998). We tested for drug effects, expectancy effects, and their interaction on both attention performance and emotion ratings, in addition to pain. We also included a manipulation of stimulus expectancy (Atlas et al, 2010) and assessed whether stimulus expectancy effects on pain interacted with either drug or treatment expectancy. The overall aim of this study was to evaluate whether opioid treatments and placebo effects operate by modulating the moment-to-moment sources of pain modulation, or whether analgesic treatments affect pain independently and additively with short-term pain modulatory factors.
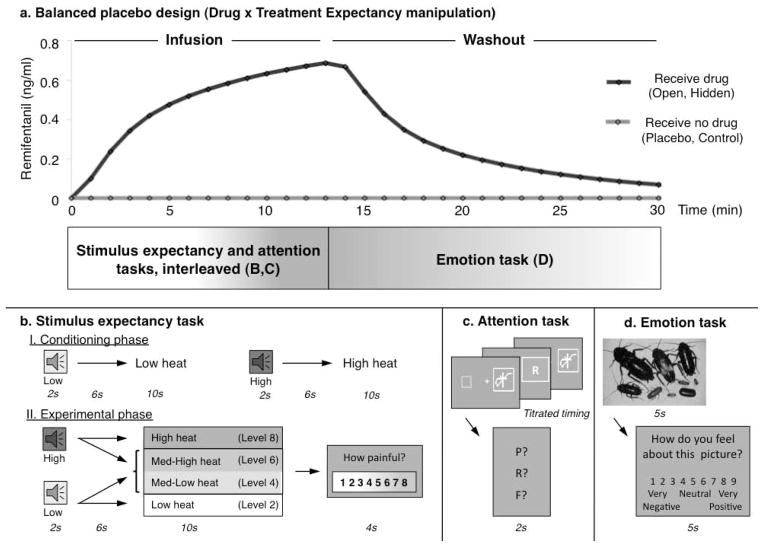
Figure 1. Experimental Design.
a) We used a balanced placebo design, wherein participants received remifentanil intravenously during two runs (Red; Open and Hidden administration) and received no remifentanil during two runs (Blue: Placebo and Control conditions). The stimulus expectancy task and the attention task were presented during drug infusion, while the emotion task was performed during the washout period. We estimated performance as a function of predicted drug concentration over time. The figure illustrates predicted drug concentration for one participant. b) Prior to the main experiment, participants went through a conditioning phase designed to manipulate stimulus expectancies, in which two auditory cues were followed by low or high intensity stimulation. During the main experiment, each cue was equally likely to be followed by its predicted level (level 2 or level 8 on an 8-point scale) or two levels of heat calibrated to elicit ratings of medium pain (level 4 or level6). Participants provided pain reports on each trial using a continuous visual analogue scale. c) Following each trial of the stimulus expectancy task (i.e. after participants provided pain ratings), participants performed three attention task trials. A target letter was presented in the midst of a chain of rapidly changing symbols, with durations determined through a titration phase. Following stimulus presentation, participants reported the letter they had seen on that trial using a forced-choice response. d) The emotion task was presented during the washout period. Participants saw negative, neutral, and positive images from the International Affective Pictures Set (Lang et al, 1998) and rated how negative or positive they felt in response to the image, using a 9-point scale.
Materials and Methods
Participants
Fifteen participants were enrolled in the study. One participant did not complete the session due to nausea, so data were analyzed from 14 participants (7 female, mean age = 22). All participants were right-handed and spoke English fluently. Participants were not enrolled if they reported a history of neurological or psychiatric disorders, psychoactive substance abuse, or prior treatment with opioids, and were screened for illicit drugs using a commercial urine drug test. All participants gave informed consent as approved by Columbia University’s Institutional Review Board and were fully debriefed following the experiment.
Remifentanil administration and pharmacokinetic modeling
All participants received intravenous remifentanil (10 μg/ml concentration) at a steady dose of .04 μg/kg/min during Open and Hidden administration (see below, “Experimental design”). We used a three-compartment pharmacokinetic model of remifentanil (Minto et al, 1997a; Minto et al, 1997b) to estimate the predicted brain (effect site) concentration for each participant over time. The resultant time course provided estimates of predicted brain concentration of remifentanil at each minute. We used linear interpolation to estimate the brain concentration during each trial for each task (i.e. every 45 seconds for analyses of pain reports and attention performance and every 11.5 seconds for analyses of emotion ratings). We normalized the Drug regressor to reflect the percentage of maximum concentration over time and modeled maximum absolute concentration (which accounts for dose, sex, weight, and age) at the subject level in group-level analyses. The dose administered in this study corresponded to an average maximum absolute concentration of 0.761 ng/ml, which is similar to the fixed dose of 0.8 ng/ml applied in previous work that used target-controlled infusion (Bingel et al, 2011) and corresponded to the average dose determined to elicit analgesia without sedation in a prior experiment that included a subject-specific dosing procedure (Study 2 of Atlas et al, 2012). Subjects received a mixture of saline and glucose (0.9% saline) during Placebo and Control runs.
Remifentanil or saline infusion proceeded for 13.5 minutes and was immediately followed by a washout period to minimize circulating remifentanil by the next run. Participants performed stimulus expectancy and attention tasks during the infusion period, provided overall ratings of intensity, unpleasantness, and pleasantness at the start of the washout period, and performed the emotion task for the next ~5 minutes of the washout period (see Figure 1). Drug concentration was reduced but remained appreciable during the emotion task, and analyses used the estimated drug concentration during each trial as a continuous variable.
The duration of the washout period ranged from seven minutes (the minimum amount of time allotted for participants to provide overall ratings of intensity, unpleasantness, and pleasantness and to complete the emotion task) to thirty-eight minutes (M = 11 minutes 51 seconds). The estimated drug concentration was reduced by 50% within four minutes of washout, due to remifentanil’s rapid elimination half-life. However, pharmacokinetic models of remifentanil predict that a 13.5-minute infusion will take one hour to return completely to baseline, indicating that carry-over effects were possible. To account for potential effects of residual remifentanil, we counterbalanced order across all participants (i.e. made sure that each condition was followed by every other condition and appeared in each potential position). In our analyses, we modeled predicted residual remifentanil carryover across runs, accounting for the duration of the washout period on a run-by-run basis, so that estimated brain remifentanil concentrations reflect the combination of the current infusion and any residual remifentanil from the prior run.
Thermal stimulation and pain ratings
Thermal stimulation was delivered to the volar surface of the left forearm using a 16×16 mm Peltier thermode (Medoc, Inc.). Each stimulus lasted 10 seconds (1.5 s ramp up and down, 7s at peak). Participants rated stimulation on a continuous, numerically anchored visual analogue scale (VAS) from 0–8 (0 = no sensation; 1 = non-painful warmth; 2 = low pain; 5 = moderate pain; 8 = maximum tolerable pain). The pain rating scale we used is simple and provides reliable and rapid measurements (Bijur et al, 2001; Chapman et al, 1985). However, it is unidimensional. Previous work has shown that some opioid analgesics may specifically target pain unpleasantness, without affecting pain intensity (Cohen et al, 2008; Kupers et al, 1991; Price et al, 1985), though other studies have shown opposite effects (Gracely et al, 1979). To acknowledge this potential dissociation, we collected retrospective ratings of overall pain intensity, unpleasantness, and pleasantness on each run after the pain task (before washout).
Experimental paradigm
Stimulus expectancy cues
As in Atlas et al., (2010), participants first went through a learning procedure prior designed to manipulate explicit stimulus expectancies (see Figure 1B). Participants were told that two cues (500 and 1000 Hz tones, counterbalanced across subjects), would predict low or high pain, respectively. Participants then performed a forced-choice task to ensure that they could accurately discriminate between auditory cues. All participants performed accurately (>90%) so no participants were excluded.
Pain calibration and conditioning procedure
Temperatures were individually calibrated using a modified version of an adaptive calibration described in previous work (Atlas et al, 2010). In the current experiment, we used this procedure to 1) ensure that participants demonstrated a reliable relationship between temperature and pain report (R2 > .40); 2) determine temperatures appropriate for each individual; 3) determine the four skin sites that showed the most reliable relationship between temperature and subjective pain; and 4) establish a relationship between auditory cues and noxious thermal stimulation.
Each participant received a series of temperatures preceded by 2-sec auditory predictive cues. All participants first received stimulations of 41 °C and 47 °C, preceded by Low-expectancy and High-expectancy cues, respectively. Participants provided pain reports on every trial, and we used these pain reports to iteratively fit a linear relationship between temperature and pain report. Participants received 12 trials calibrated to elicit low pain (VAS = 2) preceded by the low-expectancy cue, and 12 trials calibrated to elicit high pain (VAS = 8) preceded by the high-expectancy cue.
An experimenter rotated the thermode across eight skin sites during this procedure. We fit a linear function between temperature and pain reports, which allowed us to select four most reliable skin sites and determine temperatures predicted to elicit ratings of low pain (VAS rating = 2; M = 40.71°C, SD = 2.83), low-medium pain (VAS rating = 4; M = 43.11°C, SD = 2.33), medium-high pain (VAS rating = 6; M = 44.3°C, SD = 1.60), and high pain (VAS rating = 8; M = 47.25°C, SD = 1.31). We applied these temperatures during the main experiment.
Treatment expectancy manipulation
Following pain calibration and conditioning, participants went through four runs of the experiment. Two runs were conducted during remifentanil infusion, and two runs were conducted during the control condition, when standard saline solution (0.9% saline) was infused. Instruction about the infusion (Treatment Expectancy) was evenly crossed with remifentanil administration (Drug) in a 2 × 2 design. Thus, on two runs, participants were told that they would receive remifentanil, and on two runs, participants were told that they would receive saline only, with no drug. This created four conditions: Open remifentanil administration (Expect Drug, Receive Drug), Hidden remifentanil administration (Expect No Drug, Receive Drug), Placebo (Expect Drug, Receive No Drug), and Control (Expect No Drug, Receive No Drug). Main effects of Treatment Expectancy were assessed with the contrast [Open + Placebo − Hidden − Control]. Main effects of Drug were assessed with the contrast [Open + Hidden − Placebo − Control]. The interaction was assessed with the contrast [Open + Control − Placebo − Hidden], as is standard in a 2 × 2 factorial ANOVA design. Order was counterbalanced across participants so that each condition occurred in each possible position, and each condition was followed equally often by every other condition. To ensure that participants were blind to condition, 1) saline was administered during placebo and control conditions to provide sensory feedback of intravenous infusion, 2) communication between the anesthesiologist and experimenters was minimized to reduce social cues, and 3) the experimenter who most directly interacted with the participant was blind to condition.
Stimulus expectancy manipulation
During the infusion period, which lasted for 13.5 minutes for each run, participants experienced 18 thermal pain trials (10 sec duration, as described above). Noxious stimulation was preceded by a 2-sec auditory predictive cue that gave information about the upcoming noxious heat intensity as in Atlas et al, (2010), and a 6-sec anticipatory delay period (see Figure 1B). Each run consisted of 6 conditions of 3 trials each: High Expectancy Cue followed by High heat (HH), High Expectancy Cue followed by Medium-High heat (HM2), High Expectancy Cue followed by Medium-Low heat (HM1), Low Expectancy Cue followed by Medium-High heat (LM2), Low Expectancy Cue followed by Medium-Low heat (LM1), and Low Expectancy Cue followed by Low heat (LL). Participants rated perceived pain immediately following pain offset on every trial using the VAS described above. In a previous publication (Atlas et al, 2012), we reported effects of Drug and Treatment Expectancy on pain reports across all temperatures. In this paper, we analyzed pain reports only during the critical medium range of heat trials (Low Medium and High Medium) as a function of Drug, Treatment Expectancy, Stimulus Expectancy, and Temperature.
Attention task
Between stimulus expectancy trials, participants performed three trials of a simple visual letter detection task to monitor attention and alertness (Shih and Sperling, 2002). This task was adapted from Johnston et al., (2012), which measured stimulus expectancy effects on attention by presenting visual discrimination probes during noxious thermal stimulation. In the present study, visual discrimination task trials were interleaved with thermal stimulation trials and presented two seconds after pain ratings were collected. Thus we measured direct effects of Drug and Treatment Expectancy on attention, independent of pain or Stimulus Expectancy. On each trial, participants saw a set of masked images interleaved with a target letter (Shih and Sperling, 2002; see Figure 1C). Target and mask presentation durations were determined based on a titration task performed prior to the main experiment, which allowed us to calculate the exposure length required to achieve 85% accuracy (M = 62.78ms; SE = 4.89). Forced choice performance was measured on each trial with a two-second probe that presented the target letter as well as two lure options (see Figure 1C). A one-second inter-stimulus interval separated each attention task trial.
Emotion task
During the washout period, participants performed a task designed to assess emotional changes (see Figure 1D). Participants saw 108 pictures from the international affective picture set (IAPS; Lang et al, 1998). In each run, participants viewed 9 negative, 9 neutral, and 9 positive images, based on published normed ratings (Lang et al, 1998). Images were presented in random order within each run, and counterbalanced across runs. Images were displayed for 5 seconds, followed by a 5-second rating slide with the prompt “How do you feel about this picture?” (see Figure 1D). A one-second inter-stimulus interval separated each trial. One participant viewed only negative and neutral images due to technical errors and was excluded from analyses.
Debriefing
Participants went through a structured debriefing interview following the experiment. Participants answered free response questions about their overall experience (e.g. “How effective do you think the analgesic was overall?”) and were debriefed regarding the deception. Participants were then asked whether they had suspected deception prior to debriefing, and completed a forced choice task to identify each of the four runs. Notes from two participants were misplaced. Of the 12 remaining participants, 7 performed correctly on the retrospective forced choice task. However, only three participants reported suspecting deception prior to debriefing. During experimental trials, therefore, at the moderate doses we used, participants were reasonably blind to which conditions were the verum drug conditions.
Statistical Analysis
We measured four dependent variables: 1) trial-by-trial pain, 2) visual discrimination task performance, 3) emotional image ratings, and 4) overall pain intensity and affect. Overall pain ratings were analyzed with repeated-measures Analysis of Variance. All other dependent variables were analyzed with separate linear mixed-effects models, implemented using custom Matlab software and verified with the glmer function in the R package lme4 (Bates et al, 2011). Both software packages produced qualitatively identical results. The first level of each model included regressors for each predicted effect and all relevant interactions, along with a linear term for the effect of run. Drug effects were modeled as a continuous measure based on the pharmacokinetic model of remifentanil described above. The second level of each mixed effects model assessed the significance of coefficients across individuals, treating participant as a random variable. We also included second-level covariates for maximum absolute drug concentration, as described above.
Sources of pain modulation
We assessed whether trial-by-trial pain reports were modulated by Drug, Treatment Expectancy, Stimulus Expectancy, Temperature and all possible interactions between these factors. The present paper focuses on medium-range heat trials (i.e. on the subset of Low-Medium and High-Medium trials), during which all factors were fully crossed.
Effects on visual discrimination task performance
We analyzed effects on trial-by-trial performance (percent correct based on the three probes per trial) as a function of Drug, Treatment Expectancy, and their potential interaction. Because outcomes were discrete (i.e. performance on each trial could be 0%, 33%, 66%, or 100% correct), we used a bootstrapping estimation procedure (bias-corrected and accelerated; Efron and Tibshirani, 1993), with p-values calculated based on 10,000 bootstraps.
Effects on responses to emotional images
Trial-by-trial ratings were analyzed as a function of Drug, Treatment Expectancy, and Valence (negative, neutral, or positive based on normed ratings; Lang et al, 1998), and all possible interactions between these factors.
Results
Pain: Additive effects of Drug, Treatment expectancy, and Stimulus expectancy
As shown in Figure 2, all factors (Drug, Treatment Expectancy, Stimulus Expectancy and Temperature) influenced pain reports on the critical medium-range temperature trials, and their effects were additive. Remifentanil administration produced an average reduction of 1.04 VAS units (Drug effect: t = −3.89, SE = 0.27, p < 0.01), and information that remifentanil was being delivered produced an average additional reduction of .31 units (Treatment Expectancy effect: t = −2.37, SE = 0.13, p < 0.05). High pain cues were associated with an increase of 0.37 units, relative to the same temperatures preceded by low pain cues (Stimulus Expectancy effect: t = 3.33, SE =0.11, p < 0.01). Temperature also affected ratings: High-medium trials were rated 1.18 units higher than low-medium trials, regardless of cue (Temperature effect: t = 3.77, SE = 0.31, p < 0.01). There were no interactions between Drug, Treatment Expectancy, and Stimulus Expectancy, nor were there any interactions with Temperature or main effects of Run (all p’s > 0.1). Finally, individuals with higher absolute brain concentrations of remifentanil reported more pain on average (t = 2.88, SE = 1.93, p < .05). Absolute concentration of remifentanil is positively correlated with participant height and weight, suggesting that the fixed weight-adjusted dose administered in the present study was less analgesic for heavier participants.
Figure 2. Drug, treatment expectancy, and stimulus expectancy effects on pain reports under medium heat.
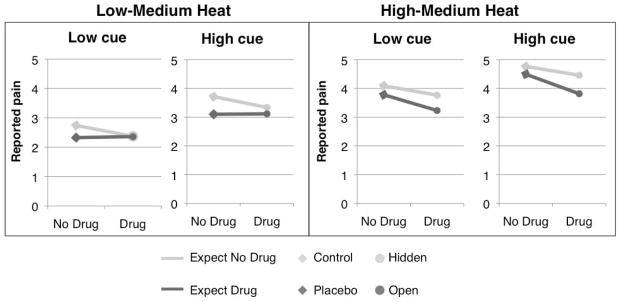
We measured pain in response to thermal stimuli calibrated to elicit ratings of low-medium pain (level 4 on an 8-point scale) and high-medium pain (level 6 on an 8-point scale). Pain reports were significantly influenced by Drug concentration (a continuous regressor, visualized in all figures as [Drug vs No Drug] for simplicity), Treatment Expectancy (Expect No Drug vs Expect Drug), Stimulus Expectancy (High cue vs Low cue), and Temperature (High-Medium Heat vs Low-Medium Heat), with additive effects. We did not observe any significant interactions between these factors.
Attention: Over-additive Drug x Treatment Expectancy interaction
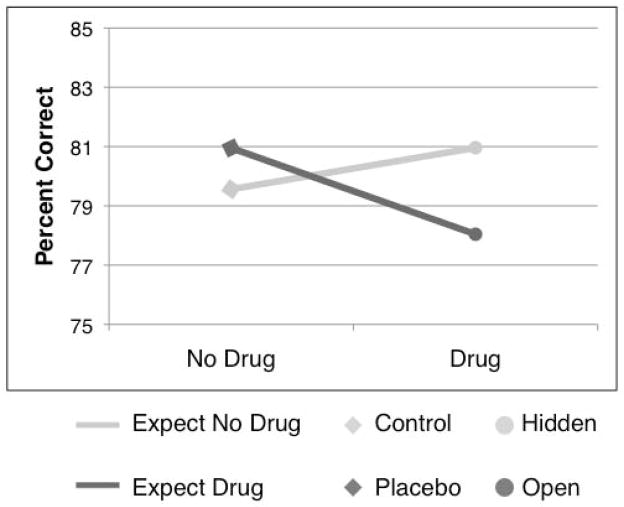
We found a significant Drug x Treatment Expectancy interaction on letter detection performance (t = −1.74, SE = 2.11, p < 0.05). As shown in Figure 3 and confirmed by post-hoc simple effects analyses, this interaction was driven by a significant drug-induced reduction in performance only when participants believed they were receiving remifentanil (Open − Placebo: t = −1.76, SE = 3.32, p < 0.05). There were no effects of drug administration when participants believed they were not receiving the drug (i.e. Hidden − Control; p > 0.5), nor were there significant simple effects of Expectancy (Open > Hidden or Placebo > Control; all p’s > 0.2). There was a marginally significant drug-induced reduction in performance (main effect of Drug; t = −1.94, SE = 2.75, p = .065). The main effect of Expectancy was not significant (p > .2).
Figure 3. Attention task performance.
We found a significant interaction between Drug and Expectancy on the visual discrimination task. Participants performed less well when they expected to receive remifentanil and received the drug than when remifentanil was delivered outside of their awareness. There was no effect of Treatment Expectancy when participants did not receive remifentanil.
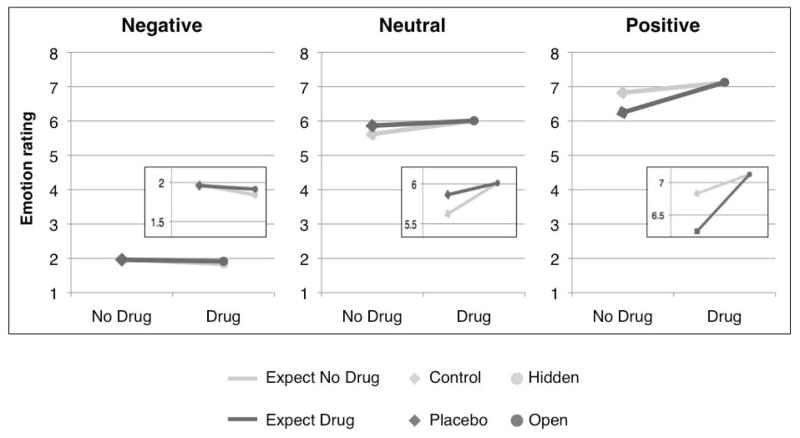
Emotional images: Drug enhances responses to both negative and positive images
As expected, image valence (based on normative ratings) strongly influenced emotion ratings (t = 16.32, SE = 0.15, p < 0.001): negative images were rated as more negative than neutral images, and positive images were rated as more positive than neutral images (see Figure 4). There were no main effects of Drug or Treatment Expectancy on ratings, nor were there any Drug x Treatment Expectancy interactions (all p’s > 0.2). However, we observed a significant Drug x Valence interaction (t = 2.3, SE = 0.21, p < .05), such that high drug concentration enhanced rated valence: Negative images were rated more negatively, and positive images were rated more positively, than when drug concentration was low (see Figure 4). We note that we found no significant Drug effects when we examined ratings separately within each valence, likely because of the limited number of trials per condition per run. Finally, individuals with higher maximum drug concentration showed stronger Drug x Valence interactions (t = 3.0, SE = 1.84, p < .05).
Figure 4. Ratings of emotional stimuli.
Drug concentration interacted with valence to influence emotion ratings. Participants rated negative images more negative and rated positive images more positive when they received remifentanil than when they received no drug. We found no effects of Treatment Expectancy on emotion ratings, nor were there significant interactions between Drug and Treatment Expectancy.
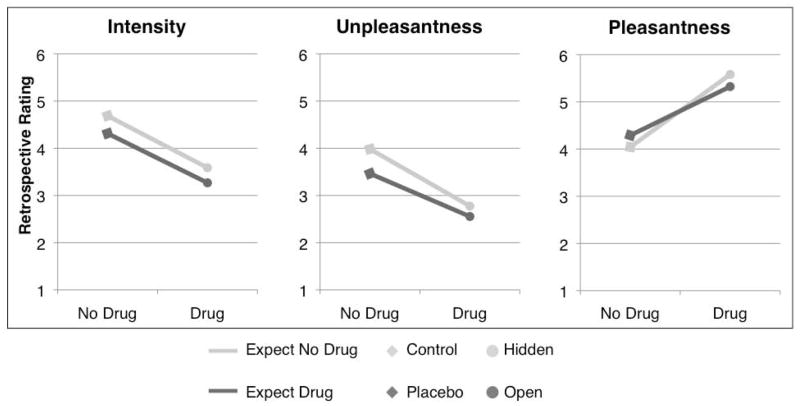
Drug effects on retrospective ratings of pain intensity and affect
Remifentanil reduced post-task ratings of pain intensity (t(10) = −3.27, p < .01) and unpleasantness (t(12) = −4.37, p < .001) and increased retrospective pleasantness ratings (t(9) = 2.82, p < .05) (see Figure 5). We found no main effect of Treatment Expectancy, and no Drug x Treatment Expectancy interactions on ratings (all p’s > 0.15).
Figure 5. Post-task pain ratings.
Remifentanil influenced overall ratings of both pain intensity and affect: Open and Hidden conditions were perceived as less intense, less unpleasant, and more pleasant than Placebo and Control conditions. Treatment expectancy had no effect on retrospective ratings.
Discussion
The gold standard clinical trial depends on the logic that psychological factors are identical between placebo and control groups. The present study used a mechanistic approach to test this assumption. We used a within-subjects balanced placebo design to isolate drug effects and placebo effects on pain, attention, and emotional responses to images. We tested for interactions in the effects of drug, treatment expectancy, and trial-by-trial stimulus expectancy on each outcome. We found that pain was influenced in an additive manner by remifentanil, expectations about remifentanil, and expectations about noxious stimulus intensity. However, attention performance showed a different pattern: Drug effects and placebo effects interacted to influence attention, such that the strongest impairment was produced by the combination of drug and treatment expectancy. Finally, emotional responses to pictures showed another, qualitatively distinct pattern. Remifentanil enhanced responses to emotional stimuli, causing participants to perceive negative images as more negative and positive images more positive.
These data reveal several new pieces of information about the nature of placebo (treatment expectancy) effects. Each outcome—pain, attention performance, and emotion ratings—was associated with a different pattern of results across drug and expectancy conditions. This implies that expectancy effects on pain are not reducible to changes in attention or emotion. Treatment expectancy had a reliable effect on pain, but a drug-dependent effect on attention, and negligible effects on emotion ratings. The placebo instructions did not increase positive emotion ratings for other stimuli.
Secondly, treatment expectancy effects were additive with stimulus expectancy effects, implying that multiple kinds of expectations—about treatments and about the stimulus itself—can jointly and independently influence pain. Previous theorists have suggested that there may be distinct types of expectations. In particular, Kirsch has drawn a distinction between stimulus expectancies and response expectancies, or expectations about one’s own nonvolitional reactions (Kirsch, 1985; Kirsch, 1997). In Kirsch’s model, placebos generate response expectancies, which in turn give rise to nonvolitional responses. This is consistent with how we conceptualize placebo effects here. We chose to refer to placebo effects as treatment expectancies rather than response expectancies, since information about analgesic treatments and information about upcoming stimuli might both induce response expectancies for reduced pain. Thus we differentiate short-term expectations about events that will occur in the environment from sustained expectations about one’s own internal state. We find that maintaining the treatment context does not affect the impact of cue-based stimulus expectancies, and likewise giving information about upcoming stimuli does not reduce the influence of sustained treatment expectancy. We have previously hypothesized that these types of expectancies are likely to depend on separate neuromodulatory systems, namely that stimulus expectancies are likely to rely on phasic dopamine responses, whereas placebo analgesia is linked to endogenous opioid binding (Atlas et al, 2012; Atlas et al, 2010). The additive effects reported here lend further support to this hypothesis. Future studies should directly examine the neurochemical bases of these two classes of expectancy.
Our results have implications for the study of attention as well. Cognitive performance during pain has received much less attention than pain in placebo and opioid pharmacology studies, but it is an important functional aspect of pain in its own right (Buhle et al, 2012; Eccleston and Crombez, 1999; Legrain et al, 2009). We found that instructions that the drug was being administered reduced performance only when participants actually received remifentanil. Likewise, remifentanil administration only affected performance when participants knew they were receiving the drug. Though studies have found mixed support for opioid drug effects on attention and simple motor performance (Zacny, 1995), the interaction reveals that changes in attention of the type observed here cannot be attributed to pharmacological properties of remifentanil itself. Instead, decrements in performance emerge only when drug administration is combined with explicit knowledge that the drug is being administered. This interaction might reflect a competition for directed attention between task demands and self-monitoring. Self-monitoring is likely to be highest when patients both expect side effects and receive feedback from actual drug-induced changes, leading to a competition for attention resources that might result in reduced performance.
The effects of remifentanil on emotion ratings challenge several existing ideas about the effects of opioids. Consistent with previous findings in other types of opioid analgesia (Gospic et al, 2008; Kupers et al, 1991; Price et al, 1985), remifentanil reduced overall ratings of pain unpleasantness. In addition to effects on the affective components of pain, remifentanil also influenced pain intensity ratings, suggesting that its effects are not limited to unpleasantness. Furthermore, we found that remifentanil also increased pleasantness ratings for pain, implying enhanced positive contextual or sensory associations with painful events. Remifentanil had more complex effects on ratings of images as well. Rather than leading to an overall shift toward positive affect, remifentanil enhanced responses to emotional stimuli, rendering negative emotional images more negative and positive emotional images more positive. It is commonly assumed that opioids increase positive affect, but they may in fact have more nuanced effects on the contextual modulation of affect, perhaps increasing the degree of contextual (or episodic memory-based) control over affect. This might explain some of the clinical manifestations of opioid treatment in some individuals, such as the evocation of strong emotional memories. The effect was strongest for individuals who received higher drug concentrations; clinicians might consider that enhanced emotional lability could be a direct consequence of remifentanil administration. This builds on findings from Gospic et al. (Gospic et al, 2008), who found that remifentanil increased the pleasantness of neutral images, but did not increase the pleasantness of negative images. They did not examine effects on positive images. Our results also suggest that it should not be assumed that drugs affect pain and emotion in similar ways, since remifentanil rendered aversive images more negative but also made pain less unpleasant.
Future directions and limitations
This work raises a number of important questions that should be addressed in future research. First, this work has important translational implications that should be directly tested in the clinic. Drugs and placebos might influence clinical outcomes primarily by means of so-called “non-specific” factors—expectations, attention, and emotional responses—and using validated paradigms from cognitive psychology provides a way to measure these factors as potential mediators.
In this paper, we used a within-subjects design, which has both advantages and disadvantages. The advantages include minimization of person-level confounds such as clinical history, response to remifentanil, and pain tolerance. These advantages are substantial, as it is difficult to adequately control for such variables in clinical studies. Disadvantages include potential complications due to carry-over effects across runs and reduced similarity with between-subjects designs typical in clinical trials. We counterbalanced order to ensure that the effects of learning and carry-over were minimized in our analyses, and while our pharmacokinetic analyses account for potential carry-over, we note that we could not model pharmacodynamics of remifentanil, which might yield a different timecourse of effects on outcomes. Important next steps will be to test this approach in between-subjects designs (i.e. subjects randomly assigned to Placebo, Control, Open, and Hidden groups) in more ecologically valid clinical settings, to assess the magnitude of these effects in clinical contexts. It will be critical to carefully consider the ethical implications of such work, as Hidden and Placebo conditions require deception, and patients assigned to Hidden administration would believe they are being denied treatment, which might reduce compliance.
We note that we did not design this study to test whether changes in attention and emotion formally mediate remifentanil-induced analgesia: The attention task and emotion task were presented after pain was administered, which precluded us from testing their formal contributions to the analgesic effects of remifentanil or treatment expectancy on pain. In previous work, we have shown that shifts in attention play a role in stimulus expectancy effects on pain, using a similar paradigm with intermixed attention probes (Johnston et al, 2012). Here, we chose to separate phases of the experiment so that we could measure pure influences of drug and expectancy on lower-level processes. Future studies that present emotion and attention probes during noxious stimulation can directly test for mediation.
We note that participants knew that the infusion period had ended prior to the emotion task, and therefore Treatment Expectancy was likely reduced at this point. Future studies that measure emotional responses during overt vs. covert drug administration can provide stronger tests of treatment expectancy effects on emotion.
Finally, we note that our sample size was rather small, due to limited remifentanil availability for research purposes at the time of the study. In addition, the effects, though they are reliable, are not large in magnitude. Effect sizes can vary based on the intensity of the manipulations and contextual factors, and so whether these effects are large enough to be clinically meaningful must be addressed in ecological studies in clinical contexts. Here, the small effects might be due to the fact that we a) used a low dose of remifentanil to reduce the potential for unblinding (i.e., awareness of which is the real drug condition); and b) our expectancy manipulation was minimal, and designed to allow experimental control rather than to mimic the magnitude and type of expectancies present in the clinic. We chose not to enhance expectancies by reinforcing our placebo / open instructions with response conditioning (i.e. reduced thermal stimulation) in order to study expectancy effects due to information alone rather those combined with conditioned responses. In addition, unblinding in some participants is still possible, and would work against the expectancy effects we observed, reducing differences between [Open − Hidden] and [Placebo − Control] comparisons. In clinical settings, prior treatment experiences (Colloca and Miller, 2011) and stronger desire for relief (Price et al, 1999) can create substantially larger expectations, and unblinding is not an issue in standard open drug administration in clinical practice. Thus, effect sizes may be larger in the clinic (Vase et al, 2002), when expectancies are maximized and drugs are administered at clinical doses.
Conclusion
Overall, this work suggests that the potential component processes that underlie placebo effects—including effects on pain, attention, and affective responses—should be considered independently, rather than grouped together as “non-specific” processes. Predictive processing (i.e., stimulus expectancy effects), attention, and emotion are affected by opioid administration and beliefs about opioid administration in distinct ways, depending on the outcome. In the future, simple analogues of these experimental tasks can be developed and measured, which might help to isolate drug effects in clinical trials. Furthermore, if we consider the separate contributions of the placebo effect’s component processes, we can develop interventions that directly target these independent factors and thereby influence clinical outcomes.
Acknowledgments
The authors would like to thank Namema Amendi and Steven Dashnaw for helpful assistance in data collection, and Stephen Shafer for assistance with the pharmacokinetic model of remifentanil. This work was funded by the NIH (NIMH RO1MH076136 and NIDA RO1DA027794, awarded to T.D.W.). The authors declare no conflict of interest.
References
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, et al. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15(5 Pt 1):3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain Mediators of Predictive Cue Effects on Perceived Pain. J Neurosci. 2010;30(39):12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Wager TD. How expectations shape pain. Neurosci Lett. 2012;520(2):140–148. doi: 10.1016/j.neulet.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable Influences of Opiates and Expectations on Pain. J Neurosci. 2012;32(23):8053–8064. doi: 10.1523/JNEUROSCI.0383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. Lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-40. 2011 http://CRAN.R-project.org/package=lme4.
- Benedetti F, Carlino E, Pollo A. How placebos change the patient’s brain. Neuropsychopharmacology. 2011;36(1):339–354. doi: 10.1038/npp.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8(12):1153–1157. doi: 10.1111/j.1553-2712.2001.tb01132.x. [DOI] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Mhuircheartaigh RN, Lee MC, Ploner M, et al. The Effect of Treatment Expectation on Drug Efficacy: Imaging the Analgesic Benefit of the Opioid Remifentanil. Science Translational Medicine. 2011;3(70):1–10. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Stevens BL, Friedman JJ, Wager TD. Distraction and Placebo: Two Separate Routes to Pain Control. Psychological Science. 2012;23(3):246–253. doi: 10.1177/0956797611427919. [DOI] [PubMed] [Google Scholar]
- Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22(1):1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- Cohen SP, Christo PJ, Wang S, Chen L, Stojanovic MP, Shields CH, et al. The effect of opioid dose and treatment duration on the perception of a painful standardized clinical stimulus. Reg Anesth Pain Med. 2008;33(3):199–206. doi: 10.1016/j.rapm.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Placebos and painkillers: is mind as real as matter? Nat Rev Neurosci. 2005;6(7):545–552. doi: 10.1038/nrn1705. [DOI] [PubMed] [Google Scholar]
- Colloca L, Miller FG. How placebo responses are formed: a learning perspective. Phil Trans R Soc B. 2011;366:1859–1869. doi: 10.1098/rstb.2010.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull. 1999;125(3):356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman and Hall; New York: 1993. [Google Scholar]
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375(9715):686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaten MA, Aslaksen PM, Finset A, Simonsen T, Johansen O. Cognitive and emotional factors in placebo analgesia. Journal of psychosomatic research. 2006;61(1):81–89. doi: 10.1016/j.jpsychores.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Frost JJW, Henry N, Jr, Dannals Robert F, Ravert Hayden T, Links Jonathan M, Wilson Alan A, Donald Burns H, Wong Dean F, McPherson Robert W, Rosenbaum Arthur E, Kuhar Michael J, Snyder Solomon H. Imaging opiate receptors in the human brain by positron tomography. Journal of computer assisted tomography. 1985;9(2):231–236. doi: 10.1097/00004728-198503000-00001. [DOI] [PubMed] [Google Scholar]
- Gospic K, Gunnarsson T, Fransson P, Ingvar M, Lindefors N, Petrovic P. Emotional perception modulated by an opioid and a cholecystokinin agonist. Psychopharmacology. 2008;197(2):295–307. doi: 10.1007/s00213-007-1032-4. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Dubner R, Deeter WR, Wolskee PJ. Clinicians’ expectations influence placebo analgesia. Lancet. 1985;1(8419):43. doi: 10.1016/s0140-6736(85)90984-5. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Dubner R, McGrath PA. Narcotic analgesia: fentanyl reduces the intensity but not the unpleasantness of painful tooth pulp sensations. Science. 1979;203(4386):1261–1263. doi: 10.1126/science.424753. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Dubner R, Wolskee PJ, Deeter WR. Placebo and naloxone can alter post-surgical pain by separate mechanisms. Nature. 1983;306(5940):264–265. doi: 10.1038/306264a0. [DOI] [PubMed] [Google Scholar]
- Johnston NE, Atlas LY, Wager TD. Opposing Effects of Expectancy and Somatic Focus on Pain. PLoS ONE. 2012;7(6):e38854. doi: 10.1371/journal.pone.0038854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AK, Friston KJ, Qi LY, Harris M, Cunningham VJ, Jones T, et al. Sites of action of morphine in the brain. Lancet. 1991;338(8770):825. doi: 10.1016/0140-6736(91)90717-4. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ. The placebo effect in alternative medicine: can the performance of a healing ritual have clinical significance? Ann Intern Med. 2002;136(11):817–825. doi: 10.7326/0003-4819-136-11-200206040-00011. [DOI] [PubMed] [Google Scholar]
- Kirsch I. Response expectancy as a determinant of experience and behavior. American Psychologist. 1985;40(11):1189–1202. [Google Scholar]
- Kirsch I. Response expectancy theory and application: A decennial review. Applied and Preventive Psychology. 1997;6(69–79) [Google Scholar]
- Kupers RC, Konings H, Adriaensen H, Gybels JM. Morphine differentially affects the sensory and affective pain ratings in neurogenic and idiopathic forms of pain. Pain. 1991;47(1):5–12. doi: 10.1016/0304-3959(91)90004-H. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol Psychiatry. 1998;44(12):1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Legrain V, Damme SV, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain. 2009;144(3):230–232. doi: 10.1016/j.pain.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Zubieta JK, Fig LM, Phan KL, Koeppe RA, Taylor SF. μ-Opioid receptors and limbic responses to aversive emotional stimuli. Proceedings of the National Academy of Sciences. 2002;99(10):7084–7089. doi: 10.1073/pnas.102174799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minto CF, Schnider TW, Egan TD, Youngs E, Lemmens HJ, Gambus PL, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997a;86(1):10–23. doi: 10.1097/00000542-199701000-00004. [DOI] [PubMed] [Google Scholar]
- Minto CF, Schnider TW, Shafer S. Pharmacokinetics and pharmacodynamics of remifentanil. II. Model application. Anesthesiology. 1997b;86(1):24–33. doi: 10.1097/00000542-199701000-00005. [DOI] [PubMed] [Google Scholar]
- Mueller M, Bjorkedal E, Kamping S. Manipulation of Expectancy and Anxiety in Placebo Research and Their Effects on Opioid-Induced Analgesia. Journal of Neuroscience. 2012;32(41):14051–14052. doi: 10.1523/JNEUROSCI.3756-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing--induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46(6):957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of analgesia in an experimental paradigm. Pain. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- Price DD, Von der Gruen A, Miller J, Rafii A, Price C. A psychophysical analysis of morphine analgesia. Pain. 1985;22(3):261–269. doi: 10.1016/0304-3959(85)90026-0. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Marlatt GA. The balanced placebo design: methodological considerations. Addictive behaviors. 1981;6(2):107–122. doi: 10.1016/0306-4603(81)90003-4. [DOI] [PubMed] [Google Scholar]
- Ross S, Krugman A, Lyerly S, Clyde D. Drugs and placebos: A model design. Psychological Reports. 1962;10:383–392. [Google Scholar]
- Shih S-I, Sperling G. Measuring and Modeling the Trajectory of Visual Spatial Attention. Psychological Review. 2002;109(2):260–305. doi: 10.1037/0033-295x.109.2.260. [DOI] [PubMed] [Google Scholar]
- Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Buchel C. Attention modulates spinal cord responses to pain. Curr Biol. 2012;22(11):1019–1022. doi: 10.1016/j.cub.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Vase L, Riley JL, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–452. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C, Kross E. An fMRI-based neurologic signature of physical pain. New England Journal of Medicine. 2013;368(15):1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP. A Review of the Effects of Opioids on Psychomotor and Cognitive Functioning in Humans. Experimental and Clinical Psychopharmacology. 1995;3(4):432–466. [Google Scholar]
- Zhang W, Luo J. The transferable placebo effect from pain to emotion: Changes in behavior and EEG activity. Psychophysiology. 2009;46(3):626–634. doi: 10.1111/j.1469-8986.2009.00786.x. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003;60(11):1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]