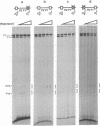
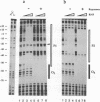
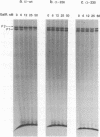
Abstract
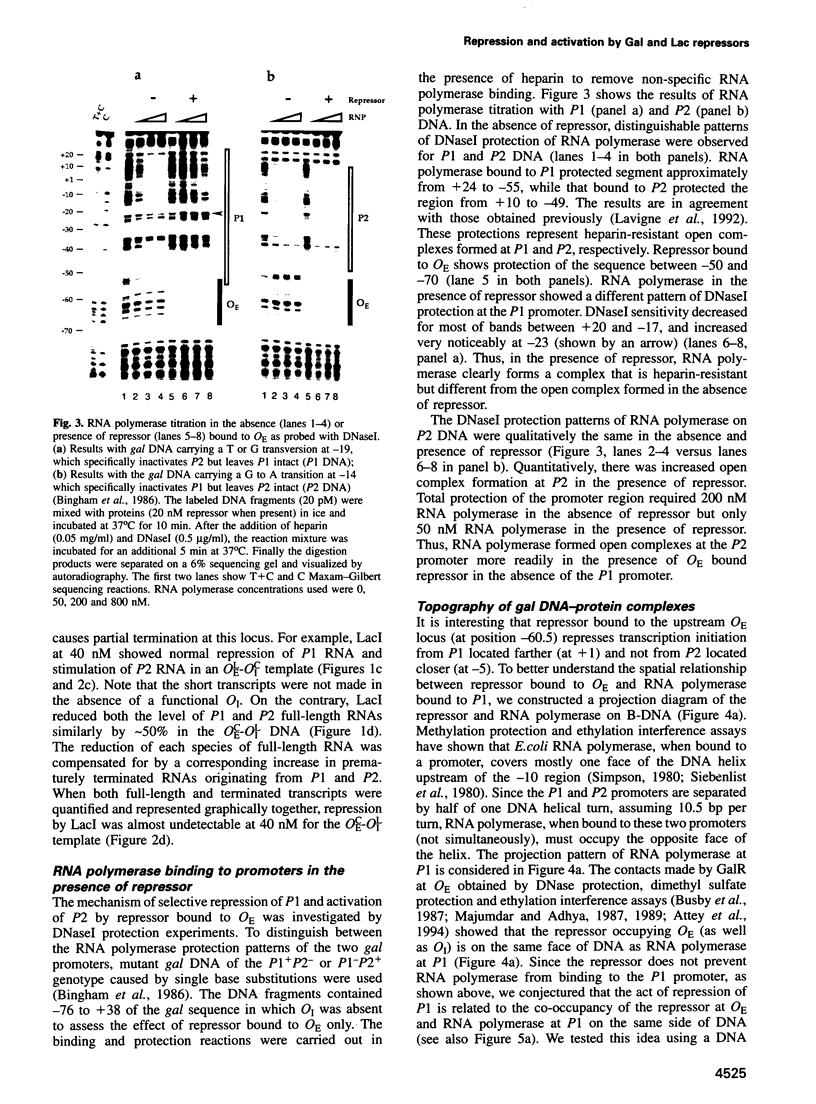
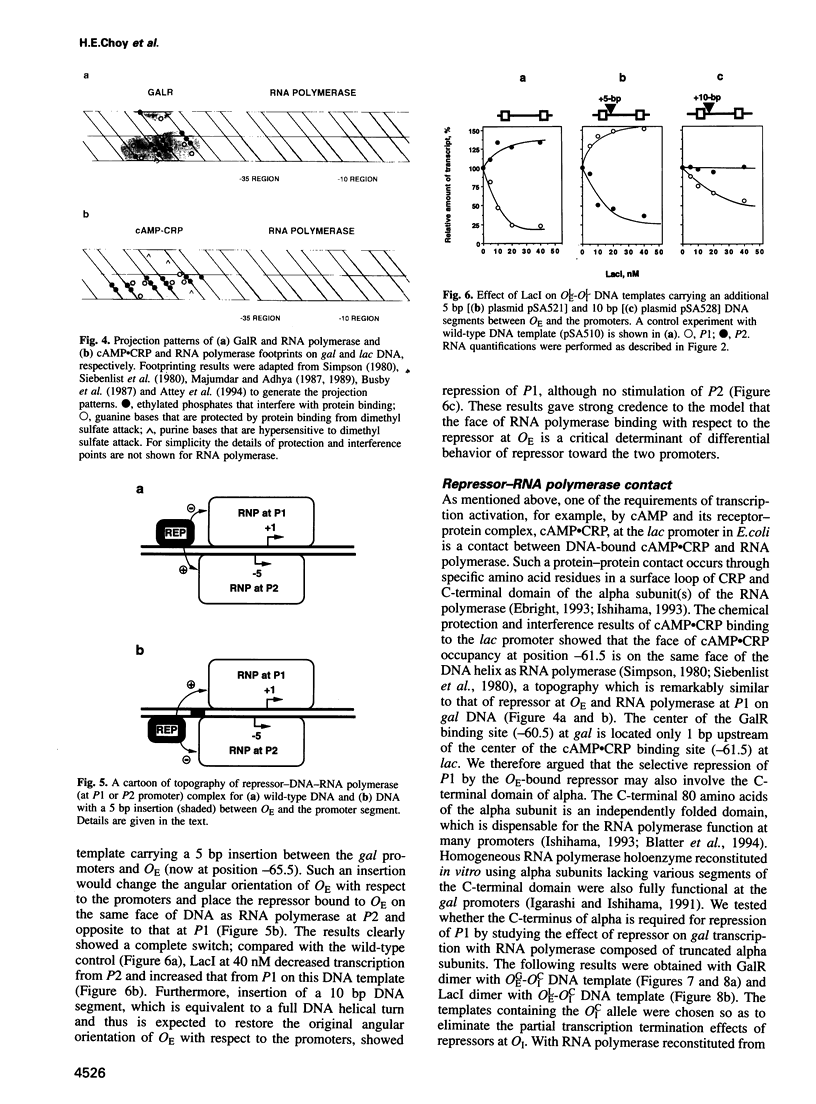
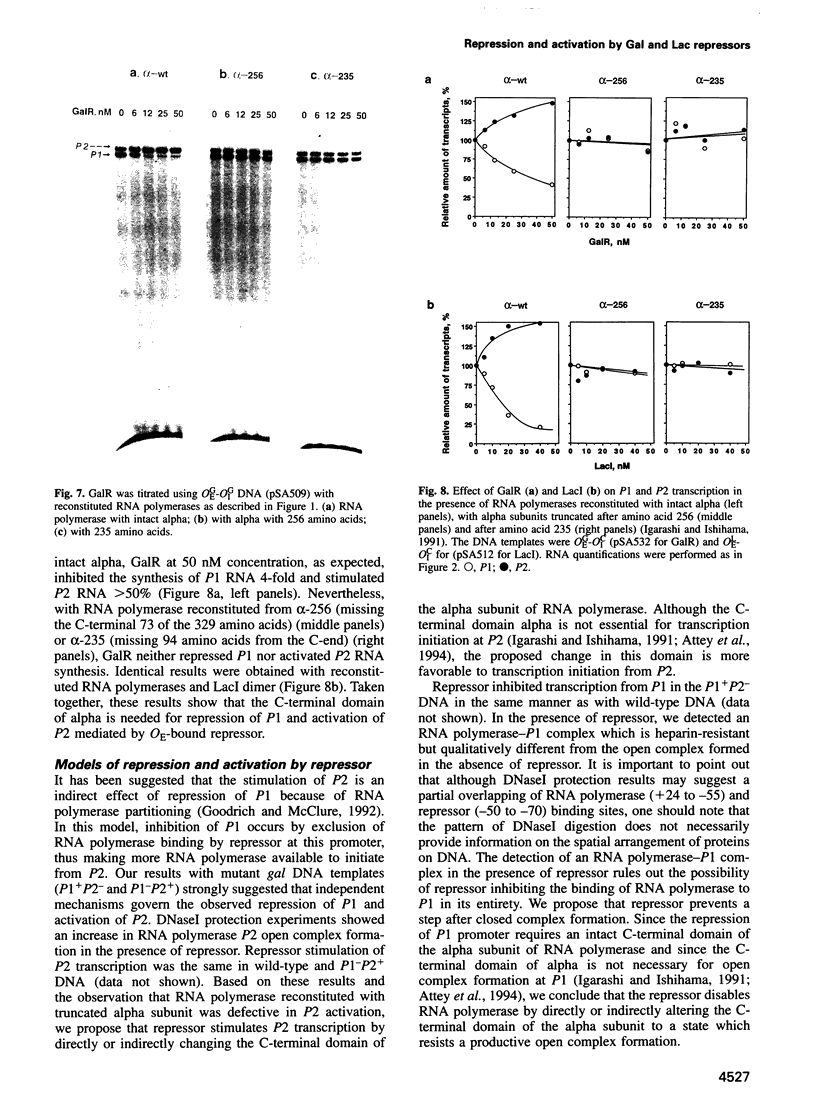
Gal or Lac repressor binding to an upstream DNA segment, in the absence of DNA looping, represses the P1 promoter located on the same face and activates the P2 promoter situated on the opposite face of the DNA helix in the gal operon. Both inhibition and stimulation of transcription requires the physical presence of the C-terminal domain of the alpha subunit of RNA polymerase although the latter is not required for transcription itself. We propose that Gal and Lac repressors inhibit or stimulate transcription initiation by disabling or stimulating RNA polymerase activity at a post-binding step by directly or indirectly altering the C-terminal alpha domain to an unfavorable state at P1 or a more favorable state at P2, respectively.
Full text
PDF
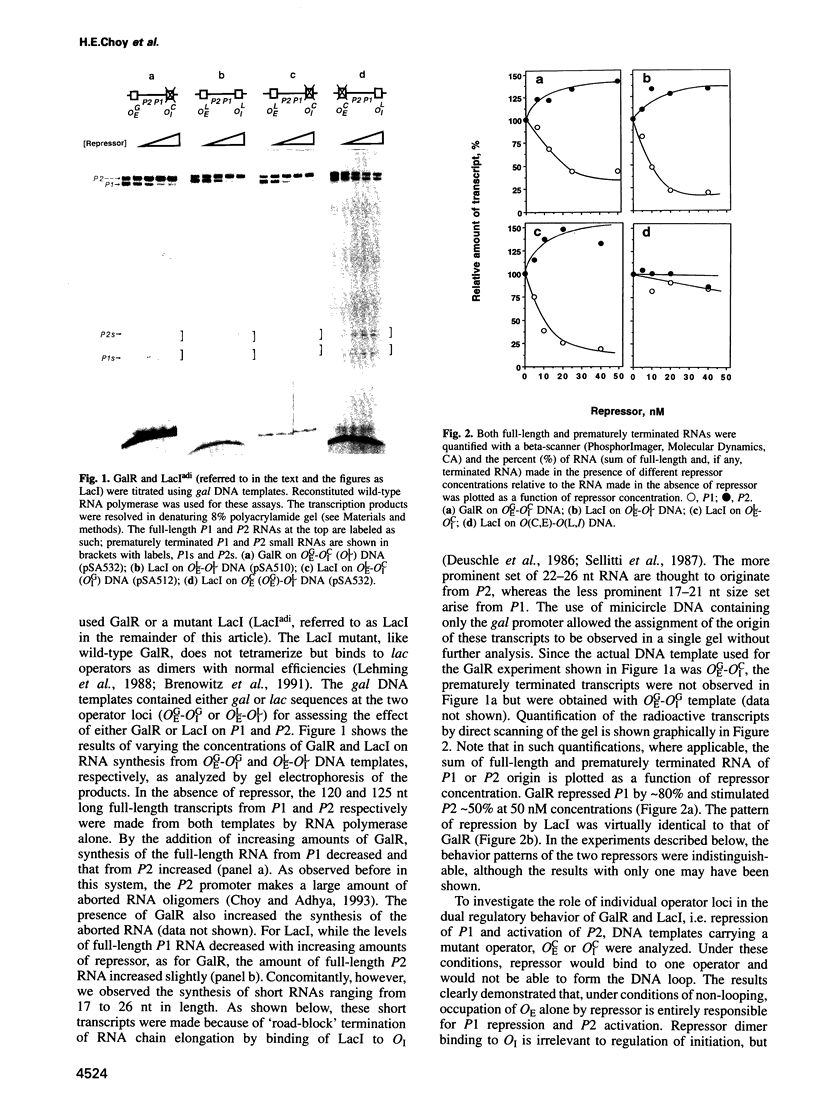


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S. Multipartite genetic control elements: communication by DNA loop. Annu Rev Genet. 1989;23:227–250. doi: 10.1146/annurev.ge.23.120189.001303. [DOI] [PubMed] [Google Scholar]
- Alberti S., Oehler S., von Wilcken-Bergmann B., Krämer H., Müller-Hill B. Dimer-to-tetramer assembly of Lac repressor involves a leucine heptad repeat. New Biol. 1991 Jan;3(1):57–62. [PubMed] [Google Scholar]
- Attey A., Belyaeva T., Savery N., Hoggett J., Fujita N., Ishihama A., Busby S. Interactions between the cyclic AMP receptor protein and the alpha subunit of RNA polymerase at the Escherichia coli galactose operon P1 promoter. Nucleic Acids Res. 1994 Oct 25;22(21):4375–4380. doi: 10.1093/nar/22.21.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham A. H., Ponnambalam S., Chan B., Busby S. Mutations that reduce expression from the P2 promoter of the Escherichia coli galactose operon. Gene. 1986;41(1):67–74. doi: 10.1016/0378-1119(86)90268-4. [DOI] [PubMed] [Google Scholar]
- Blatter E. E., Ross W., Tang H., Gourse R. L., Ebright R. H. Domain organization of RNA polymerase alpha subunit: C-terminal 85 amino acids constitute a domain capable of dimerization and DNA binding. Cell. 1994 Sep 9;78(5):889–896. doi: 10.1016/s0092-8674(94)90682-3. [DOI] [PubMed] [Google Scholar]
- Brenowitz M., Jamison E., Majumdar A., Adhya S. Interaction of the Escherichia coli Gal repressor protein with its DNA operators in vitro. Biochemistry. 1990 Apr 3;29(13):3374–3383. doi: 10.1021/bi00465a033. [DOI] [PubMed] [Google Scholar]
- Brenowitz M., Mandal N., Pickar A., Jamison E., Adhya S. DNA-binding properties of a lac repressor mutant incapable of forming tetramers. J Biol Chem. 1991 Jan 15;266(2):1281–1288. [PubMed] [Google Scholar]
- Brodolin K. L., Studitsky V. M., Mirzabekov A. D. Conformational changes in E. coli RNA polymerase during promoter recognition. Nucleic Acids Res. 1993 Dec 11;21(24):5748–5753. doi: 10.1093/nar/21.24.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S., Spassky A., Chan B. RNA polymerase makes important contacts upstream from base pair -49 at the Escherichia coli galactose operon P1 promoter. Gene. 1987;53(2-3):145–152. doi: 10.1016/0378-1119(87)90002-3. [DOI] [PubMed] [Google Scholar]
- Bushman F. D., Shang C., Ptashne M. A single glutamic acid residue plays a key role in the transcriptional activation function of lambda repressor. Cell. 1989 Sep 22;58(6):1163–1171. doi: 10.1016/0092-8674(89)90514-x. [DOI] [PubMed] [Google Scholar]
- Bölker M., Kahmann R. The Escherichia coli regulatory protein OxyR discriminates between methylated and unmethylated states of the phage Mu mom promoter. EMBO J. 1989 Aug;8(8):2403–2410. doi: 10.1002/j.1460-2075.1989.tb08370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy H. E., Adhya S. Control of gal transcription through DNA looping: inhibition of the initial transcribing complex. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11264–11268. doi: 10.1073/pnas.89.23.11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy H. E., Adhya S. RNA polymerase idling and clearance in gal promoters: use of supercoiled minicircle DNA template made in vivo. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):472–476. doi: 10.1073/pnas.90.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle U., Gentz R., Bujard H. lac Repressor blocks transcribing RNA polymerase and terminates transcription. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4134–4137. doi: 10.1073/pnas.83.12.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebright R. H. Transcription activation at Class I CAP-dependent promoters. Mol Microbiol. 1993 May;8(5):797–802. doi: 10.1111/j.1365-2958.1993.tb01626.x. [DOI] [PubMed] [Google Scholar]
- Frantz B., O'Halloran T. V. DNA distortion accompanies transcriptional activation by the metal-responsive gene-regulatory protein MerR. Biochemistry. 1990 May 22;29(20):4747–4751. doi: 10.1021/bi00472a001. [DOI] [PubMed] [Google Scholar]
- Fritz H. J., Bicknäse H., Gleumes B., Heibach C., Rosahl S., Ehring R. Characterization of two mutations in the Escherichia coli galE gene inactivating the second galactose operator and comparative studies of repressor binding. EMBO J. 1983;2(12):2129–2135. doi: 10.1002/j.1460-2075.1983.tb01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J. A., McClure W. R. Regulation of open complex formation at the Escherichia coli galactose operon promoters. Simultaneous interaction of RNA polymerase, gal repressor and CAP/cAMP. J Mol Biol. 1992 Mar 5;224(1):15–29. doi: 10.1016/0022-2836(92)90573-3. [DOI] [PubMed] [Google Scholar]
- Haber R., Adhya S. Interaction of spatially separated protein-DNA complexes for control of gene expression: operator conversions. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9683–9687. doi: 10.1073/pnas.85.24.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heltzel A., Lee I. W., Totis P. A., Summers A. O. Activator-dependent preinduction binding of sigma-70 RNA polymerase at the metal-regulated mer promoter. Biochemistry. 1990 Oct 16;29(41):9572–9584. doi: 10.1021/bi00493a011. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Ishihama A. Bipartite functional map of the E. coli RNA polymerase alpha subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell. 1991 Jun 14;65(6):1015–1022. doi: 10.1016/0092-8674(91)90553-b. [DOI] [PubMed] [Google Scholar]
- Irani M. H., Orosz L., Adhya S. A control element within a structural gene: the gal operon of Escherichia coli. Cell. 1983 Mar;32(3):783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- Ishihama A. Protein-protein communication within the transcription apparatus. J Bacteriol. 1993 May;175(9):2483–2489. doi: 10.1128/jb.175.9.2483-2489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb A., Busby S., Buc H., Garges S., Adhya S. Transcriptional regulation by cAMP and its receptor protein. Annu Rev Biochem. 1993;62:749–795. doi: 10.1146/annurev.bi.62.070193.003533. [DOI] [PubMed] [Google Scholar]
- Kuhnke G., Krause A., Heibach C., Gieske U., Fritz H. J., Ehring R. The upstream operator of the Escherichia coli galactose operon is sufficient for repression of transcription initiated at the cyclic AMP-stimulated promoter. EMBO J. 1986 Jan;5(1):167–173. doi: 10.1002/j.1460-2075.1986.tb04192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuldell N., Hochschild A. Amino acid substitutions in the -35 recognition motif of sigma 70 that result in defects in phage lambda repressor-stimulated transcription. J Bacteriol. 1994 May;176(10):2991–2998. doi: 10.1128/jb.176.10.2991-2998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S., North A. K., Weiss D. S. Prokaryotic transcriptional enhancers and enhancer-binding proteins. Trends Biochem Sci. 1991 Nov;16(11):397–402. doi: 10.1016/0968-0004(91)90163-p. [DOI] [PubMed] [Google Scholar]
- Lanzer M., Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne M., Kolb A., Buc H. Transcription activation by cAMP receptor protein (CRP) at the Escherichia coli gal P1 promoter. Crucial role for the spacing between the CRP binding site and the -10 region. Biochemistry. 1992 Oct 13;31(40):9647–9656. doi: 10.1021/bi00155a018. [DOI] [PubMed] [Google Scholar]
- Li M., Moyle H., Susskind M. M. Target of the transcriptional activation function of phage lambda cI protein. Science. 1994 Jan 7;263(5143):75–77. doi: 10.1126/science.8272867. [DOI] [PubMed] [Google Scholar]
- Majumdar A., Adhya S. Effect of ethylation of operator-phosphates on Gal repressor binding. DNA contortion by repressor. J Mol Biol. 1989 Jul 20;208(2):217–223. doi: 10.1016/0022-2836(89)90383-5. [DOI] [PubMed] [Google Scholar]
- Majumdar A., Adhya S. Probing the structure of gal operator-repressor complexes. Conformation change in DNA. J Biol Chem. 1987 Sep 25;262(27):13258–13262. [PubMed] [Google Scholar]
- Majumdar A., Rudikoff S., Adhya S. Purification and properties of Gal repressor:pL-galR fusion in pKC31 plasmid vector. J Biol Chem. 1987 Feb 15;262(5):2326–2331. [PubMed] [Google Scholar]
- Mandal N., Su W., Haber R., Adhya S., Echols H. DNA looping in cellular repression of transcription of the galactose operon. Genes Dev. 1990 Mar;4(3):410–418. doi: 10.1101/gad.4.3.410. [DOI] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Pittard A. J., Davidson B. E. TyrR protein of Escherichia coli and its role as repressor and activator. Mol Microbiol. 1991 Jul;5(7):1585–1592. doi: 10.1111/j.1365-2958.1991.tb01904.x. [DOI] [PubMed] [Google Scholar]
- Ryu S., Garges S., Adhya S. An arcane role of DNA in transcription activation. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8582–8586. doi: 10.1073/pnas.91.18.8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlax P. J., Capp M. W., Record M. T., Jr Inhibition of transcription initiation by lac repressor. J Mol Biol. 1995 Jan 27;245(4):331–350. doi: 10.1006/jmbi.1994.0028. [DOI] [PubMed] [Google Scholar]
- Sellitti M. A., Pavco P. A., Steege D. A. lac repressor blocks in vivo transcription of lac control region DNA. Proc Natl Acad Sci U S A. 1987 May;84(10):3199–3203. doi: 10.1073/pnas.84.10.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Simpson R. B. Interaction of the cAMP receptor protein with the lac promoter. Nucleic Acids Res. 1980 Feb 25;8(4):759–766. [PMC free article] [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Summers A. O. Untwist and shout: a heavy metal-responsive transcriptional regulator. J Bacteriol. 1992 May;174(10):3097–3101. doi: 10.1128/jb.174.10.3097-3101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]