Abstract
Background
Youth exposed to extreme adverse life conditions have blunted cortisol responses to stress.
Purpose
To examine whether growing up in highly stigmatizing environments similarly shapes stigmatized individuals’ physiological responses to identity-related stress.
Methods
We recruited 74 lesbian, gay, and bisexual young adults (mean age=23.68) from 24 states with varying levels of structural stigma surrounding homosexuality. State-level structural stigma was coded based on several dimensions, including policies that exclude sexual minorities from social institutions (e.g., same-sex marriage). Participants were exposed to a laboratory stressor, the Trier Social Stress Test (TSST), and neuroendocrine measures were collected.
Results
LGB young adults who were raised in highly stigmatizing environments as adolescents evidenced a blunted cortisol response following the TSST compared to those from low-stigma environments.
Conclusions
The stress of growing up in environments that target gays and lesbians for social exclusion may exert biological effects that are similar to traumatic life experiences.
Keywords: stigma, HPA axis reactivity, sexual orientation
Researchers interested in the causes of social inequalities in health have long focused on chronic stress as a potential mechanism through which harmful social environments increase risk for negative health outcomes (1, 2). One pathway that has received particular attention in this literature involves changes in the functioning of the hypothalamic-pituitary-adrenocortical (HPA) axis, which is activated specifically by social and evaluative threats (3) and results in a coordinated neuroendocrine response that culminates in the production of cortisol. HPA axis function is influenced by a wide range of adverse social experiences, including poverty (4, 5) and childhood maltreatment (6–10).
Stigma and discrimination are characteristics of the social environment that also contribute to health disparities between minority and majority group members (11, 12). Although stigma has been conceptualized as a chronic stressor that makes adaptational demands on stigmatized individuals (13, 14), there is a paucity of research examining the impact of stigma on HPA axis functioning. Indeed, the majority of research on relationships between stigma, discrimination, and physiological responses to stress has focused on cardiovascular reactivity and functioning (15). Recent studies have begun to address this gap through two methodological approaches. Diurnal rhythm studies use observational designs to examine whether different forms of stigma are correlated with measures of cortisol taken at various points throughout the day. For example, African Americans who perceived more discrimination experienced a steeper (i.e., healthier) diurnal slope in cortisol compared to African Americans who perceived less discrimination (16). In contrast, gay men who disclosed their sexual orientation in the workplace evidenced heightened levels of workday cortisol compared to those who concealed their sexual orientation (17), suggesting that in certain settings disclosing a stigmatized identity can result in physiological dysregulation. Studies of HPA axis reactivity, on the other hand, typically utilize experimental designs to manipulate exposure to stigma, discrimination, or other types of social and evaluative stressors in the laboratory. In a recent example of this work, women who chronically perceived more sexism exhibited higher cortisol output when being evaluated by a man than women who did not chronically perceive sexism (18).
This research has provided important initial insights into the relationships between stigma and HPA axis functioning but has focused on individual (e.g., stigma consciousness, self-stigma) and interpersonal (e.g., discrimination, disclosure) forms of stigma. Importantly, stigma can also occur above the individual and interpersonal levels of analysis. This concept of macro-level stigma, which has been termed structural stigma, refers to societal conditions and institutional practices that constrain stigmatized individuals’ opportunities, resources, and wellbeing (11, 19). Examples of structural stigma include Jim Crow laws, which became a prominent means of maintaining white privilege in Southern states (20), as well as “redlining” policies that systematically contributed to racial residential segregation in urban neighborhoods (21). Structural stigma and individual-level stigma are conceptualized as distinct constructs (11). Although studies that include measures of both structural and individual forms of stigma are rare, there is some evidence that the two variables are not strongly correlated. For instance, a recent study found that there was no relationship between Latinos’ perceptions of discrimination and states with more negative immigration policies (i.e., perceptions of discrimination did not vary across geographic localities) (22). The lack of statistical correlation is not altogether surprising, given that most measures of perceived stigma capture interpersonal events and interactions, rather than structural conditions.
The extent to which structural forms of stigma affect HPA axis functioning is largely unknown. The current study therefore expands the existing literature by evaluating whether exposure to structural stigma alters HPA reactivity among members of a stigmatized group, namely lesbian, gay, and bisexual (LGB) individuals. We focus on LGB populations because this group currently confronts multiple forms of structural stigma in the United States. Indeed, states currently vary substantially in the policies, laws, and social environments that create unfairness and structured exclusion of gays and lesbians. Researchers have taken advantage of this state-level variation to create measures of structural stigma, and several recent studies utilizing these measures have documented robust associations between structural stigma and poor health outcomes. For instance, LGB adults who lived in states that passed constitutional amendments banning same-sex marriage in 2004 experienced a 37% increase in mood disorders, a 42% increase in alcohol use disorders and a 248% increase in generalized anxiety disorders in the 12 months following the passage of the amendments (23). Additionally, sexual orientation disparities in psychiatric morbidity are significantly greater in states without hate crime and employment non-discrimination laws that include sexual orientation as a protected class status (24). This research suggests that living in particular states can structure opportunities and resources differently for LGB individuals and that the U.S. state is a meaningful areal unit in which to examine variation in structural stigma. The degree to which structural stigma influences other aspects of health and physiological functioning remains unknown, however.
In this study, we therefore asked the following question: Can growing up in highly stigmatizing states shape stigmatized individuals’ subsequent physiological responses to identity-related stress? To answer this question, we recruited LGB respondents from a large number of states with varying levels of structural stigma surrounding homosexuality and then exposed these participants to a well-validated laboratory stressor that produces a reliable cortisol response. By linking measures of structural stigma to individual-level data on HPA axis reactivity, we were able to evaluate for the first time the extent to which exposure to structural stigma during adolescence alters neuroendocrine responses to identity-related stress. As a secondary aim, we examined whether structural stigma was associated with HPA axis reactivity over and above perceived stigma, measured at the individual level. To our knowledge, no previous study has examined whether different forms of stigma (structural, individual) are independently associated with neuroendocrine functioning.
Methods
Participants
We recruited 77 LGB young adults (ages 18–30; mean age=23.68, SD=4.12) from colleges and the broader community in a large metropolitan city. Fliers that were used to recruit participants stated: “Are you gay, lesbian, or bisexual? If so, you can earn up to $25 for participating in a study on life experiences.” Further, participants were told that the purpose of the study was to “understand connections between daily experiences, your bodily activity, and health.” Thus, there was no indication that the study was about stigma or other constructs of interest in the study.
Three respondents were excluded because they did not participate in the laboratory stressor (see below). The final sample (n=74) was fairly evenly split by gender (54% female). Nearly 60% of the sample (n=43) identified as non-White (Table 1). The average age of disclosure to various people in the respondent’s life was as follows: (1) to family: M=17.56 (SD=3.61); (2) to a straight friend: M=16.62 (SD=2.96); and (3) to a friend who was lesbian, gay, or bisexual: M=16.62 (SD=2.86). Thus, on average, our sample was both aware of their sexual orientation and had disclosed it to at least one person during their adolescence.
Table 1.
Descriptive Statistics for Study Variables
| Variable | |
|---|---|
| Demographics |
N or Mean (SD) |
| Sex | |
| Female | 40 (54%) |
| Male | 34 (46%) |
| Race/Ethnicity | |
| White | 31 (42%) |
| Non-White | 43 (58%) |
| Sexual Orientation | |
| Lesbian/Gay | 42 (57%) |
| Bisexual | 32 (43%) |
| Age | 23.68 (4.12) |
| Stigma Variables | Mean (SD) |
| Structural Stigma | 3.66 (2.85) |
| Perceived stigma | 1.78 (0.53) |
Procedures
All participants were run between the hours of 2–7 p.m., given that cortisol reaches its diurnal nadir during this time. Twenty-four hours in advance of their scheduled visit, participants were emailed asking them to refrain from activities that could influence their cortisol on the day of the visit, including brushing their teeth or drinking caffeinated beverages within 4 hours of their scheduled time and exercising at any point during that day.
After providing informed consent, participants sat comfortably for a 5-minute resting period, following which the first saliva sample was collected. Participants then completed a battery of self-report questionnaires, including demographics, information for covariates, and stigma items (see below). Next, participants completed the Trier Social Stress Test (TSST)(25), a social-evaluative threat task that produces a reliable cortisol response (3). The TSST was administered following standard procedures, including a preparation, speech, and math component (25–27). Participants were told they would prepare and deliver a 5-minute speech, which would be videotaped, to a panel of two interviewers who were introduced as researchers with “extensive experience in evaluating speech.” In reality, these two interviewers were confederates who were trained to behave neutrally (i.e., to provide neither positive nor negative feedback during the speech). In order to make the speech identity-relevant, and to ensure that the social evaluation was salient, the topic of the speech was to “discuss an experience in which you were rejected based on your sexual orientation.”
Immediately following the speech task, participants completed a five-minute math task in which they were given a three-digit number and told to serially subtract backwards from the number in steps of 7. If the participant made an error, they were told that they must begin the task from the beginning. The second saliva sample was taken 20 minutes after the speech task started (reactivity), and the final saliva sample was taken 20 minutes after the math task ended (recovery).
Measures
Structural Stigma
We used an existing measure of structural stigma, which in previous studies was associated with suicide attempts (28) and tobacco use (29) among LGB youth. This measure is composed of 4 different items that together create an index of the social environment surrounding LGB populations. The first item was the density of same-sex partner households by state. Data were obtained from the Census Bureau’s Census 2000 Summary File. This measure assessed where gay and lesbian couples live relative to the general population, depicting the extent to which they are over- or under-represented in a particular state. The index was calculated using the total number of households, as well as the number of households headed by a male and female same-sex unmarried partner couple for each census tract or county (30). These numbers were summed over the entire state. This total was then transformed into a relative proportion. For example, a value of 2.0 means that same-sex couples were twice as likely to be living in a particular state compared to typical households.
The second item was the proportion of Gay Straight Alliances (GSAs) per public high school in the state. Data on GSAs were obtained from Gay, Lesbian, and Straight Education Network for the year 2006; the number of public high schools in the state was obtained from the National Center for Education Statistics. We divided the number of GSAs by the number of public high schools in that state to create this item.
Third, we included 5 state-level policies related to sexual orientation in the year 2000: (1) absence of constitutional amendments banning same-sex marriage; (2) employment non-discrimination policies that include sexual orientation; (3) hate crime policies that include sexual orientation as a protected class status; (4) a non-discrimination policy that extended to LGB students, and/or a statute banning bullying based explicitly on sexual orientation; and (5) statutes that do not explicitly restrict gay and lesbian couples from adoption). Each policy was coded 0/1 for presence or absence; these values were then summed, with a possible range from 0–5.
The fourth measure was public opinion toward sexual minorities in each U.S. state. Lax and Phillips (31) aggregated responses from 41 national polls from the Roper Center’s iPol archive, dating from 1999–2008. These polls, which were random national samples conducted by various organizations (e.g., Gallup, Pew), yielded approximately 80,000 responses. Policy-specific opinions were collected for the following areas: gay adoption, hate crimes, health benefits, discrimination in jobs and housing, marriage, sodomy, and civil unions (e.g., "Do you think there should be adoption rights for gay and lesbian couples?"). We used the mean value for these 7 opinions by state.
A factor analysis of the 4 items (density of same-sex couples, presence of GSA’s, policies, and attitudes) indicated that they loaded onto a single factor (factor loadings ranged from 0.79 to 0.97). The items also demonstrated good internal consistency (α=0.77), indicating that they could be combined into a single measure. Because the different indicators were measured on different scales, each of the 4 items was standardized (i.e., M=0, SD=1); we then summed the z-transformed score of each item to create an overall index of structural stigma in that state. Values for structural stigma ranged from −4.69 to 8.23, indicating substantial variation in structural stigma across the 24 states. Positive scores indicate more supportive social climates, and therefore lower levels of structural stigma. The mean for the structural stigma measure was 3.66 (SD=2.85); thus, overall, the 24 states tended to have relatively low levels of structural stigma.
Respondents were asked their state of residence during the ages of 10–18; for those who lived in more than one location, they were asked the length of time lived in each state. Respondents lived in 24 states during adolescence. Analyses were conducted on the state of longest residence. We examined the structural stigma scale in two ways in our analysis. First, we examined it as a continuous measure, to determine whether structural stigma was associated with cortisol reactivity across the entire distribution of structural stigma scores. Second, we created tertiles of the scale, with the top tertile (n=29) representing states with particularly low levels of structural stigma (e.g., Massachusetts, New York) and the bottom two-thirds (n=45) representing states with relatively high levels of structural stigma (e.g., Mississippi, Georgia, Ohio).
Perceived Stigma
We used a 6-item version of the Perceived Devaluation-Discrimination Scale (32) (α=0.81) to measure stigma at the individual level of analysis. The items were originally written for individuals with mental illness but were adapted for LGB respondents. Respondents were asked whether they strongly agree (0), agree (1), disagree (2), or strongly disagree (3) with statements indicating that most people devaluate or discriminate against people who are gay or lesbian (e.g., “Most people think less of gay people”).
Neuroendocrine Measures
Neuroendocrine samples were obtained with cryovial tubes (Salimetrics) using the drool method. Participants expectorated approximately 1 ml of saliva into a cryovial with a plastic straw. Saliva samples were stored immediately at −80°C until they were shipped overnight on dry ice to a laboratory in Boston, Massachusetts, where they were assayed for salivary free cortisol, using a commercially available luminescence immunoassay (CLIA; IBL-Hamburg, Hamburg, Germany). Intra-assay (2.73%) and inter-assay (9.22%) coefficients of variance were both acceptable.
To examine cortisol reactivity, we used each of the three cortisol measures (baseline, reactivity, recovery) to compute area under the curve (AUC) with respect to increase using standard procedures (33, 34). AUC with respect to increase represents the time-dependent change in cortisol relative to the baseline resting value (33, 34). Approximately two-thirds (62.2%) of participants exhibited an increase in cortisol during the TSST. Other studies using the TSST with participants in the same age range as those in our study also found considerable heterogeneity in stress responses, with approximately one-quarter to one-third of participants failing to exhibit a cortisol response to the task (35).
Following standard procedures for examining time-dependent increases in cortisol (34), respondents who did not exhibit a cortisol increase were coded as zero on the AUC measure. This procedure was used to examine cortisol reactivity only among participants who exhibited a cortisol response to the TSST. We note, however, that including respondents who did not exhibit a cortisol increase in the analyses did not change the interpretation of the study’s finding (i.e., the direction and magnitude of the effect were similar). Cortisol AUC among those who exhibited a cortisol response to the TSST ranged from a low of 0.3 to a high of 515.0 (M=88.10, SD=124.25).
Statistical Analysis
We examined the association of structural stigma with cortisol reactivity in two ways. First, we examined the continuous measure of structural stigma as a predictor of cortisol AUC using linear regression. Second, we examined the structural stigma scale divided into tertiles. We compared cortisol reactivity for participants living in high structural stigma environments (the lower two tertiles of the measure) during adolescence to that of participants living in low-stigma environments (the highest tertile of the measure). To do so, we conducted a univariate analysis of covariance (ANCOVA) with structural stigma as a between-subjects variable. All analyses included a standard set of covariates used in cortisol studies (36), including sex, age, race (White vs. Non-White), waking time on morning of the experiment, as well as any smoking, exercise, and caffeine use on the day of the experiment. The final model also included perceived stigma as an additional covariate.
Results
Descriptive Statistics
Table 1 depicts descriptive statistics for the structural and perceived stigma variables. On average, respondents perceived high levels of stigma against gays and lesbians (M=1.78, SD=0.53). For instance, nearly half of the sample (45.9%) strongly agreed or agreed with the statement that “Most people look down on gay people.” The correlation between structural and perceived stigma was small (r = 0.13) and not statistically significant (p = 0.28).
Relationships between structural stigma and cortisol response
We first examined the continuous measure of structural stigma as a predictor of cortisol AUC. After controlling for the standard set of covariates, higher state-level structural stigma (i.e., a lower score on structural stigma index) was not significantly associated with reduced cortisol AUC among those who exhibited a cortisol response to the task, β = 0.12, p = 0.26.
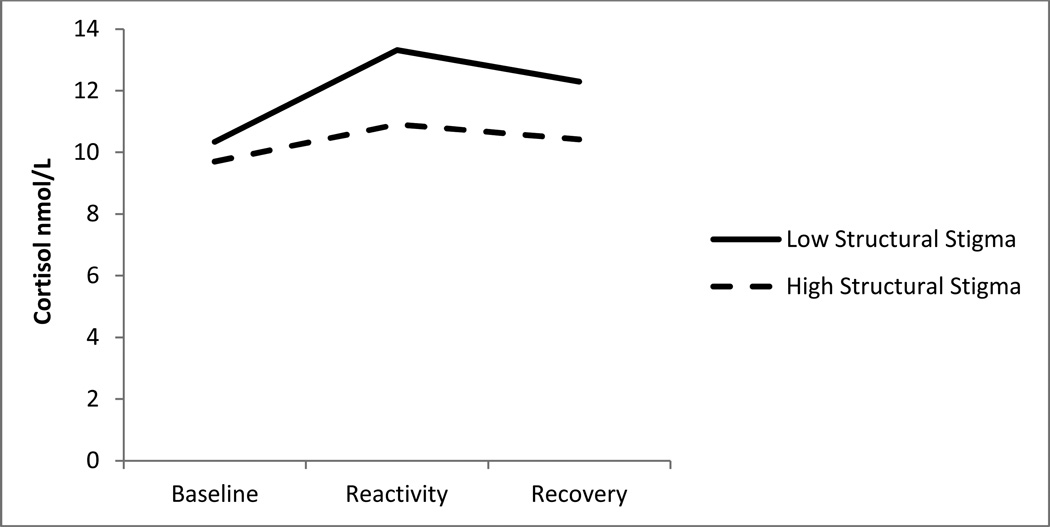
In the second set of analyses, we examined the structural stigma scale divided into tertiles and compared cortisol reactivity for participants living in high structural stigma environments (the lower two tertiles of the measure) during adolescence to that of participants living in low-stigma environments (the highest tertile of the measure). After controlling for covariates, state-level structural stigma during adolescence was associated with cortisol reactivity to the TSST, F(1,71) = 4.57, p = .037, η2 = 0.07 (see Figure 1). Cortisol AUC for individuals living in low structural stigma states (adjusted M = 124.68) was greater than cortisol AUC for individuals living in high-stigma states (adjusted M = 62.68), indicating blunted cortisol responses for those from states with high structural stigma.
Figure 1. Blunted Cortisol Response Associated with Exposure to Structural Stigma.
Notes. Cortisol responses to the Trier Social Stress Test. Low structural stigma represents individuals living in states in the top tertile of supportiveness during adolescence, and high structural stigma represents individuals living in states in the bottom two tertiles. Y-axis refers to cortisol in nmol/L. The figure does not have a relation to the statistical model; it is simply a representation of the mean cortisol values at each time point for the high and low objective stigma groups. The figure is provided so that readers can more easily interpret the results of the ANCOVA model.
In a final model that included perceived stigma as an additional covariate (Table 2), structural stigma remained significantly associated with cortisol reactivity, F(1,70) = 4.45, p = .039, η2 = 0.07. In contrast, perceived stigma was not independently associated with cortisol reactivity in this model (p > .05).
Table 2.
Fully-adjusted Model Predicting Cortisol Area Under the Curve as a Function of Structural and Perceived Stigma
| df | F | p-value | η2 | |
|---|---|---|---|---|
| Independent Variable | ||||
| Structural Stigma | 1 | 4.45 | .039 | .07 |
| Covariates | ||||
| Gender | 1 | 7.83 | .007 | .11 |
| Age | 1 | 2.01 | .161 | .03 |
| Race | 1 | 0.66 | .418 | .01 |
| Waking Time | 1 | 4.46 | .039 | .07 |
| Smoking | 1 | 0.96 | .331 | .02 |
| Exercise | 1 | 1.12 | .294 | .02 |
| Caffeine Use | 1 | 0.02 | .882 | .00 |
| Perceived Stigma | 1 | 3.39 | .071 | .05 |
| Error | 62 | |||
| Total | 70 | |||
Notes. Structural stigma is reverse coded such that higher scores indicate low structural stigma.
Discussion
Cortisol has been widely studied in the context of general life stressors (37). Surprisingly, despite the relevance of cortisol to the study of stigma and other minority stressors, very few studies have examined stigma and HPA axis reactivity among LGB populations. Those addressing this topic have focused exclusively on stigma at the interpersonal level of analysis, through examining the effect of disclosure on cortisol response in LGB individuals (17, 38). In the present study, we extend this literature and show for the first time that prior exposure to structural stigma shapes subsequent HPA axis reactivity among members of a stigmatized group. Specifically, LGB young adults who were raised in highly stigmatizing environments as adolescents evidenced a blunted cortisol response following the TSST, compared to those from low-stigma environments. We found a relationship between structural stigma and cortisol reactivity using a dichotomous measure of structural stigma, but not with a continuous measure, suggesting that only high levels of structural stigma are associated with cortisol reactivity, rather than a relationship between structural stigma and cortisol reactivity existing along the entire distribution of exposure to structural stigma (i.e., the relationship is not linear).
These results are consistent with numerous studies documenting that youths exposed to extreme adverse life conditions, such as childhood maltreatment, have blunted cortisol in response to stress (e.g., (6, 7)). Similarly, among a sample of healthy college students, those with a greater number of adverse life events were more likely to have a diminished cortisol response to the TSST (39). Individuals with post-traumatic stress disorder (PTSD) and other forms of severe trauma also appear to have basal hypocortisolism (40). In a seminal article that synthesized the literature on stress and the HPA axis, Miller and colleagues (37) posited several factors that may result in diminished HPA axis reactivity, including chronic stressors, stress that is severe and persistent, and stress that results in feelings of shame. Each of these factors (chronicity, severity, and persistence of stress, feelings of shame) are core components of minority stress among LGB populations (13). Thus, the stress of growing up in environments that target gays and lesbians for social exclusion may exert biological effects that are similar to traumatic life experiences.
Why might exposure to structural stigma in adolescence result in a blunted cortisol response to identity-related stress in early adulthood? A similar pattern of cortisol hypo-response following psychosocial stress has been observed among individuals exposed to other types of adverse environments, including child maltreatment, poverty, and deprivation (4, 5, 8–10). There are several neurobiological mechanisms that might explain this pattern. One explanation is that corticotrophin releasing hormone (CRH) receptors in the pituitary are down-regulated following chronic exposure to stressors or traumatic events as a result of CRH hyper-secretion in the hypothalamus (41, 42). Another possibility is that increased production of cortisol as a result of chronic stress exposure results in heightened negative feedback sensitivity to glucocorticoids, a process mediated by glucocorticoid receptors in the hippocampus, which inhibit CRH production in the hypothalamus and terminate the HPA axis response to a stressor (43–46).
We raise three key points to clarify the interpretation of these results regarding the relationship between structural stigma and cortisol response in LGB young adults. First, the HPA axis response to chronic stress may vary across development. In a longitudinal study, girls exposed to sexual abuse exhibited hyper-cortisolism in childhood and early adolescence that eventually transformed into a pattern of hypo-cortisolism in mid- to late adolescence (47). Conversely, child maltreatment has been linked to elevated cortisol reactivity in samples of adults (48). Second, interpretation of whether a particular pattern of cortisol reactivity is adaptive or maladaptive is challenging, and previous studies have found that psychosocial stress exposure is associated with both blunted and elevated cortisol reactivity (6, 7, 48, 49). Thus, we cannot assume that the blunted pattern of reactivity observed here is either adaptive or maladaptive. Third, the timing of exposure to stress may also determine the nature of cortisol response to stressors. In a cohort study in the Netherlands, exposure to adversity in childhood was associated with heightened cortisol response to a psychosocial stressor in adolescence whereas exposure to adverse environments in adolescence was associated with a blunted cortisol response (50), similar to the pattern of blunted response observed here in relation to structural stigma during adolescence. However, we cannot test whether the relationship between structural stigma and cortisol response was specific to the developmental period of adolescence, because we do not have data on where the LGB youth lived during childhood (they were asked about state of residence from ages 10–18).
Second, the topic of the speech in the TSST involved an identity relevant topic, rather than a more general stress task (e.g., giving a speech about a historical figure or current event). There are some strengths to this approach, including the fact that social evaluation is a key component of sexual minority stigma (51) and that stressors involving social evaluation are common experiences regardless of sexual orientation. However, we cannot make claims about generalizability of these results across multiple types of stressors, since only one was examined in this study. Future studies should examine whether the relationships between structural stigma and cortisol response observed here are found in other stressful contexts that are not related to a sexual minority identity.
As a secondary aim, we took advantage of the rare opportunity to examine whether individual and structural measures of stigma were independently associated with HPA axis functioning. When both forms of stigma were entered as predictors, only structural stigma remained significantly associated with cortisol reactivity. Thus, structural stigma was a stronger correlate of HPA axis functioning than subjective appraisals of stigma. There are several possible reasons for these results. Given that most of our respondents perceived that homosexuality is stigmatized, there could be a restricted range to this variable. In addition, a relatively small sample size, and the addition of several covariates—particularly those (like structural stigma) that are strongly related to the outcome—increases the likelihood that other variables in the model become non-significant. Further, there are reporting biases with subjective measures of stigma (52) that could affect the ability to detect relationships between perceived stigma and cortisol reactivity. For instance, if some of the psychological processes that stigmatized individuals engage in to reduce the harmful effects of stigma, such as coping efforts (53), are associated with both a tendency to report stigma and with HPA axis reactivity, this could lead to an underestimate of the effect of perceived stigma on cortisol response. Finally, our measure of perceived stigma, the Perceived Devaluation-Discrimination Scale, captures only one aspect of stigma at the individual level. It is possible that other dimensions of individual and interpersonal forms of stigma, such as discrimination experiences, could affect HPA axis reactivity, a possibility that should be explored in future research.
Similar to other studies (22), we found that individual and structural forms of stigma were not strongly correlated. Thus, it was not possible to examine perceived stigma as a mediator of the relationship between structural stigma and cortisol reactivity. There are mechanisms other than perceived stigma through which structural stigma could influence cortisol response. For instance, it is likely that gays and lesbians living in high structural stigma states confront greater gay-related victimization and violence than those living in low structural stigma states; exposure to victimization and other stressors in turn have been associated with cortisol reactivity in general population samples (54). Future studies are needed to identify pathways linking structural stigma to HPA axis activation.
This study has a number of limitations. First, the participants were obtained via convenience sampling and are not a representative sample of the states in which they were raised. This not only restricts generalizability of the results, but may also introduce biases in the relationship between structural stigma and HPA axis reactivity. It is difficult to know how this sampling strategy would potentially bias our results, however. On the one hand, it is possible that we captured individuals who found the environments in their initial state of residence especially difficult to endure. In this case, our sample of individuals from high structural stigma states might be especially likely to exhibit a blunted cortisol response, which would overestimate the relationship between structural stigma and HPA axis functioning. On the other hand, most respondents in this study moved to a city that is known for its supportive climate for gays and lesbians and had been living there between one and twelve years. Since low structural stigma environments were associated with a typical stress response to the TSST (i.e., an elevated cortisol response), it is possible that some of the adverse effects of high structural stigma environments were reduced (or reversed) in our sample. In this case, our results would underestimate the associations between structural stigma and HPA axis functioning.
Second, the participants were comfortable in disclosing their sexual orientation to researchers. Although disclosure has been associated with HPA axis functioning in prior studies (17), degree of sexual orientation disclosure was not associated with HPA axis reactivity in our sample (results not shown but available upon request) and is therefore unlikely to bias our results. Third, the LGB young adults in this sample come from only 24 states. However, the structural stigma scale for all 50 states ranges from −5.07 to 8.23, which is very similar to the range observed with our sample (−4.69 to 8.23). Moreover, this restricted range would reduce statistical power to detect an effect.
Fourth, because we were interested in examining how growing up in a stigmatizing environment during adolescence affected later HPA axis reactivity among LGB young adults, it was important that our measure of structural stigma occur when our respondents were adolescents. Two of the four variables comprising the structural stigma variable were obtained in the year 2000 when our sample was, on average, 13 years of age, but information on density of GSAs and the state-level attitudes only became available a few years later. Nevertheless, all indicators of structural stigma occurred when our sample was, on average, under the age of 18. Moreover, when we excluded the oldest participants (for whom the structural stigma variables could have occurred during young adulthood) from the analyses, the results remained unchanged. Thus, although the 4 structural stigma variables come from slightly different years, this did not appear to bias the study results.
Finally, although we were able to control the stressor in the laboratory, we could not experimentally manipulate exposure to structural stigma. Thus, these data represent correlational relationships, and causality cannot be inferred. Nevertheless, because it is unethical to randomly assign individuals to environments with and without structural stigma, the approach used in the current study may be the most feasible method to test these relationships.
Conclusion
Our results suggest that structural stigma should be included in the growing list of adverse social environments that have been associated with alterations in neuroendocrine functioning (5, 6, 55). Exposure to the chronic stress of structural stigma during adolescence appears to disrupt later HPA axis functioning among LGB young adults, and this effect is independent of individual appraisals of stigma. Previous research has indicated that structural forms of stigma are associated with a variety of negative health outcomes in LGB populations (23, 24, 28), but the mechanisms underlying the relationships between structural stigma and health remain inadequately understood. Given the associations of HPA axis dysregulation with both psychiatric disorders (40) and with a range of adverse physical health outcomes, including metabolic syndrome and cardiovascular disease (56), it is possible that this dysregulation is a mechanism linking structural stigma to poor health in LGB individuals, a possibility that should be explored in future research. Future studies are also needed to understand the ways in which other social contexts surrounding LGB youth – including peers, parents, and neighborhoods – may serve to buffer LGB adolescents against the negative psychological and physiological consequences of exposure to structural stigma.
Acknowledgements
The authors acknowledge funding from the National Institutes of Drug Abuse (K01DA032558), the National Institutes of Mental Health (K01MH092526), and a seed grant from the Robert Wood Johnson Foundation Health & Society Scholars program.
Footnotes
Conflicts of Interest Statement
The authors have no conflicts of interest to disclose.
References
- 1.Taylor SE, Repetti RL, Seeman T. Health psychology: what is an unhealthy environment and how does it get under the skin? Annu Rev Psychol. 1997;48:411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- 2.Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- 3.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 4.Evans GW, Kim P. Childhood poverty and health: cumulative risk exposure and stress dysregulation. Psychol Sci. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol Sci. 2010;21:31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- 6.Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: associations with stress reactivity and regulation in 10-12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMillan HL, Georgiades K, Duku EK, et al. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biol Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from romanian orphanages. Dev Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 9.Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Link BG, Phelan JC. Conceptualizing Stigma. Annu Rev Sociol. 2001;27:363–385. [Google Scholar]
- 12.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896:173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 13.Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(6):74–697. doi: 10.1037/0033-2909.129.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Major B, O'Brien LT. The social psychology of stigma. Annu Rev Psychol. 2005;56:393–421. doi: 10.1146/annurev.psych.56.091103.070137. [DOI] [PubMed] [Google Scholar]
- 15.Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller-Rowell TE, Doan SN, Eccles JS. Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology. 2012;37:107–118. doi: 10.1016/j.psyneuen.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huebner DM, Davis MC. Gay and bisexual men who disclose their sexual orientations in the workplace have higher workday levels of salivary cortisol and negative affect. Ann Behav Med. 2005;30:260–267. doi: 10.1207/s15324796abm3003_10. [DOI] [PubMed] [Google Scholar]
- 18.Townsend SS, Major B, Gangi CE, Mendes WB. From "in the air" to "under the skin": cortisol responses to social identity threat. Pers Soc Psychol Bull. 2011;37:151–164. doi: 10.1177/0146167210392384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrigan PW, Watson AC, Heyrman ML, et al. Structural stigma in state legislation. Psychiatr Serv. 2005;56:557–563. doi: 10.1176/appi.ps.56.5.557. [DOI] [PubMed] [Google Scholar]
- 20.Feagin JR. Racist America: Roots, Current Realities, and Future Reparations. New York: Routledge; 2000. [Google Scholar]
- 21.Massey D, Denton N. American Apartheid: Segregation and the Making of the Underclass. Cambridge: Harvard University Press; 1993. [Google Scholar]
- 22.Hopkins DJ, Mummolo J, Esses V, Kaiser C, Morrow H, McDermott M. Out of context: the absence of spatial variation in U.S. immigrants’ perceptions of discrimination. SSRN Working Paper 2108173. 2013 Available at: http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2108173.
- 23.Hatzenbuehler ML, McLaughlin KA, Keyes KM, Hasin DS. The impact of institutional discrimination on psychiatric disorders in lesbian, gay, and bisexual populations: a prospective study. Am J Public Health. 2010;100:452–459. doi: 10.2105/AJPH.2009.168815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatzenbuehler ML, Keyes KM, Hasin DS. State-level policies and psychiatric morbidity in lesbian, gay, and bisexual populations. Am J Public Health. 2009;99:2275–2281. doi: 10.2105/AJPH.2008.153510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 26.Jamieson JP, Nock MK, Mendes WB. Mind over matter: reappraising arousal improves cardiovascular and cognitive responses to stress. J Exp Psychol Gen. 2012;141:417–422. doi: 10.1037/a0025719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruenewald TL, Kemeny ME, Aziz N, Fahey JL. Acute threat to the social self: shame, social self-esteem, and cortisol activity. Psychosom Med. 2004;66:915–924. doi: 10.1097/01.psy.0000143639.61693.ef. [DOI] [PubMed] [Google Scholar]
- 28.Hatzenbuehler ML. The social environment and suicide attempts in lesbian, gay, and bisexual youth. Pediatrics. 2011;127:896–903. doi: 10.1542/peds.2010-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatzenbuehler ML, Wieringa NF, Keyes KM. Community-level determinants of tobacco use disparities in lesbian, gay, and bisexual youth: results from a population-based study. Arch Pediatr Adolesc Med. 2011;165:527–532. doi: 10.1001/archpediatrics.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gates GJ, Ost J. The Gay and Lesbian Atlas. Washington, D.C: The Urban Institute Press; 2004. [Google Scholar]
- 31.Lax JR, Phillips JH. Gay rights in the states: public opinion and policy responsiveness. Am Polit Sci Rev. 2009;103:367–386. [Google Scholar]
- 32.Link BG. Understanding labeling effects in the area of mental-disorders: an assessment of the effects of expectations of rejection. Am Sociol Rev. 1987;52:96–112. [Google Scholar]
- 33.Fekedulegn DB, Andrew ME, Burchfiel CM, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosom Med. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- 34.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 35.Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol. 2004;25:69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Mendes WB, Gray HM, Mendoza-Denton R, Major B, Epel ES. Why egalitarianism might be good for your health: physiological thriving during stressful intergroup encounters. Psychol Sci. 2007;18:991–998. doi: 10.1111/j.1467-9280.2007.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 38.Juster RP, Smith NG, Ouellet E, Sindi S, Lupien SJ. Sexual orientation and disclosure in relation to psychiatric symptoms, diurnal cortisol, and allostatic load. Psychosom Med. 2013;75:103–116. doi: 10.1097/PSY.0b013e3182826881. [DOI] [PubMed] [Google Scholar]
- 39.Elzinga BM, Roelofs K, Tollenaar MS, et al. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Yehuda R, Bierer LM, Schmeidler J, et al. Low cortisol and risk for PTSD in adult offspring of holocaust survivors. Am J Psychiatry. 2000;157:1252–1259. doi: 10.1176/appi.ajp.157.8.1252. [DOI] [PubMed] [Google Scholar]
- 41.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 42.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 44.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 45.Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- 46.Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004;29:389–404. doi: 10.1016/s0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 47.Putnam EW. Second International Conference of Child Abuse and Neglect. Leuven; 2007. The psychobiological effects of sexual abuse: A 20 year prospective study. [Google Scholar]
- 48.Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 49.Bremner JD, Vythilingam M, Vermetten E, et al. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- 50.Bosch NM, Riese H, Reijneveld SA, et al. Timing matters: long term effects of adversities from prenatal period up to adolescence on adolescents' cortisol stress response. The TRAILS study. Psychoneuroendocrinology. 2012;37:1439–1447. doi: 10.1016/j.psyneuen.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Pachankis JE, Goldfried MR, Ramrattan ME. Extension of the rejection sensitivity construct to the interpersonal functioning of gay men. J Consult Clin Psychol. 2008;76:306–317. doi: 10.1037/0022-006X.76.2.306. [DOI] [PubMed] [Google Scholar]
- 52.Meyer IH. Prejudice as stress: conceptual and measurement problems. Am J Public Health. 2003;93:262–265. doi: 10.2105/ajph.93.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crocker J, Major B. Social stigma and self-esteem: the self-protective properties of stigma. Psychol Rev. 1989;96:608–630. [Google Scholar]
- 54.Peters E, Riksen-Walraven JM, Cillessen AH, de Weerth C. Peer rejection and HPA activity in middle childhood: friendship makes a difference. Child Dev. 2011;82:1906–1920. doi: 10.1111/j.1467-8624.2011.01647.x. [DOI] [PubMed] [Google Scholar]
- 55.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nijm J, Jonasson L. Inflammation and cortisol response in coronary artery disease. Ann Med. 2009;41:224–233. doi: 10.1080/07853890802508934. [DOI] [PubMed] [Google Scholar]