Abstract
Retinitis pigmentosa (RP) is a devastating form of retinal degeneration, with significant social and professional consequences. Molecular genetic information is invaluable for an accurate clinical diagnosis of RP due to its high genetic and clinical heterogeneity. Using a gene capture panel that covers 163 of the currently known retinal disease genes, including 48 RP genes, we performed a comprehensive molecular screening in a collection of 123 RP unsettled probands from a wide variety of ethnic backgrounds, including 113 unrelated simplex and 10 autosomal recessive RP (arRP) cases. As a result, 61 mutations were identified in 45 probands, including 38 novel pathogenic alleles. Interestingly, we observed that phenotype and genotype were not in full agreement in 21 probands. Among them, eight probands were clinically reassessed, resulting in refinement of clinical diagnoses for six of these patients. Finally, recessive mutations in CLN3 were identified in five retinal degeneration patients, including four RP probands and one cone-rod dystrophy (CRD) patient, suggesting that CLN3 is a novel non-syndromic retinal disease gene. Collectively, our results underscore that, due to the high molecular and clinical heterogeneity of RP, comprehensive screening of all retinal disease genes is effective in identifying novel pathogenic mutations and provides an opportunity to discover new genotype-phenotype correlations. Information gained from this genetic screening will directly aid in patient diagnosis, prognosis, and treatment, as well as allowing appropriate family planning and counseling.
Keywords: retinitis pigmentosa, blindness, retinal genes, whole exome sequencing, next generation sequencing, retinal capture NGS
INTRODUCTION
Retinitis pigmentosa (RP; MIM# 268000) is one of the most common forms of inherited retinal degeneration affecting 1 in 3,000 people worldwide (Hamel 2006; Hartong et al. 2006). Patients with RP lose vision due to the degeneration of rod photoreceptors followed by cone photoreceptors death throughout the retina (Hamel 2006; Hartong et al. 2006). The genetic basis of RP is highly heterogeneous. Currently RP is known to be caused by mutations in over 50 genes (RetNet https://sph.uth.edu/retnet/). The inheritance of RP is also complex, with autosomal dominant (ad), autosomal recessive (ar), X-linked (xl), digenic and even mitochondrial forms (Fahim et al. 1993; Kajiwara et al. 1994; Dryja et al. 1997; Mansergh et al. 1999). Furthermore, almost half of all RP cases are simplex in which the inheritance pattern cannot be reliably determined due to missing information and/or limited pedigree size (Fahim et al. 1993). This is further complicated by the extensive clinical and genetic overlap between RP and other retinal diseases. First of all, different mutations in the same gene can cause different diseases. For example, mutations in CRX, CRB1, IMPDH1, RDH12, RPE65, TULP1, and SPATA7, can cause either RP or other retinal diseases, including Leber congenital amaurosis (LCA) and cone rod dystrophy (CRD) (RetNet https://sph.uth.edu/retnet/). Furthermore, genes associated with certain syndromic diseases can also be linked to non-syndromic RP and in some cases the different phenotypes can be caused by exactly the same mutation. For example, mutation c.1169T>G, p.(M390R) in BBS1, previously known to cause Bardet-Biedl syndrome (BBS), was recently identified in non-syndromic RP patients as well (Estrada-Cuzcano et al. 2012). As a result, new or even reported mutations in genes previously known to cause other forms of retinal dystrophy may also cause RP. In order to solve these cases, it is imperative to perform a comprehensive molecular diagnosis which includes both known RP genes and other retinal diseases genes.
Current methods for the molecular diagnosis of RP such as Sanger sequencing and Arrayed Primer Extension (APEX) have limitations. Sanger sequencing, while highly accurate, is time consuming and costly, making it impractical for testing a large number of genes in a large numbers of patients. The latest APEX array, utilizing homogeneous multiplex PCR and four-color single-base extension technology, can detect hundreds of known mutations in parallel (Kurg et al. 2000). However, it is only designed to efficiently detect known mutations in known genes. For example, the commercially available APEX arRP array includes 710 known mutations in 28 autosomal recessive RP disease genes (Asper Biotech http://www.asperbio.com/). With a similar array, a typical genetic diagnostic rate of less than 15% was achieved (Avila-Fernandez et al. 2010).
In contrast, next generation sequencing (NGS) technology provides a new approach for molecular diagnosis of RP. Several recent studies reported the new NGS-based molecular diagnosis of RP, in which approximately 100 inherited retinal disease genes were targeted and sequenced (Simpson et al. 2011; Neveling et al. 2012; O’Sullivan et al. 2012; Shanks et al. 2012; Glockle et al. 2013). Their results showed a genetic diagnostic rate of approximately 50%, which is significantly higher than that achieved by conventional methods. Furthermore, NGS allows for the screening of other known retinal disease genes in addition to RP disease genes without significantly increasing the cost. This is particularly important due to the extensive clinical and genetic overlap between RP and other retinal diseases which is described above and indeed recent finding suggests that many RP patients carry mutations in other retinal disease genes due to a combination of novel genotype/phenotype correlations, late onset of syndromic features, and clinical misdiagnosis (Fu et al. 2013).
In our study, we performed a comprehensive molecular screening of 123 RP probands, including 113 unrelated simplex and 10 autosomal recessive RP (arRP) cases, using a custom designed 163-gene panel that includes 48 known RP causative genes and 115 other retinal disease genes. Causative mutations were found in 45 probands and 38 novel pathogenic alleles were identified. In eight out of 21 cases with inconsistent molecular and clinical diagnoses, clinical reassessments were performed. As a result, clinical diagnoses for six cases were refined based on the molecular diagnosis and subsequent clinical reassessment. In addition, CLN3 was identified as a novel disease gene for non-syndromic retinal diseases as supported by five unrelated patient families in this study. Collectively, our results demonstrate the power and importance of combining comprehensive molecular screening and clinical information to accurately diagnose genetically and clinically heterogeneous diseases such as RP.
MATERIAL AND METHODS
Clinical diagnosis of RP patients
Probands and other family members (when available) were ascertained primarily at (1) the UC San Diego Shiley Eye Center (La Jolla, CA), (2) the Retina Foundation of the Southwest (Dallas, TX), (3) the McGill Ocular Genetics Clinic and Lab at the Montreal Children’s Hospital, McGill University Health Centre (Montreal, Quebec, Canada), (4) the Jules Stein Eye Institute, UCLA School of Medicine (Los Angeles, CA), (5) the Kellogg Eye Center, University of Michigan (Ann Arbor, MI), (6) and the Department of Ophthalmology & Center for Vision and Vascular Science (Belfast, UK). Informed consent was obtained from all patients in accordance to the tenets of the Declaration of Helsinki. Probands underwent complete ophthalmologic exams and imaging studies including visual acuity testing, Goldmann visual field testing, fundoscopy, electrophysiological testing (ERG), Goldman applanation tonometry, indirect ophthalmoscopy, optical coherence tomography (OCT), fundus autofluorescence (FAF), fundus photography, and fluorescein angiography. Pedigrees were constructed based on patient interviews. A peripheral blood or a saliva sample was taken from every proband and additional family member when available. Genomic DNA was isolated from peripheral blood and saliva samples as previously described (Sohocki et al. 2001; Bowne et al. 2011), or as instructed by the manufacturer (Qiagen Inc).
Design of the capture panel
A capture panel of retinal disease genes was previously developed and assessed by our group (Wang et al. 2013). The panel covers 2560 exons and corresponding splice junctions of 163 known retinal disease genes, with a total of 649,804 bp in design region. In total, 48 RP genes were targeted, including all 30 arRP genes that had been reported at the time of panel design (Supplemental table 1). Of the 2560 exons, 49 were not captured efficiently due to technical challenges (average coverage < 5X; Supplemental Table 2). In addition, 21 exons in EYS and 51 exons in USH2A were missing in our capture panel design (Supplemental Table 2).
The sensitivity of our method was tested using HapMap sample NA11831, as described in (Wang et al. 2013). Briefly, this method can detect 99.5% of SNPs originally found in the genotyping array data. At around 50X coverage, we can achieve nearly saturated sensitivity with a relatively low cost (cost is linear to the depth of coverage).
Library preparation and capture sequencing
Pre-capture Illumina libraries were generated as previously described (Koenekoop et al. 2012). NimbleGen SeqCap EZ Hybridization and Wash Kits were used for panel capture according to the manufacturer’s protocol. In general, 24 to 44 pre-capture libraries were pooled together for each capture reaction. After capture, DNA libraries were quantified and sequenced on an Illumina HiSeq 2000, according to the manufacturer’s protocols.
Bioinformatics analysis
100-bp paired-end reads were obtained. Data were processed as previously described (Koenekoop et al. 2012). In Particular, dbNSFP was used to functionally predict the effects of missense variants (Liu et al. 2011). Variants with predictions of “damaging” or “conserved” from no fewer than four algorithms were considered as putatively pathogenic. Variants with predictions of “benign” or “non-conserved” from no fewer than four algorithms were considered as benign. Exceptions were made in familial cases where segregation tests supported the pathogenicity of certain missense alleles.
Sanger validation and segregation test
For each identified mutation, a 500-bp flanking sequence at each side was obtained from the UCSC genome browser. RepeatMasker was used to mask the repetitive region (Smit et al. 1996–2010). Primer 3 was used to design a pair of primers at least 50 bp upstream and downstream from the mutation (Rozen and Skaletsky 2000). After PCR amplification, the amplicons were sequenced on an ABI 3730xl or 3500XL Genetic Analyzer. Family members were also Sanger-sequenced when available.
RESULTS
Collection of DNA from RP patients
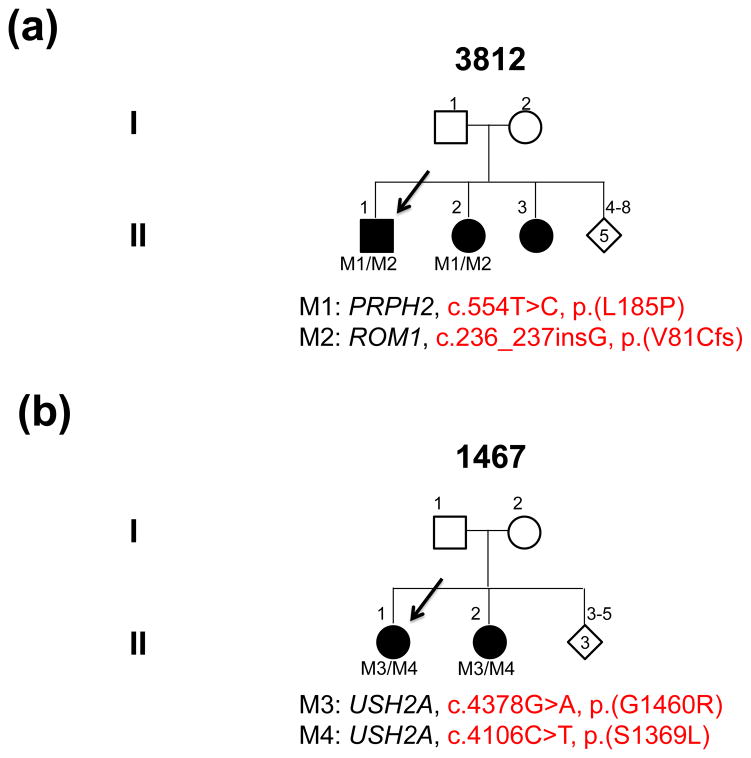
A total of 123 RP probands, including 113 unrelated simplex and 10 arRP cases, were collected. Individuals were selected for the study based on a clinical diagnosis of RP after extensive workups. Pedigrees from 113 simplex RP patients showed no family history of retinal disease, while 10 arRP families had pedigrees supporting a recessive mode of inheritance with either multiple affected individuals or evidence of consanguinity (Fig. 3, Fig. 5a, and Supplemental Fig. 1).
Fig. 3.
Pedigrees and mutations segregating in family 3812 (a) and family 1467 (b)
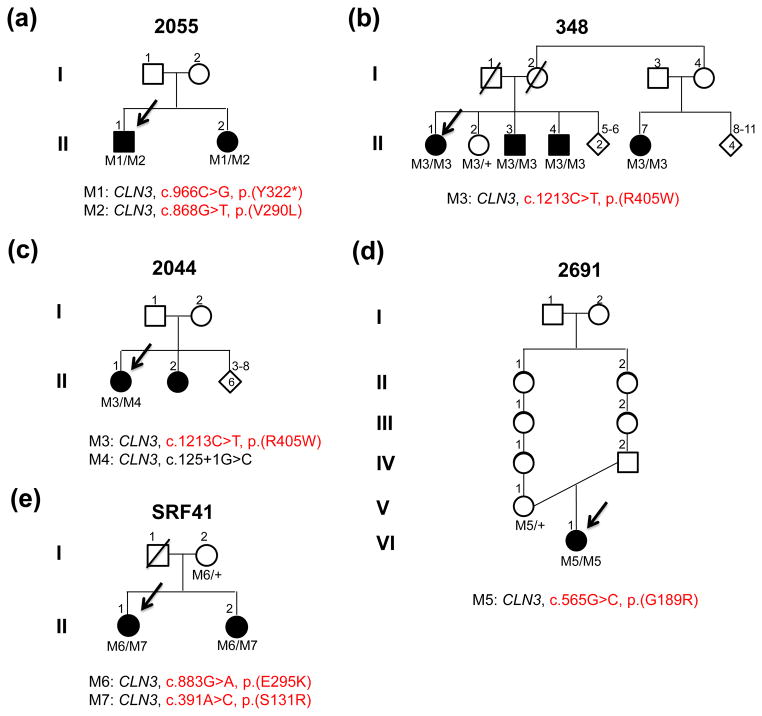
Fig. 5.
Pedigrees and mutations segregating in family 2055 (a), family 348 (b), family 2044 (c), family 2691 (d) and family SRF41 (e)
Capture sequencing and data processing of 123 samples
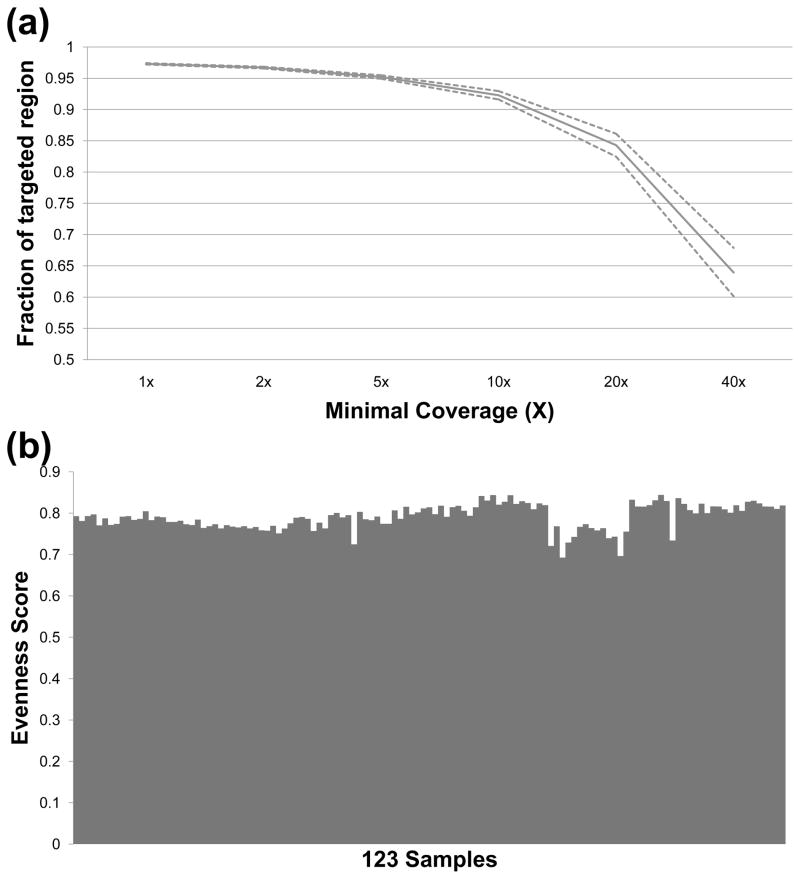
To identify causative mutations in these RP patients, we performed targeted capture sequencing of 163 retinal disease genes using a custom designed capture panel as described in the material and methods section “design of the capture panel”. As shown in Fig. 1, high quality results were obtained. For each sample, an average of 2.1 million reads were generated, approximately 20% of which were mapped to the targeted regions. The mean and median coverage for the targeted regions were 77X and 70X, respectively. Furthermore, 92% of the targeted regions had at least 10X coverage, and 84% of the targeted regions had at least 20X coverage (Fig. 1a). To determine if our sequence coverage over the targeted regions is evenly distributed, an evenness score was calculated for our data as previously described (Mokry et al. 2010). On average, an evenness score of 0.80 was achieved, indicating a near uniform distribution of our captured reads over the targeted regions (Fig. 1b).
Fig. 1.
High quality sequencing results were obtained. (a) The solid curve shows the fraction of targeted region (y-axis) covered by at least certain coverage (x-axis). Dashed lines show the 95% confidence interval. (b) The evenness score of capture sequencing results from 123 RP samples
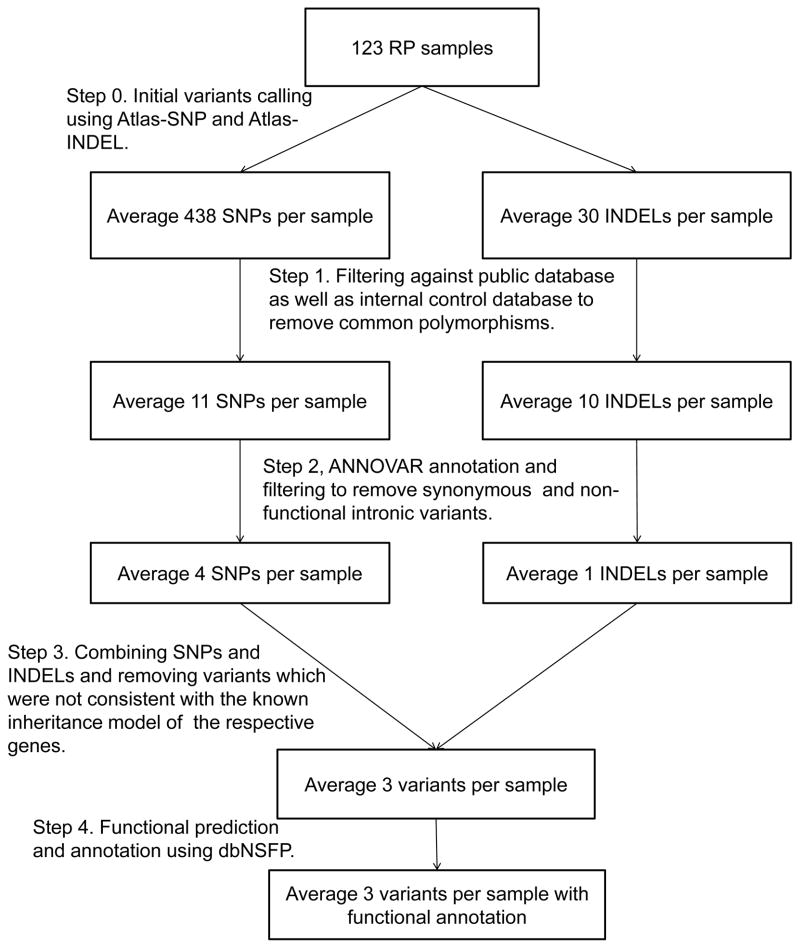
A previously described automatic variant calling, filtering, and annotation pipeline was used to process the capture sequencing data from all 123 samples (Fig. 2) (Koenekoop et al. 2012). On average, 468 raw variants were initially called for each sample. After filtering out common polymorphisms with frequency >0.5% in any of the variant databases queried, including 1000 Genome build 201105 and 201011 (Genomes Project 2010), dbSNP135 (National Center for Biotechnology Information build 135), NHLBI Exome Sequencing database (NHLBI GO Exome Sequencing Project (ESP) ESP6500SI), NIEHS Exome Sequencing database (NIEHS Environmental Genome Project NIEHS95), and an internal control database of 997 exomes, an average of 21 rare variants per sample remained, including five non-synonymous variants. To assess the pathogenicity of these rare non-synonymous variants, each variant was searched against the HGMD database to identify previously reported mutations (Stenson et al. 2003). Furthermore, in silico prediction for each variant was performed using dbNSFP (Liu et al. 2011).
Fig. 2.
Automatic pipeline used to filter and annotate variants
Identification of pathogenic mutations
In order to systematically identify putative pathogenic mutations for each case, we applied a stepwise mutation identification strategy as previously described (Fu et al. 2013; Wang et al. 2013). As a result, 45 probands (42 simplex cases and 3 familial cases) were found to carry pathogenic mutations in known RP genes (Table 1, 31 cases) and other retinal disease genes (Table 2, 14 cases). All identified pathogenic mutations were validated by Sanger sequencing. Segregation tests were performed where applicable.
Table 1.
Summary of 31 probands carrying pathogenic mutations in known RP genes
| ID | Type | Gene | NM ID | Genotype | cDNA change | Protein change | Reference |
|---|---|---|---|---|---|---|---|
| Group with higher confidence | |||||||
| 1235001 | Simplex | RHO | NM_000539 | Heterozygous | c.1040C>T | p.(P347L) | (Dryja et al. 1990) |
| 690001 | Simplex | RPGR | NM_001034853 | Hemizygous | c.1377_1378del | p.(L460Ifs) | (Zito et al. 1999) |
| 3812 | arRP | PRPH2 | NM_000322 | Heterozygous | c.554T>C | p.(L185P) | (Kajiwara et al. 1994) |
| ROM1 | NM_000327 | Heterozygous | c.236_237insG | p.(V81Cfs) | (Kajiwara et al. 1994) | ||
| 1268001 | Simplex | PRPF31 | NM_015629 | Heterozygous | c.636delG | p.(M212Ifs) | (Sullivan et al. 2006) |
| 1313001 | Simplex | USH2A | NM_206933 | Homozygous | c.2299delG | p.(E767Sfs) | (Eudy et al. 1998) |
| UTAD319_01 | Simplex | RPE65 | NM_000329 | Homozygous | c.893delA | p.(K298Sfs) | (Zernant et al. 2005) |
| 1249001 | Simplex | BBS1 | NM_024649 | Homozygous | c.1169T>G | p.(M390R) | (Mykytyn et al. 2002) |
| UTAD468_01 | Simplex | BBS1 | NM_024649 | Homozygous | c.1169T>G | p.(M390R) | (Mykytyn et al. 2002) |
| RFS095_5294 | Simplex | RDH12 | NM_152443 | Heterozygous | c.806_810del | p.(A269Gfs) | (Janecke et al. 2004) |
| Heterozygous | c.495_499del | p.(A166Cfs) | (Stone 2007) | ||||
| UTAD311_01 | Simplex | RDH12 | NM_152443 | Heterozygous | c.806_810del | p.(A269Gfs) | (Janecke et al. 2004) |
| Heterozygous | c.63_66del | p.(I22Gfs) | (Stone 2007) | ||||
| RFS054_2701 | Simplex | ABCA4 | NM_000350 | Heterozygous | c.3113C>T | p.(A1038V) | (Allikmets et al. 1997) |
| Heterozygous | c.4577C>T | p.(T1526M) | (Lewis et al. 1999) | ||||
| Heterozygous | c.1622T>C | p.(L541P) | (Rozet et al. 1998) | ||||
| Heterozygous | c.658C>T | p.(R220C) | (Webster et al. 2001) | ||||
| UTAD452_01 | Simplex | SNRNP200 | NM_014014 | Heterozygous | c.2041C>T | p.(R681C) | (Benaglio et al. 2011) |
| UTAD312_01 | Simplex | USH2A | NM_206933 | Heterozygous | c.486-1G>A | p.(?) | Novel |
| Heterozygous | c.2276G>T | p.(C759F) | (Rivolta et al. 2000) | ||||
| 1239 | Simplex | MERTK | NM_006343 | Homozygous | c.91_97del7 | p.(P31Rfs) | Novel |
| 532001 | Simplex | MERTK | NM_006343 | Homozygous | c.390G>A | p.(W130*) | Novel |
| 688001 | Simplex | RPGR | NM_001034853 | Hemizygous | c.2158C>T | p.(Q720*) | Novel |
| 689001 | Simplex | RPGR | NM_001034853 | Hemizygous | c.2359G>T | p.(G787*) | Novel |
| 693001 | Simplex | PRPF31 | NM_015629 | Heterozygous | c.763C>T | p.(Q255*) | Novel |
| 1053001 | Simplex | RPGR | NM_001034853 | Heterozygous | c.1375_1376del | p.(V459Lfs) | Novel |
| 1280001 | Simplex | CRX | NM_000554 | Heterozygous | c.431_443del13 | p.(L146Qfs) | Novel |
| 1300001 | Simplex | PRPF31 | NM_015629 | Heterozygous | c.616G>T | p.(E206*) | Novel |
| 1305001 | Simplex | PROM1 | NM_006017 | Heterozygous | c.2011A>T | p.(K671*) | Novel |
| Heterozygous | c.510-1G>A | p.(?) | Novel | ||||
| 10786001 | Simplex | CNGA1 | NM_000087 | Heterozygous | c.1885C>T | p.(R629*) | Novel |
| Heterozygous | c.117C>A | p.(C39*) | Novel | ||||
| RFS195_6068 | Simplex | PRPF8 | NM_006445 | Heterozygous | c.6970delG | p.(E2324Rfs) | Novel |
| UTAD494_01 | Simplex | PROM1 | NM_006017 | Heterozygous | c.730C>T | p.(R244*) | Novel |
| Heterozygous | c.1354_1355ins T | p.(Y452Lfs) | Novel | ||||
| Group with lower confidence | |||||||
| 1152001 | Simplex | PDE6B | NM_000283 | Heterozygous | c.1237C>T | p.(Q413*) | Novel |
| Heterozygous | c.2399T>C | p.(L800P) | Novel | ||||
| UTAD382_01 | Simplex | ABCA4 | NM_000350 | Heterozygous | c.2588G>C | p.(G863A) | (Allikmets et al. 1997) |
| Heterozygous | c.4532C>G | p.(P1511R) | Novel | ||||
| UTAD779_01 | Simplex | PRCD | NM_001077620 | Homozygous | c.49C>T | p.(R17C) | Novel |
| 1467 | arRP | USH2A | NM_206933 | Heterozygous | c.4378G>A | p.(G1460R) | Novel |
| Heterozygous | c.4106C>T | p.(S1369L) | Novel | ||||
| RFS051_1930 | Simplex | TULP1 | NM_003322 | Heterozygous | c.1112+2T>C | p.(?) | Novel |
| Heterozygous | c.1376T>C | p.(I459T) | Novel | ||||
| UTAD725_01 | Simplex | RDH12 | NM_152443 | Heterozygous | c.806_810del | p.(A269Gfs) | (Janecke et al. 2004) |
| Heterozygous | c.167C>A | p.(A56D) | Novel | ||||
Table 2.
Summary of 14 probands carrying pathogenic mutations in other retinal disease genes, not previously associated with non-syndromic RP
| ID | Type | Gene | NM ID | Genotype | cDNA change | Protein change | Reference |
|---|---|---|---|---|---|---|---|
| Group with higher confidence | |||||||
| UTAD339_01 | Simplex | NPHP1 | NM_000272 | Homozygous | c.1027G>A | p.(G343R) | (Caridi et al. 2006) |
| 459001 | Simplex | RDH5 | NM_002905 | Homozygous | c.839G>A | p.(R280H) | (Gonzalez-Fernandez et al. 1999) |
| 918001 | Simplex | CYP4V2 | NM_207352 | Heterozygous | c.802-8_810del17insGC | in-frame deletion of exon7 | (Wada et al. 2005) |
| Heterozygous | c.1091-2A>G | p.(?) | (Li et al. 2004) | ||||
| 920001 | Simplex | CYP4V2 | NM_207352 | Heterozygous | c.802-8_810del17insGC | in-frame deletion of exon7 | (Wada et al. 2005) |
| Heterozygous | c.1091-2A>G | p.(?) | (Li et al. 2004) | ||||
| 1302001 | Simplex | CYP4V2 | NM_207352 | Heterozygous | c.802-8_810del17insGC | in-frame deletion of exon7 | (Wada et al. 2005) |
| Heterozygous | c.1020G>A | p.(W340*) | (Wada et al. 2005) | ||||
| 558001 | Simplex | BBS2 | NM_031885 | Homozygous | c.471G>A | p.(?) | Novel |
| 1191001 | Simplex | JAG1 | NM_000214 | Heterozygous | c.1455_1456insT G | p.(R486*) | Novel |
| UTAD566_01 | Simplex | NPHP4 | NM_015102 | Heterozygous | c.111G>A | p.(W37*) | Novel |
| Heterozygous | c.3506delC | p.(P1169Qfs) | Novel | ||||
| Group with lower confidence | |||||||
| 1304001 | Simplex | CYP4V2 | NM_207352 | Heterozygous | c.802-8_810del17insGC | in-frame deletion of exon7 | (Wada et al. 2005) |
| Heterozygous | c.1027T>G | p.(Y343D) | Novel | ||||
| 11407001 | Simplex | BBS5 | NM_152384 | Homozygous | c.148C>A | p.(L50I) | Novel |
| 1895001 | Simplex | CDHR1 | NM_033100 | Homozygous | c.2027T>A | p.(I676N) | Novel |
| 1205001 | Simplex | GPR98 | NM_032119 | Heterozygous | c.3443G>A | p.(G1148D) | Novel |
| Heterozygous | c.8226T>G | p.(I2742M) | Novel | ||||
| UTAD451_01 | Simplex | USH1C | NM_005709 | Heterozygous | c.793G>A | p.(D265N) | Novel |
| Heterozygous | c.653G>T | p.(G218V) | Novel | ||||
| 2055 | arRP | CLN3 | NM_001042432 | Heterozygous | c.966C>G | p.(Y322*) | Novel |
| Heterozygous | c.868G>T | p.(V290L) | Novel | ||||
Pathogenic mutations in known RP genes were found in 31 cases
As shown in Table 1, pathogenic mutations in known RP genes were found in 31 probands (29 simplex cases and two familial cases). Among them, 25 probands (24 simplex cases and one familial case) carry either known pathogenic mutations or novel loss-of-function (LOF) mutations (nonsense, frameshift, or splicing mutations) in known RP genes (higher confidence). In addition, six other probands (five simplex cases and one familial case) were identified to carry one or more novel putative pathogenic missense mutations in known RP genes (lower confidence). The pathogenicity of the novel missense alleles were all supported by the in silico program dbNSFP (Liu et al. 2011). Detailed criteria can be found in the Material and Methods section under Bioinformatics analysis.
In the 31 probands (29 simplex cases and two familial cases), a total of 43 pathogenic mutations were identified in 19 known RP genes, including 18 previously reported alleles, 18 novel LOF mutant alleles, and seven novel missense alleles. The pathogenicity predictions for the novel missense mutations are listed in Supplemental Table 3. Among the 29 simplex cases solved in this step, seven (24%) were due to mutations in autosomal dominant genes. Four out of the 29 simplex cases (14%) turned out to be X-linked with mutations in RPGR. The remaining 18 simplex cases (62%) harbor mutations in autosomal recessive genes.
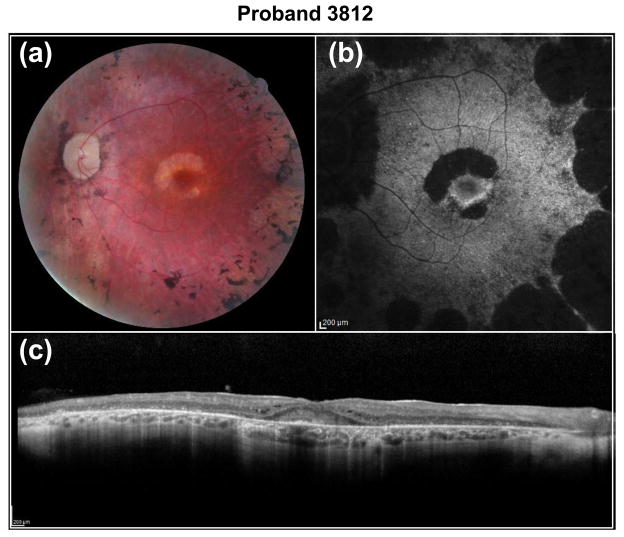
The two familial cases solved at this step are proband 3812 and proband 1467. Proband 3812 carries known digenic RP mutations. The pair of heterozygous mutations, c.554T>C, p.(L185P) in PRPH2 and c.236_237insG, p.(V81Cfs) in ROM1, was previously reported to cause digenic RP (Kajiwara et al. 1994). Segregation testing in two of the affected siblings indicated that the mutations co-segregated with the disease phenotype (Fig. 3a). The clinical phenotype in proband 3812 is quite unique. Fundus images revealed an extensive maculopathy in a horsehoe pattern (Fig. 4a), with absent fundus autofluorescence pericentrally (Fig. 4b) and marked retinal remodelling with extensive debris and cystoid macular edema in the fovea (Fig. 4c). Proband 1467 from one of our arRP families carries two novel compound heterozygous missense variants in USH2A. The first allele, c.4378G>A, p.(G1460R), affects a conserved amino acid and was predicted to be detrimental (Supplemental Table 3). The second allele, c.4106C>T, p.(S1369L), was predicted to be benign (Supplemental Table 3). Nevertheless, we still considered it as a putatively pathogenic mutation in this proband since it segregates with the disease phenotype in the affected siblings (Fig. 3b).
Fig. 4.
Fundus images of proband 3812. Shown are (a) fundus photograph, (b) fundus autofluorescence (FAF) and (c) optical coherence tomography (OCT) images. The images of OS reveal an extensive maculopathy in a horsehoe pattern, with absent FAF pericentrally and marked retinal remodelling with extensive debris and CME in the fovea
Pathogenic mutations in other retinal disease genes were found in 14 cases
As shown in Table 2, 14 probands (13 simplex cases and one familial case) carry pathogenic mutations in other retinal disease genes not previously associated with RP. Among them, eight probands (all are simplex) carry either known pathogenic mutations or novel LOF mutations in other retinal disease genes (higher confidence). In addition, six probands (five simplex cases and one familial case) carry one or more putatively pathogenic missense mutations in other retinal disease genes (lower confidence).
In the 14 cases (13 simplex cases and one familial case), a total of 18 pathogenic mutations in 11 other retinal disease genes (BBS2, BBS5, CDHR1, CLN3, CYP4V2, GPR98, JAG1, NPHP1, NPHP4, RDH5, and USH1C) were identified, including five previously reported alleles, five novel LOF mutant alleles, and eight novel missense alleles. The pathogenicity predictions for the novel missense mutations are listed in Supplemental Table 3. Among the 13 simplex cases solved in this step, one carries a heterozygous mutation in an autosomal dominant gene while the remaining probands carry mutations in recessive genes. The only familial case solved in this step, proband 2055, indeed carries compound heterozygous mutations in a recessive gene.
Collectively, as shown in Table 1 and Table 2, we identified a total of 61 pathogenic mutations in 45 probands, including 42 simplex cases and three familial cases. Thirty-eight of the 61 pathogenic mutations identified were novel while the remaining 23 mutations had been previously reported. The mutations identified spread among 30 genes, including 19 known RP genes and 11 other retinal disease genes. Among the 42 solved simplex cases, 30 (71%) were due to mutations in autosomal recessive genes, eight cases (19%) carry mutations in autosomal dominant genes, and the remaining four cases (10%) all have mutations in RPGR, which is an X-linked gene. Two of the arRP familial cases carry mutations in autosomal recessive genes, as expected, while the third carries digenic mutations whose inheritance pattern is similar to that of autosomal recessive disease.
Clinical reassessment of probands with inconsistent molecular and clinical diagnoses
Interestingly, an inconsistency between molecular information and the initial clinical diagnosis was observed for a total of 21 probands (Supplemental Table 4). Specifically, for seven probands, although mutations have been found in known RP disease genes, the particular mutations identified in the patients had previously been associated with retinal diseases other than RP. The remaining 14 probands carry mutations in other retinal disease genes not previously associated with RP. In order to untangle the inconsistency in these 21 cases, we performed a clinical reassessment of all available patients. In eight of the 21 probands, we successfully obtained enough clinical information for a reliable reassessment. Two probands (2055 and UTAD468_01) were confirmed as RP patients and the clinical diagnoses of six probands (1249001, 1191001, 1313001, UTAD319_01, RFS095_5294, and RFS054_2701) were refined to other diseases based on additional clinical information. Importantly, among the two reconfirmed RP probands, one proband (2055) carries mutations in CLN3.
Identification of CLN3 as a novel non-syndromic retinal disease gene
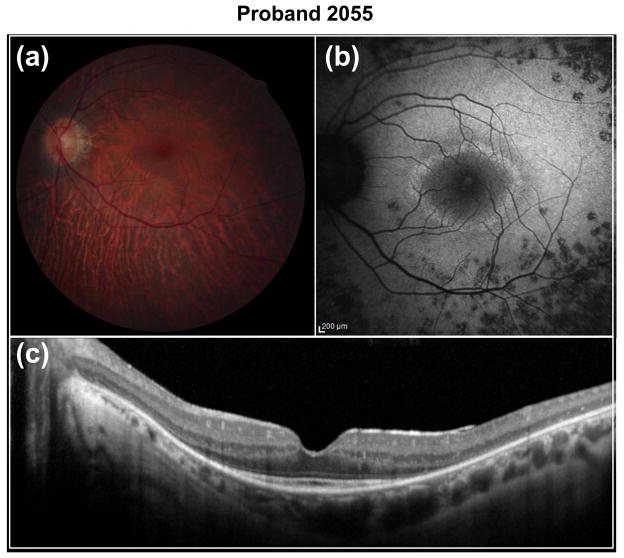
CLN3 was previously identified as a causative gene for neuronal ceroid lipofuscinoses (NCL, also known as Batten disease) (The-International-Batten-Disease-Consortium 1995), a devastating systemic disease characterized by rapid psychomotor deterioration, seizures, failure to thrive, microcephaly, ataxia, and vision loss due to photoreceptor degeneration. Typically, the onset of Batten disease is in early childhood and patients with this disease die prematurely, usually before age 40. However, one family with a milder form of Batten disease has been described (Sarpong et al. 2009). In our study, we found compound heterozygous variants in CLN3 in proband 2055 from one of our arRP families originating from Sicily. The first allele is a novel nonsense variant, c.966C>G, p.(Y322*), which introduces a premature stop codon in exon 14, predicted to produce an mRNA, potentially triggering nonsense-mediated decay. The second allele is a novel missense variant, c.868G>T, p.(V290L). Although the missense variant was predicted to be benign (Supplemental Table 3), we considered it as a potentially pathogenic mutation since it segregated with the disease phenotype in the two affected siblings (Fig. 5a). Clinical reassessment revealed a typical RP phenotype for proband 2055 (Fig. 6) and his affected sibling (Supplemental Fig. 2). No signs or symptoms of Batten disease were found in either case even at the ages of 40 and 45 years, respectively. No other potential disease-causing mutations were found in the 163 retinal disease genes sequenced in the proband.
Fig. 6.
Fundus images of proband 2055. Shown are (a) fundus photograph, (b) fundus autofluorescence (FAF) and (c) optical coherence tomography (OCT) images. The retinal photograph of the OS shows mild choroidal sclerosis and peripheral pigment mottling. FAF shows a central hyper-fluorescent ring surrounded by essentially normal FAF, surrounded by mottled FAF outside the arcades. OCT shows extensive IS/OS junction loss, except in the fovea
In order to further validate the potential association between CLN3 and non-syndromic retinal degeneration, we Sanger sequenced the coding region of CLN3 in an additional RP and CRD patient cohort. A total of four patients were found to carry putatively pathogenic mutations in CLN3, including three RP probands and one CRD proband. The first RP patient, proband 348 from an arRP family with four affected members, carries a novel homozygous missense variant, c.1213C>T, p.(R405W), in CLN3. This Mohawk family is a Native American Indian family living in Quebec. The variant affects a conserved amino acid and was predicted to be detrimental by in silico analyses (Supplemental Table 3) and co-segregated with the disease (Fig. 5b). Upon clinical reassessment and extensive physical exams, we confirmed that all the patients in this family are otherwise healthy, without any signs of NCL in their late 50s and 60s. The second RP patient, proband 2044 (from an arRP family, a native from Mexico, Fig. 5c), carries novel compound heterozygous variants in CLN3. The first allele is a splicing variant, c.125+1G>C, which affects the splice donor site of intron 2. Known splicing mutations affecting the same splicing site (c.125+5G>A and c.126-1G>A) were previously reported in patients with NCL, suggesting the functional importance of this splicing site (Kousi et al. 2012). The second allele is the same variant found in proband 348 described above (c.1213C>T, p.(R405W)). Clinical reassessment of proband 2044 confirmed the RP phenotype (Supplemental Fig. 3) and found no symptoms of Batten disease at the age of 57. The third RP patient, proband 2691 from a Mexican family, carries a homozygous missense variant c.565G>C, p.(G189R) in CLN3. The allele was predicted to be damaging and affect a conserved amino acid based on our in silico analyses (Supplemental Table 3). Homozygosity mapping (Supplemental Fig. 4) and segregation test (Fig. 5d) further support the pathogenicity of the variant found in the family. Clinical reassessment was also performed and found no symptoms other than retinal degeneration at the age of 10 (Supplemental Fig. 5). In addition to the RP probands, one CRD family was also identified. The CRD proband, SRF41, from a Chinese family, carries compound heterozygous missense variants, c.883G>A, p.(E295K) and c.391A>C, p.(S131R), in CLN3.
Both alleles were predicted to be detrimental (Supplemental Table 3) and co-segregated with the disease phenotype (Fig. 5e). Clinical reassessment of the two affected siblings confirmed the phenotypes (Supplemental Fig. 6); no significant signs of neurological impairment were found at the age of 20.
Altogether, our results indicate that mutations in CLN3 can lead to non-syndromic retinal degeneration, establishing CLN3 as a new non-syndromic retinal disease gene. The c.1213C>T, p.(R405W) mutation may be a recurrent mutation in CLN3 resulting in RP.
Refinement of the clinical diagnoses of six probands
Based on the molecular results and clinical reassessments, we refined the clinical diagnoses of six probands (1249001, 1191001, 1313001, UTAD319_01, RFS095_5294, and RFS054_2701) to other diseases including Alagille syndrome (AGS), BBS, LCA, Stargardt macular dystrophy (STGD), and Usher syndrome type II. For example, proband 1249001 carries a homozygous missense mutation, c.1169T>G, p.(M390R), in BBS1 which was previously reported to cause both BBS and RP (Mykytyn et al. 2002; Estrada-Cuzcano et al. 2012). Upon clinical reassessment, proband 1249001 was found to have three features of BBS including RP, polydactyly, and obesity, strongly suggesting that the patient actually has BBS. Another example comes from proband 1191001 who carries a novel heterozygous insertion, c.1455_1456insTG, p.(R486*), in JAG1. The variant introduces a premature stop codon, likely resulting in a null allele due to protein truncation and/or nonsense-mediated decay. Previously, single copy LOF mutations in JAG1 have been reported in patients with AGS, a rare disease with defects in the liver, heart and kidneys (Li et al. 1997). Patients with AGS often have biliary atresia, tetralogy of Fallot, and a wide variety of kidney diseases. We reassessed the patient’s clinical phenotype and found that the patient had a history of congenital aortic aneurysm and Grave’s thyroid disease in addition to RP, indicating it is more appropriate to diagnose this patient as AGS rather than non-syndromic RP. Similarly, the clinical diagnosis of proband 1313001 was refined to Usher syndrome type II; probands UTAD319_01 and RFS095_5294 were re-classified as LCA patients; the clinical diagnosis of proband RFS054_2701 was adjusted to STGD.
Confirmation of phenotypic variations under the same pathogenic allele
Proband UTAD468_01 carries the well known homozygous mutation c.1169T>G, p.(M390R) in BBS1 which was initially known to cause BBS and was later discovered to also cause non-syndromic RP (Estrada-Cuzcano et al. 2012). After clinical reassessment, we confirmed non-syndromic RP in this patient, as we found none of the common phenotypic features of BBS, including polydactyly, obesity, and kidney disease at the age of 23. The same BBS1 mutation was observed in proband 1249001 described above, whose clinical diagnosis was refined to BBS based on the clinical reassessment. As a result, our observation of both scenarios confirmed the previous finding that this particular allele, c.1169T>G, p.(M390R), in BBS1 can give rise to either BBS or non-syndromic RP, probably due to genetic modifiers or environmental differences.
DISCUSSION
In this study, NGS-based molecular diagnosis was applied to a set of 123 unsettled RP patients, most of which are simplex cases with no or limited inheritance information. Sporadic or simplex RP cases are more difficult to genotype, as the inheritance mode is unknown. Sporadic RP cases can be autosomal recessive, de novo autosomal dominant, or x-linked. Despite this added complexity, we were able to provide a successful molecular diagnosis in 37% (45/123) of the probands. Among the 42 solved simplex cases, 30 (71%) were due to homozygous or compound heterozygous mutations in autosomal recessive genes, eight (19%) carry heterozygous mutations in autosomal dominant genes, and the remaining four (10%) all carry mutations in RPGR, which is an X-linked gene. A total of 61 pathogenic mutations were identified in our study, including 38 novel mutations.
Our data, for the first time, link mutations in CLN3 with the non-syndromic retinal degenerative diseases arRP and CRD. CLN3 mutations were first found in patients with NCL, a rare early-onset, devastating, autosomal recessive neurodegenerative disorder. In our study, four RP families and one CRD family were identified to carry either homozygous or compound heterozygous mutations in CLN3. All available patients in these families showed typical retinal phenotypes with no additional signs of NCL. This data indicates that mutations in CLN3 can cause non-syndromic retinal degeneration, which implies a more favorable prognosis for patients carrying these mutations in CLN3.
This is not the first time that a “syndromic” gene was later found to cause isolated retinal disease without syndromic features. CLN3 now joins a growing list of this kind of genes. Other examples include BBS1, which can cause either BBS or non-syndromic RP when mutated (Estrada-Cuzcano et al. 2012) and CEP290, which can cause Joubert syndrome or isolated LCA when mutated (den Hollander et al. 2006). This new phenomenon extends our understanding of genotype-phenotype correlations. How a mutated gene can cause a complex, extensive syndrome in one patient and isolated RP in another is currently not understood, but it is postulated to result from the complex interaction between genetic modifiers and environmental differences (Wang et al. 2011).
One of the utilities of molecular information is to improve diagnostic accuracy when coupled with clinic information. This is particularly important for heterogeneous diseases, such as RP, whose clinical phenotypes overlap with other similar diseases. Indeed, out of the 45 probands with positive molecular diagnoses, 21 show potential inconsistency with their initial clinical diagnoses. Reassessment of the clinical information was performed for eight probands in this study. As a result, the clinical diagnoses of six probands were refined to other retinal-related diseases or syndromes based on the mutation information.
Such a high refinement rate underlines the challenge of accurate diagnosis based on clinic phenotype alone and emphasizes the value of comprehensive molecular diagnosis. In RP, there are several reasons for this challenge. First, RP is a clinically heterogeneous disease with many phenotypic features that overlap with other retinal and syndromic diseases. Second, in the case of syndromic RP, patients may manifest only RP at the time of diagnosis but later in life develop other syndromic symptoms. In other words, the initial phenotype at the first medical visit may be dominated by a visual phenotype, while other syndromic features may develop over time. This makes a comprehensive molecular diagnosis valuable for clinicians by allowing them to refine the clinical diagnosis. Together, our results underscore that molecular diagnosis can aid accurate clinical diagnosis, and an unbiased and systematic diagnosis can guide better management of the patient and disease.
It is interesting that 19% (8/42) of our solved simplex cases actually carry heterozygous mutations in autosomal dominant genes. Simplex cases are often thought to be autosomal recessive since the patients’ parents are assumed to be unaffected. However, in some cases, de novo dominant mutations may occur resulting in affected offsprings with unaffected parents (Neveling et al. 2012). Furthermore, it is also possible that the dominant mutations have incomplete penetrance, causing the parents to not manifest disease phenotypes, despite carrying the mutations. Missing parental clinical information may lead to dominant simplex cases, as well. Unfortunately, parental samples were not available to determine if the dominant mutations found in the simplex cases are de novo.
It is also worth noting that 78 probands, including 71 simplex cases and seven familial cases, remain unsolved. The unsolved cases may be due to the following reasons. (1) Five out of the seven unsolved familial cases had been previously pre-screened using either SNP based linkage experiment or the APEX arRP chip, and for all five cases no mutations or linkage regions were identified in known RP genes in these prescreening steps (Supplemental Table 5). This partially explains our relatively low solving rate for these arRP familial cases. (2) Simplex cases of RP are known to have a lower solving rate than arRP, adRP or xlRP, partially due to the fact that there is little information about the patients’ families. Simplex cases can be caused by dominant alleles; however, it is very hard to prove the pathogenicity of novel missense dominant alleles given a lack of family data. Indeed, in 14 of the 71 unsolved simplex cases, we identified single heterozygous missense variants with unknown significance in dominant genes (Supplemental Table 6). The variants were completely absent in any of the databases in our pipeline and were predicted to be detrimental by our in silico analysis. However, since single rare heterozygous variants could be private polymorphisms, we could not confidently call whether or not these variants are pathogenic without additional functional studies. (3) Some exonic regions in known retinal disease genes were not included in our design or were not captured efficiently due to technical challenges (Supplemental Table 2). Further whole exome sequencing (WES) which was performed for 35 of the 78 negative samples revealed two additional cases carrying putatively pathogenic mutations in USH2A (data not shown) suggesting that our initial approach might miss at least four cases due to limitations in the capture. (4) Other technical and methodological limitations exist. For example, RPGR-ORF15 is highly repetitive and GC-rich and our approach (like other approaches) has difficulties in detecting mutations in this region (though we still managed to detect two cases carrying mutations in RPGR-ORF15). In addition, deep intronic mutations, copy-number variations, structural variations, and large exonic deletions could not be detected by our approach as well.
In summary, our study suggests that a comprehensive diagnostic strategy including the screening of all retinal disease genes, instead of screening only those known to cause RP, may be more suitable for patients with RP. Applying this tool to a large patient cohort provides an excellent opportunity to identify families in which new disease-causing genes may be found. Furthermore, molecular diagnosis can aid in clinical diagnosis, and an unbiased and systematic diagnosis can better guide disease management. In addition to RP, these concepts likely apply to other genetically and clinically heterogeneous diseases.
Supplementary Material
Acknowledgments
We gratefully acknowledge all participating patients and their family members. NGS was conducted at the Functional Genomic Core (FGC) facility at Baylor College of Medicine supported by NIH shared instrument grant 1S10RR026550 to R.C. This work was supported by grants from the Retinal Research Foundation, Foundation Fighting Blindness and the National Eye Institute (R01EY022356) to R.C.. K.Z. acknowledges supports from the National Eye Institute (P30EY022589, R01EY021374, and RO1EY018660), the King Abdulaziz City for Science and Technology through the UC San Diego Center of Excellence in Nanomedicine, Veterans Affairs Merit Award, Research to Prevent Blindness, and Burroughs Wellcome Fund Clinical Scientist Award in Translational Medicine. R.K.K. acknowledges supports from the Foundation Fighting Blindness Canada, the CIHR, Reseau Vision, FRSQ, NIH, and McGill University Health Centre. S.P.D acknowledges grants from the Foundation Fighting Blindness and National Institutes of Health Grant EY007142. F.W. is supported by predoctoral fellowship: The Burroughs Wellcome Fund, The Houston Laboratory and Population Sciences Training Program in Gene Environment Interaction. J.Z. is supported by NIH training grant T32 EY007102 and NLM training fellowship T15 LM007093. X.W. is supported by predoctoral fellowship: The Burroughs Wellcome Fund, The Houston Laboratory and Population Sciences Training Program in Gene Environment Interaction. We thank Ms. Kristin Rauscher for assistance in sample preparation. We thank Mr. Naimesh Solanki for assistance in sequencing library preparation. We sincerely thank Ms. Renee Pigeon and Ms. Shirley Briand for their help in coordinating the patients.
References
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15 (3):236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- Avila-Fernandez A, Cantalapiedra D, Aller E, Vallespin E, Aguirre-Lamban J, Blanco-Kelly F, Corton M, Riveiro-Alvarez R, Allikmets R, Trujillo-Tiebas MJ, Millan JM, Cremers FP, Ayuso C. Mutation analysis of 272 Spanish families affected by autosomal recessive retinitis pigmentosa using a genotyping microarray. Mol Vis. 2010;16:2550–2558. [PMC free article] [PubMed] [Google Scholar]
- Benaglio P, McGee TL, Capelli LP, Harper S, Berson EL, Rivolta C. Next generation sequencing of pooled samples reveals new SNRNP200 mutations associated with retinitis pigmentosa. Hum Mutat. 2011;32 (6):E2246–2258. doi: 10.1002/humu.21485. [DOI] [PubMed] [Google Scholar]
- Biotech A. [Accessed 04/15/2013]; http://www.asperbio.com/
- Bowne SJ, Humphries MM, Sullivan LS, Kenna PF, Tam LC, Kiang AS, Campbell M, Weinstock GM, Koboldt DC, Ding L, Fulton RS, Sodergren EJ, Allman D, Millington-Ward S, Palfi A, McKee A, Blanton SH, Slifer S, Konidari I, Farrar GJ, Daiger SP, Humphries P. A dominant mutation in RPE65 identified by whole-exome sequencing causes retinitis pigmentosa with choroidal involvement. Eur J Hum Genet. 2011;19 (10):1074–1081. doi: 10.1038/ejhg.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caridi G, Dagnino M, Trivelli A, Emma F, Perfumo F, Ghiggeri GM. Stop codon at arginine 586 is the prevalent nephronopthisis type 1 mutation in Italy. Nephrol Dial Transplant. 2006;21 (8):2301–2303. doi: 10.1093/ndt/gfl277. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Koenekoop RK, Yzer S, Lopez I, Arends ML, Voesenek KE, Zonneveld MN, Strom TM, Meitinger T, Brunner HG, Hoyng CB, van den Born LI, Rohrschneider K, Cremers FP. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79 (3):556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja TP, Hahn LB, Kajiwara K, Berson EL. Dominant and digenic mutations in the peripherin/RDS and ROM1 genes in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1997;38 (10):1972–1982. [PubMed] [Google Scholar]
- Dryja TP, McGee TL, Hahn LB, Cowley GS, Olsson JE, Reichel E, Sandberg MA, Berson EL. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med. 1990;323 (19):1302–1307. doi: 10.1056/NEJM199011083231903. [DOI] [PubMed] [Google Scholar]
- Estrada-Cuzcano A, Koenekoop RK, Senechal A, De Baere EB, de Ravel T, Banfi S, Kohl S, Ayuso C, Sharon D, Hoyng CB, Hamel CP, Leroy BP, Ziviello C, Lopez I, Bazinet A, Wissinger B, Sliesoraityte I, Avila-Fernandez A, Littink KW, Vingolo EM, Signorini S, Banin E, Mizrahi-Meissonnier L, Zrenner E, Kellner U, Collin RW, den Hollander AI, Cremers FP, Klevering BJ. BBS1 mutations in a wide spectrum of phenotypes ranging from nonsyndromic retinitis pigmentosa to Bardet-Biedl syndrome. Arch Ophthalmol. 2012;130 (11):1425–1432. doi: 10.1001/archophthalmol.2012.2434. [DOI] [PubMed] [Google Scholar]
- Eudy JD, Weston MD, Yao S, Hoover DM, Rehm HL, Ma-Edmonds M, Yan D, Ahmad I, Cheng JJ, Ayuso C, Cremers C, Davenport S, Moller C, Talmadge CB, Beisel KW, Tamayo M, Morton CC, Swaroop A, Kimberling WJ, Sumegi J. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science. 1998;280 (5370):1753–1757. doi: 10.1126/science.280.5370.1753. [DOI] [PubMed] [Google Scholar]
- Fahim AT, Daiger SP, Weleber RG. Retinitis Pigmentosa Overview. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. GeneReviews. Seattle (WA): 1993. [Google Scholar]
- Fu Q, Wang F, Wang H, Xu F, Zaneveld JE, Ren H, Keser V, Lopez I, Tuan HF, Salvo JS, Wang X, Zhao L, Wang K, Li Y, Koenekoop RK, Chen R, Sui R. Next-generation sequencing-based molecular diagnosis of a chinese patient cohort with autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2013;54 (6):4158–4166. doi: 10.1167/iovs.13-11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genomes Project C. A map of human genome variation from population-scale sequencing. Nature. 2010;467 (7319):1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockle N, Kohl S, Mohr J, Scheurenbrand T, Sprecher A, Weisschuh N, Bernd A, Rudolph G, Schubach M, Poloschek C, Zrenner E, Biskup S, Berger W, Wissinger B, Neidhardt J. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez F, Kurz D, Bao Y, Newman S, Conway BP, Young JE, Han DP, Khani SC. 11-cis retinol dehydrogenase mutations as a major cause of the congenital night-blindness disorder known as fundus albipunctatus. Mol Vis. 1999;5:41. [PubMed] [Google Scholar]
- Hamel C. Retinitis pigmentosa. Orphanet J Rare Dis. 2006;1:40. doi: 10.1186/1750-1172-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368 (9549):1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Janecke AR, Thompson DA, Utermann G, Becker C, Hubner CA, Schmid E, McHenry CL, Nair AR, Ruschendorf F, Heckenlively J, Wissinger B, Nurnberg P, Gal A. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet. 2004;36 (8):850–854. doi: 10.1038/ng1394. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Berson EL, Dryja TP. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science. 1994;264 (5165):1604–1608. doi: 10.1126/science.8202715. [DOI] [PubMed] [Google Scholar]
- Koenekoop RK, Wang H, Majewski J, Wang X, Lopez I, Ren H, Chen Y, Li Y, Fishman GA, Genead M, Schwartzentruber J, Solanki N, Traboulsi EI, Cheng J, Logan CV, McKibbin M, Hayward BE, Parry DA, Johnson CA, Nageeb M, Poulter JA, Mohamed MD, Jafri H, Rashid Y, Taylor GR, Keser V, Mardon G, Xu H, Inglehearn CF, Fu Q, Toomes C, Chen R Finding of Rare Disease Genes Canada C. Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nat Genet. 2012;44 (9):1035–1039. doi: 10.1038/ng.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousi M, Lehesjoki AE, Mole SE. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum Mutat. 2012;33 (1):42–63. doi: 10.1002/humu.21624. [DOI] [PubMed] [Google Scholar]
- Kurg A, Tonisson N, Georgiou I, Shumaker J, Tollett J, Metspalu A. Arrayed primer extension: solid-phase four-color DNA resequencing and mutation detection technology. Genet Test. 2000;4 (1):1–7. doi: 10.1089/109065700316408. [DOI] [PubMed] [Google Scholar]
- Lewis RA, Shroyer NF, Singh N, Allikmets R, Hutchinson A, Li Y, Lupski JR, Leppert M, Dean M. Genotype/Phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR, in Stargardt disease. Am J Hum Genet. 1999;64 (2):422–434. doi: 10.1086/302251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Jiao X, Munier FL, Schorderet DF, Yao W, Iwata F, Hayakawa M, Kanai A, Shy Chen M, Alan Lewis R, Heckenlively J, Weleber RG, Traboulsi EI, Zhang Q, Xiao X, Kaiser-Kupfer M, Sergeev YV, Hejtmancik JF. Bietti crystalline corneoretinal dystrophy is caused by mutations in the novel gene CYP4V2. Am J Hum Genet. 2004;74 (5):817–826. doi: 10.1086/383228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, Costa T, Pierpont ME, Rand EB, Piccoli DA, Hood L, Spinner NB. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16 (3):243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- Liu X, Jian X, Boerwinkle E. dbNSFP: a lightweight database of human nonsynonymous SNPs and their functional predictions. Hum Mutat. 2011;32 (8):894–899. doi: 10.1002/humu.21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansergh FC, Millington-Ward S, Kennan A, Kiang AS, Humphries M, Farrar GJ, Humphries P, Kenna PF. Retinitis pigmentosa and progressive sensorineural hearing loss caused by a C12258A mutation in the mitochondrial MTTS2 gene. Am J Hum Genet. 1999;64 (4):971–985. doi: 10.1086/302344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokry M, Feitsma H, Nijman IJ, de Bruijn E, van der Zaag PJ, Guryev V, Cuppen E. Accurate SNP and mutation detection by targeted custom microarray-based genomic enrichment of short-fragment sequencing libraries. Nucleic Acids Res. 2010;38 (10):e116. doi: 10.1093/nar/gkq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn K, Nishimura DY, Searby CC, Shastri M, Yen HJ, Beck JS, Braun T, Streb LM, Cornier AS, Cox GF, Fulton AB, Carmi R, Luleci G, Chandrasekharappa SC, Collins FS, Jacobson SG, Heckenlively JR, Weleber RG, Stone EM, Sheffield VC. Identification of the gene (BBS1) most commonly involved in Bardet-Biedl syndrome, a complex human obesity syndrome. Nat Genet. 2002;31 (4):435–438. doi: 10.1038/ng935. Database of Single Nucleotide Polymorphisms (dbSNP) (build 135) http://www.ncbi.nlm.nih.gov/SNP/ [DOI] [PubMed] [Google Scholar]
- Neveling K, Collin RW, Gilissen C, van Huet RA, Visser L, Kwint MP, Gijsen SJ, Zonneveld MN, Wieskamp N, de Ligt J, Siemiatkowska AM, Hoefsloot LH, Buckley MF, Kellner U, Branham KE, den Hollander AI, Hoischen A, Hoyng C, Klevering BJ, van den Born LI, Veltman JA, Cremers FP, Scheffer H. Next-generation genetic testing for retinitis pigmentosa. Hum Mutat. 2012;33 (6):963–972. doi: 10.1002/humu.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHLBI GO Exome Sequencing Project (ESP) S, WA (ESP6500SI) http://evs.gs.washington.edu/EVS/. http://evs.gs.washington.edu/niehsExome/(NIEHS95)
- O’Sullivan J, Mullaney BG, Bhaskar SS, Dickerson JE, Hall G, O’Grady A, Webster A, Ramsden SC, Black GC. A paradigm shift in the delivery of services for diagnosis of inherited retinal disease. [Accessed 04/15/2013];J Med Genet. 2012 49 (5):322–326. doi: 10.1136/jmedgenet-2012-100847. http://www.sph.uth.tmc.edu/Retnet. [DOI] [PubMed] [Google Scholar]
- Rivolta C, Sweklo EA, Berson EL, Dryja TP. Missense mutation in the USH2A gene: association with recessive retinitis pigmentosa without hearing loss. Am J Hum Genet. 2000;66 (6):1975–1978. doi: 10.1086/302926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Rozet JM, Gerber S, Souied E, Perrault I, Chatelin S, Ghazi I, Leowski C, Dufier JL, Munnich A, Kaplan J. Spectrum of ABCR gene mutations in autosomal recessive macular dystrophies. Eur J Hum Genet. 1998;6 (3):291–295. doi: 10.1038/sj.ejhg.5200221. [DOI] [PubMed] [Google Scholar]
- Sarpong A, Schottmann G, Ruther K, Stoltenburg G, Kohlschutter A, Hubner C, Schuelke M. Protracted course of juvenile ceroid lipofuscinosis associated with a novel CLN3 mutation (p.Y199X) Clin Genet. 2009;76 (1):38–45. doi: 10.1111/j.1399-0004.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- Shanks ME, Downes SM, Copley RR, Lise S, Broxholme J, Hudspith KA, Kwasniewska A, Davies WI, Hankins MW, Packham ER, Clouston P, Seller A, Wilkie AO, Taylor JC, Ragoussis J, Nemeth AH. Next-generation sequencing (NGS) as a diagnostic tool for retinal degeneration reveals a much higher detection rate in early-onset disease. Eur J Hum Genet. 2012 doi: 10.1038/ejhg.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DA, Clark GR, Alexander S, Silvestri G, Willoughby CE. Molecular diagnosis for heterogeneous genetic diseases with targeted high-throughput DNA sequencing applied to retinitis pigmentosa. J Med Genet. 2011;48 (3):145–151. doi: 10.1136/jmg.2010.083568. [DOI] [PubMed] [Google Scholar]
- Smit A, Hubley R, Green P. RepeatMasker Open-3.0. 1996–2010 http://www.repeatmasker.org.
- Sohocki MM, Daiger SP, Bowne SJ, Rodriquez JA, Northrup H, Heckenlively JR, Birch DG, Mintz-Hittner H, Ruiz RS, Lewis RA, Saperstein DA, Sullivan LS. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat. 2001;17 (1):42–51. doi: 10.1002/1098-1004(2001)17:1<42::AID-HUMU5>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, Abeysinghe S, Krawczak M, Cooper DN. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat. 2003;21 (6):577–581. doi: 10.1002/humu.10212. [DOI] [PubMed] [Google Scholar]
- Stone EM. Leber congenital amaurosis - a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2007;144 (6):791–811. doi: 10.1016/j.ajo.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Sullivan LS, Bowne SJ, Birch DG, Hughbanks-Wheaton D, Heckenlively JR, Lewis RA, Garcia CA, Ruiz RS, Blanton SH, Northrup H, Gire AI, Seaman R, Duzkale H, Spellicy CJ, Zhu J, Shankar SP, Daiger SP. Prevalence of disease-causing mutations in families with autosomal dominant retinitis pigmentosa: a screen of known genes in 200 families. Invest Ophthalmol Vis Sci. 2006;47 (7):3052–3064. doi: 10.1167/iovs.05-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The-International-Batten-Disease-Consortium. Isolation of a novel gene underlying Batten disease, CLN3. Cell. 1995;82 (6):949–957. doi: 10.1016/0092-8674(95)90274-0. [DOI] [PubMed] [Google Scholar]
- Wada Y, Itabashi T, Sato H, Kawamura M, Tada A, Tamai M. Screening for mutations in CYP4V2 gene in Japanese patients with Bietti’s crystalline corneoretinal dystrophy. Am J Ophthalmol. 2005;139 (5):894–899. doi: 10.1016/j.ajo.2004.11.065. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang H, Cao M, Li Z, Chen X, Patenia C, Gore A, Abboud EB, Al-Rajhi AA, Lewis RA, Lupski JR, Mardon G, Zhang K, Muzny D, Gibbs RA, Chen R. Whole-exome sequencing identifies ALMS1, IQCB1, CNGA3, and MYO7A mutations in patients with Leber congenital amaurosis. Hum Mutat. 2011;32 (12):1450–1459. doi: 10.1002/humu.21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H, Sun V, Tuan HF, Keser V, Wang K, Ren H, Lopez I, Zaneveld JE, Siddiqui S, Bowles S, Khan A, Salvo J, Jacobson SG, Iannaccone A, Wang F, Birch D, Heckenlively JR, Fishman GA, Traboulsi EI, Li Y, Wheaton D, Koenekoop RK, Chen R. Comprehensive molecular diagnosis of 179 Leber congenital amaurosis and juvenile retinitis pigmentosa patients by targeted next generation sequencing. J Med Genet. 2013 doi: 10.1136/jmedgenet-2013-101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster AR, Heon E, Lotery AJ, Vandenburgh K, Casavant TL, Oh KT, Beck G, Fishman GA, Lam BL, Levin A, Heckenlively JR, Jacobson SG, Weleber RG, Sheffield VC, Stone EM. An analysis of allelic variation in the ABCA4 gene. Invest Ophthalmol Vis Sci. 2001;42 (6):1179–1189. [PubMed] [Google Scholar]
- Zernant J, Kulm M, Dharmaraj S, den Hollander AI, Perrault I, Preising MN, Lorenz B, Kaplan J, Cremers FP, Maumenee I, Koenekoop RK, Allikmets R. Genotyping microarray (disease chip) for Leber congenital amaurosis: detection of modifier alleles. Invest Ophthalmol Vis Sci. 2005;46 (9):3052–3059. doi: 10.1167/iovs.05-0111. [DOI] [PubMed] [Google Scholar]
- Zito I, Thiselton DL, Gorin MB, Stout JT, Plant C, Bird AC, Bhattacharya SS, Hardcastle AJ. Identification of novel RPGR (retinitis pigmentosa GTPase regulator) mutations in a subset of X-linked retinitis pigmentosa families segregating with the RP3 locus. Hum Genet. 1999;105 (1–2):57–62. doi: 10.1007/s004399900110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.