Abstract
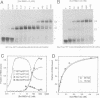
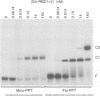
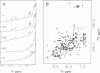
Sex determination and X chromosome dosage compensation in Drosophila melanogaster are directed by the Sex-lethal (Sxl) protein. In part, Sxl functions by regulating the splicing of the transformer pre-mRNA by binding to a 3' splice site polypyrimidine tract. Polypyrimidine tracts are essential for splicing of metazoan pre-mRNAs. To unravel the mechanism of splicing regulation at polypyrimidine tracts we analyzed the interaction of Sxl with RNA. The RNA binding activity of Sxl was mapped to the two ribonucleoprotein consensus sequence domains of the protein. Quantitation of binding showed that both RNA binding domains (RBDs) were required in cis for site-specific RNA binding. Individual RBDs interacted with RNA more weakly and had lost the ability to discriminate between wild-type and mutant transformer polypyrimidine tracts. Structural elements in one of the RBDs that are likely to interact with a polypyrimidine tract were identified using nuclear magnetic resonance techniques. In addition, our data suggest that multiple imino protons of the transformer polypyrimidine tract were involved in hydrogen bonding. Interestingly, in vitro Sxl bound with equal affinity to polypyrimidine tracts of pre-mRNAs that it does not regulate in vivo. We discuss the implications of this finding for the mechanism through which Sxl may gain selectivity for particular polypyrimidine tracts in vivo.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B. S. Sex in flies: the splice of life. Nature. 1989 Aug 17;340(6234):521–524. doi: 10.1038/340521a0. [DOI] [PubMed] [Google Scholar]
- Bell L. R., Horabin J. I., Schedl P., Cline T. W. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991 Apr 19;65(2):229–239. doi: 10.1016/0092-8674(91)90157-t. [DOI] [PubMed] [Google Scholar]
- Bell L. R., Maine E. M., Schedl P., Cline T. W. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988 Dec 23;55(6):1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- Birney E., Kumar S., Krainer A. R. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993 Dec 25;21(25):5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelens W., Scherly D., Beijer R. P., Jansen E. J., Dathan N. A., Mattaj I. W., van Venrooij W. J. A weak interaction between the U2A' protein and U2 snRNA helps to stabilize their complex with the U2B" protein. Nucleic Acids Res. 1991 Feb 11;19(3):455–460. doi: 10.1093/nar/19.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp D., Horabin J. I., Lersch R. A., Cline T. W., Schedl P. Expression of the Sex-lethal gene is controlled at multiple levels during Drosophila oogenesis. Development. 1993 Jul;118(3):797–812. doi: 10.1242/dev.118.3.797. [DOI] [PubMed] [Google Scholar]
- Burd C. G., Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994 Jul 29;265(5172):615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- Cline T. W. The Drosophila sex determination signal: how do flies count to two? Trends Genet. 1993 Nov;9(11):385–390. doi: 10.1016/0168-9525(93)90138-8. [DOI] [PubMed] [Google Scholar]
- Dombroski A. J., Walter W. A., Gross C. A. Amino-terminal amino acids modulate sigma-factor DNA-binding activity. Genes Dev. 1993 Dec;7(12A):2446–2455. doi: 10.1101/gad.7.12a.2446. [DOI] [PubMed] [Google Scholar]
- Endrizzi J. A., Cronk J. D., Wang W., Crabtree G. R., Alber T. Crystal structure of DCoH, a bifunctional, protein-binding transcriptional coactivator. Science. 1995 Apr 28;268(5210):556–559. doi: 10.1126/science.7725101. [DOI] [PubMed] [Google Scholar]
- Flickinger T. W., Salz H. K. The Drosophila sex determination gene snf encodes a nuclear protein with sequence and functional similarity to the mammalian U1A snRNP protein. Genes Dev. 1994 Apr 15;8(8):914–925. doi: 10.1101/gad.8.8.914. [DOI] [PubMed] [Google Scholar]
- García-Blanco M. A., Jamison S. F., Sharp P. A. Identification and purification of a 62,000-dalton protein that binds specifically to the polypyrimidine tract of introns. Genes Dev. 1989 Dec;3(12A):1874–1886. doi: 10.1101/gad.3.12a.1874. [DOI] [PubMed] [Google Scholar]
- Garrett D. S., Lodi P. J., Shamoo Y., Williams K. R., Clore G. M., Gronenborn A. M. Determination of the secondary structure and folding topology of an RNA binding domain of mammalian hnRNP A1 protein using three-dimensional heteronuclear magnetic resonance spectroscopy. Biochemistry. 1994 Mar 15;33(10):2852–2858. doi: 10.1021/bi00176a015. [DOI] [PubMed] [Google Scholar]
- Gill S. C., von Hippel P. H. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989 Nov 1;182(2):319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Granadino B., Campuzano S., Sánchez L. The Drosophila melanogaster fl(2)d gene is needed for the female-specific splicing of Sex-lethal RNA. EMBO J. 1990 Aug;9(8):2597–2602. doi: 10.1002/j.1460-2075.1990.tb07441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlach M., Wittekind M., Beckman R. A., Mueller L., Dreyfuss G. Interaction of the RNA-binding domain of the hnRNP C proteins with RNA. EMBO J. 1992 Sep;11(9):3289–3295. doi: 10.1002/j.1460-2075.1992.tb05407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K. B. Interaction of RNA hairpins with the human U1A N-terminal RNA binding domain. Biochemistry. 1994 Aug 23;33(33):10076–10088. doi: 10.1021/bi00199a035. [DOI] [PubMed] [Google Scholar]
- Hoffman B. E., Grabowski P. J. U1 snRNP targets an essential splicing factor, U2AF65, to the 3' splice site by a network of interactions spanning the exon. Genes Dev. 1992 Dec;6(12B):2554–2568. doi: 10.1101/gad.6.12b.2554. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Giguere V., Segui P., Evans R. M. Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell. 1987 Apr 10;49(1):39–46. doi: 10.1016/0092-8674(87)90753-7. [DOI] [PubMed] [Google Scholar]
- Howe P. W., Nagai K., Neuhaus D., Varani G. NMR studies of U1 snRNA recognition by the N-terminal RNP domain of the human U1A protein. EMBO J. 1994 Aug 15;13(16):3873–3881. doi: 10.1002/j.1460-2075.1994.tb06698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Hoshijima K., Sakamoto H., Shimura Y. Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature. 1990 Mar 29;344(6265):461–463. doi: 10.1038/344461a0. [DOI] [PubMed] [Google Scholar]
- Jessen T. H., Oubridge C., Teo C. H., Pritchard C., Nagai K. Identification of molecular contacts between the U1 A small nuclear ribonucleoprotein and U1 RNA. EMBO J. 1991 Nov;10(11):3447–3456. doi: 10.1002/j.1460-2075.1991.tb04909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaar R., Roche S. E., Beall E. L., Green M. R., Rio D. C. The conserved pre-mRNA splicing factor U2AF from Drosophila: requirement for viability. Science. 1993 Oct 22;262(5133):569–573. doi: 10.1126/science.7692602. [DOI] [PubMed] [Google Scholar]
- Kim J. L., Nikolov D. B., Burley S. K. Co-crystal structure of TBP recognizing the minor groove of a TATA element. Nature. 1993 Oct 7;365(6446):520–527. doi: 10.1038/365520a0. [DOI] [PubMed] [Google Scholar]
- Kim Y., Geiger J. H., Hahn S., Sigler P. B. Crystal structure of a yeast TBP/TATA-box complex. Nature. 1993 Oct 7;365(6446):512–520. doi: 10.1038/365512a0. [DOI] [PubMed] [Google Scholar]
- Lee A. L., Kanaar R., Rio D. C., Wemmer D. E. Resonance assignments and solution structure of the second RNA-binding domain of sex-lethal determined by multidimensional heteronuclear magnetic resonance. Biochemistry. 1994 Nov 22;33(46):13775–13786. doi: 10.1021/bi00250a031. [DOI] [PubMed] [Google Scholar]
- McKeown M. Sex differentiation: the role of alternative splicing. Curr Opin Genet Dev. 1992 Apr;2(2):299–303. doi: 10.1016/s0959-437x(05)80288-6. [DOI] [PubMed] [Google Scholar]
- Nagai K., Oubridge C., Jessen T. H., Li J., Evans P. R. Crystal structure of the RNA-binding domain of the U1 small nuclear ribonucleoprotein A. Nature. 1990 Dec 6;348(6301):515–520. doi: 10.1038/348515a0. [DOI] [PubMed] [Google Scholar]
- Nelissen R. L., Will C. L., van Venrooij W. J., Lührmann R. The association of the U1-specific 70K and C proteins with U1 snRNPs is mediated in part by common U snRNP proteins. EMBO J. 1994 Sep 1;13(17):4113–4125. doi: 10.1002/j.1460-2075.1994.tb06729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolov D. B., Hu S. H., Lin J., Gasch A., Hoffmann A., Horikoshi M., Chua N. H., Roeder R. G., Burley S. K. Crystal structure of TFIID TATA-box binding protein. Nature. 1992 Nov 5;360(6399):40–46. doi: 10.1038/360040a0. [DOI] [PubMed] [Google Scholar]
- Oliver B., Kim Y. J., Baker B. S. Sex-lethal, master and slave: a hierarchy of germ-line sex determination in Drosophila. Development. 1993 Nov;119(3):897–908. doi: 10.1242/dev.119.3.897. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Meneely P. M. Sex determination and dosage compensation: lessons from flies and worms. Science. 1994 May 13;264(5161):924–932. doi: 10.1126/science.8178152. [DOI] [PubMed] [Google Scholar]
- Rio D. C. Accurate and efficient pre-mRNA splicing in Drosophila cell-free extracts. Proc Natl Acad Sci U S A. 1988 May;85(9):2904–2908. doi: 10.1073/pnas.85.9.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin B., Green M. R. Specific and stable intron-factor interactions are established early during in vitro pre-mRNA splicing. Cell. 1985 Nov;43(1):131–142. doi: 10.1016/0092-8674(85)90018-2. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Zamore P. D., Green M. R. A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell. 1988 Jan 29;52(2):207–219. doi: 10.1016/0092-8674(88)90509-0. [DOI] [PubMed] [Google Scholar]
- Sakashita E., Sakamoto H. Characterization of RNA binding specificity of the Drosophila sex-lethal protein by in vitro ligand selection. Nucleic Acids Res. 1994 Oct 11;22(20):4082–4086. doi: 10.1093/nar/22.20.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz H. K. The genetic analysis of snf: a Drosophila sex determination gene required for activation of Sex-lethal in both the germline and the soma. Genetics. 1992 Mar;130(3):547–554. doi: 10.1093/genetics/130.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels M. E., Bopp D., Colvin R. A., Roscigno R. F., Garcia-Blanco M. A., Schedl P. RNA binding by Sxl proteins in vitro and in vivo. Mol Cell Biol. 1994 Jul;14(7):4975–4990. doi: 10.1128/mcb.14.7.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherly D., Boelens W., Dathan N. A., van Venrooij W. J., Mattaj I. W. Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B'' and their cognate RNAs. Nature. 1990 Jun 7;345(6275):502–506. doi: 10.1038/345502a0. [DOI] [PubMed] [Google Scholar]
- Scherly D., Boelens W., van Venrooij W. J., Dathan N. A., Hamm J., Mattaj I. W. Identification of the RNA binding segment of human U1 A protein and definition of its binding site on U1 snRNA. EMBO J. 1989 Dec 20;8(13):4163–4170. doi: 10.1002/j.1460-2075.1989.tb08601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Valcárcel J., Green M. R. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science. 1995 May 26;268(5214):1173–1176. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- Sosnowski B. A., Belote J. M., McKeown M. Sex-specific alternative splicing of RNA from the transformer gene results from sequence-dependent splice site blockage. Cell. 1989 Aug 11;58(3):449–459. doi: 10.1016/0092-8674(89)90426-1. [DOI] [PubMed] [Google Scholar]
- Steinmann-Zwicky M. Sex determination in Drosophila: the X-chromosomal gene liz is required for Sxl activity. EMBO J. 1988 Dec 1;7(12):3889–3898. doi: 10.1002/j.1460-2075.1988.tb03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcárcel J., Singh R., Zamore P. D., Green M. R. The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature. 1993 Mar 11;362(6416):171–175. doi: 10.1038/362171a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Giaever G. N. Action at a distance along a DNA. Science. 1988 Apr 15;240(4850):300–304. doi: 10.1126/science.3281259. [DOI] [PubMed] [Google Scholar]
- Wang J., Bell L. R. The Sex-lethal amino terminus mediates cooperative interactions in RNA binding and is essential for splicing regulation. Genes Dev. 1994 Sep 1;8(17):2072–2085. doi: 10.1101/gad.8.17.2072. [DOI] [PubMed] [Google Scholar]
- Wittekind M., Görlach M., Friedrichs M., Dreyfuss G., Mueller L. 1H, 13C, and 15N NMR assignments and global folding pattern of the RNA-binding domain of the human hnRNP C proteins. Biochemistry. 1992 Jul 14;31(27):6254–6265. doi: 10.1021/bi00142a013. [DOI] [PubMed] [Google Scholar]
- Zamore P. D., Patton J. G., Green M. R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992 Feb 13;355(6361):609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- van Gelder C. W., Gunderson S. I., Jansen E. J., Boelens W. C., Polycarpou-Schwarz M., Mattaj I. W., van Venrooij W. J. A complex secondary structure in U1A pre-mRNA that binds two molecules of U1A protein is required for regulation of polyadenylation. EMBO J. 1993 Dec 15;12(13):5191–5200. doi: 10.1002/j.1460-2075.1993.tb06214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]