Abstract
Mas-related G-protein-coupled receptor subtype C (MrgC) may play an important role in pain sensation. However, the distribution of MrgC receptors in different subpopulations of rodent dorsal root ganglion (DRG) neurons has not been clearly demonstrated owing to a lack of MrgC-selectively antibody. It is also unclear whether peripheral nerve injury induces different time-dependent changes in MrgC expression in injured and uninjured DRG neurons. Here we showed that MrgC immunoreactivity is distributed in both IB4-positive (non-peptidergic) and calcitonin gene-related peptide-positive (peptidergic) DRG neurons in mice and rats. Importantly, the MrgC mRNA level and MrgC immunoreactivity were both decreased in the injured L5 DRG compared to corresponding levels in the contralateral (uninjured) DRG in rats on days 14 and 30 after an L5 spinal nerve ligation. In contrast, mRNA and protein levels of MrgC were increased in the adjacent uninjured L4 DRG. Thus, nerve injury may induce temporal changes in MrgC expression that differ between injured and uninjured DRG neurons. In animal behavior tests, chronic constriction injury of the sciatic nerve induced mechanical pain hypersensitivity in wild-type mice and Mrg-clusterΔ−/− mice (Mrg KO). However, the duration of mechanical hypersensitivity was longer in the Mrg KO mice than in their wild-type littermates, indicating that activation of Mrgs may constitute an endogenous mechanism that inhibits the maintenance of neuropathic pain. These findings extend our knowledge about the distribution of MrgC in rodent DRG neurons and the regulation of its expression by nerve injury.
Keywords: MrgC, dorsal root ganglion, neuropathic pain, nerve injury
INTRODUCTION
G protein-coupled receptors are important drug targets for treatment of pathological pain conditions. Mas-related G-protein-coupled receptors (Mrg) are orphan G protein-coupled receptors that may play a role in pain sensation (Dong et al., 2001; Lembo et al., 2002). Of the rodent Mrg receptors (A-D), MrgC (mouse MrgC11 and rat homolog rMrgC) is expressed specifically in small-diameter dorsal root ganglion (DRG) neurons, which are presumably nociceptive afferent neurons. MrgC can function as a receptor for peptides that terminate in RF/Y-G or RF/Y-amide, such as bovine adrenal medulla peptide (BAM). Intriguingly, some MrgC ligands belong to the family of endogenous opioid peptides known to be involved in pain transmission (e.g., BAM22 and BAM8-22) (Dong et al., 2001; Lembo et al., 2002). Intrathecal administration of BAM8-22, an agonist of MrgC, was shown to induce analgesia in rodent models of inflammatory and neuropathic pain (Guan et al., 2010a; Jiang et al., 2013). Thus, Mrgs, especially MrgC, may modulate nociceptive processing after tissue and nerve injury.
Nociceptive DRG neurons possess a high degree of molecular diversity. Calcitonin gene-related peptide (CGRP) and lectin IB4 are histochemical markers that are commonly used to differentiate peptidergic and non-peptidergic DRG neurons. Despite a potential role of MrgC in modulating pain transmission, the distribution of MrgC receptors in rodent DRG neurons has not been clearly demonstrated, largely owing to a lack of MrgC antibody whose specificity has been verified in Mrg-mutant animals. It is also unclear whether nerve injury induces time-dependent changes in MrgC expression that differ between injured and uninjured DRG. A previous study showed that spinal nerve ligation (SNL) decreased MrgC mRNA level in injured DRG, but not in adjacent uninjured DRG, at day 14 post-SNL (Gustafson et al., 2005). Yet, it remains unclear if the decreased mRNA in injured DRG recovers at later time points (e.g., maintenance/recovery phase of neuropathic pain) and whether MrgC mRNA is upregulated in uninjured DRG. It is also unknown if changes in MrgC mRNA correlate with changes in protein expression.
Recently, we generated an MrgC-specific antibody to examine colocalization of MrgC and MrgA3 by immunohistochemical analysis(Han et al., 2013). However, MrgC may be expressed in a larger population of DRG neurons than MrgA3 is, and this antibody has not been used to examine the distribution of MrgC in different subsets of DRG neurons. In light of possible species differences, we conducted a double-staining immunohistochemistry study to characterize and compare the distribution of MrgC receptor in DRG neurons in mice and rats. We then used real-time reverse transcriptase–polymerase chain reaction (RT–PCR) and immunohistochemistry techniques to test the hypothesis that nerve injury differentially alters the temporal expression of MrgC in injured and uninjured DRG neurons in rats at different time points after an L5 SNL. Our previous study suggested that Mrgs may function as endogenous inhibitors of inflammatory pain (Guan et al., 2010a). Here, we tested Mrg-clusterΔ−/− mice (Mrg KO), in which all nociceptive neuron-expressing Mrg genes (including MrgC) have been deleted, to determine if activation of Mrgs also inhibits the development or maintenance of neuropathic pain.
EXPERIMENTAL PROCEDURES
All procedures were reviewed and approved by the Johns Hopkins University Animal Care and Use Committee as consistent with the National Institutes of Health Guide for the Use of Experimental Animals to ensure minimal animal use and discomfort. Animals received food and water ad libitum and were housed in isolator cages on a 12-h day–night cycle.
Animals and surgery
Mrg-clusterΔ−/− (Mrg KO) mice
Chimeric Mrg KO mice were produced by blastocyst injection of positive embryonic stem cells. The Mrg KO mice were generated by mating chimeric mice to C57BL/6 mice. The progeny were backcrossed to C57BL/6 mice for at least five generations. Mrg KO mice have a deletion of 845 kb in chromosome 7, which contains 12 intact Mrg genes, including MrgC11 (Liu et al., 2009; Guan et al., 2010a).
L5 spinal nerve ligation (SNL) model of neuropathic pain in rats
Male Sprague-Dawley rats (200–350 g, Harlan, Indianapolis, IN) were anesthetized with 2% isoflurane. The left L5 spinal nerve was ligated with a 6-0 silk suture and cut distally as described previously (Kim et al., 1997; Guan et al., 2008). The muscle layer was closed with 4-0 chromic gut suture, and the skin closed with metal clips. In a sham-operated control group, the surgical procedure was identical to that described above, except that the L5 spinal nerve was not ligated or cut. In addition, to prevent possible irritation or damage to the left L5 spinal nerve, we did not remove the left transverse process of the L6 vertebra. Sham-operated rats in different groups were combined for analysis.
Sciatic chronic constriction injury (CCI) model of neuropathic pain in mice
Adult male C57BL/6 mice (25-35 g) were anesthetized with 2% isoflurane delivered through a nose cone. Under aseptic conditions, the left sciatic nerve at the middle thigh level was separated from the surrounding tissue and loosely tied with three nylon sutures (9-0 nonabsorbable monofilament, S&T AG, Neuhausen, Switzerland) as described previously (Guan et al., 2010a). The distance between two adjacent ligatures was approximately 0.5 mm.
Real-time quantitative polymerase chain reaction (RT-PCR)
RT-PCR was carried out with a modified version of a previously described method (Ueno et al., 2002) by using Trizol reagent (Invitrogen, Carlsbad, CA). Purified RNA was quantified on the GeneQuant UV spectrophotometer (Amersham Pharmacia Biotech, Piscataway, NJ) at 260 nm absorbance and assessed for purity by using the 260/280 nm ratio. DNA from individual DRGs was reverse transcribed with the Superscript First Strand Synthesis System (Invitrogen). PCR amplifications were carried out with a GeneAmp 5700 thermal cycler (Perkin-Elmer, Waltham, MA). The reaction mixture contained 1× SYBR PCR buffer (Perkin-Elmer), 200 μM each of dATP, dCTP, and dGTP, 400 μM dUTP, 0.025 U/μl AmpliTaq Gold, 0.01 U/μl AmpEraseUNG (uracil-N-glycosylase), 3 mM MgCl2, and 200 nM of each primer in a volume of 50 μl. PCR conditions were 94°C for 3 min and 40 cycles of 94°C for 15 s, 52°C for 30 s, and 72°C for 45 s. The MrgC-specific intron-spanning primers (to avoid genomic contamination) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers were designed by using the Primer Express 1.0 Software program (Perkin-Elmer, MrgC-F: CAGCACAAGTCAGCTCCTCAAC; MrgC-R: ATGCCCATGAGAAAGGACAGAACC; GAPDH-F: TGCACCACCAACTGCTTAG; GAPDH-R: GGATGCAGGGATGATGTTC). Melting curve analysis was applied to all final PCR products after the cycling protocol. For each sample, we also carried out PCR reactions with RNA that had not been reverse transcribed to exclude genomic DNA contamination. The PCR products were separated on a 3% (w/v) agarose/Tris-acetate-EDTA gel to confirm the product size. Samples were run in triplicate, and threshold cycle (Ct) values from each reaction were averaged. Tissues from different experimental groups were processed together.
Immunohistochemistry
The animals were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and perfused intracardially with 0.1 M phosphate-buffered saline (PBS; pH 7.4, 4°C) followed by fixative (4% formaldehyde and 14% [v/v] saturated picric acid in PBS, 4°C). DRG and spinal cord tissues were cryoprotected in 20% sucrose (24 h) before being serially cut into 25-μm sections and placed onto slides. The slides were pre-incubated in blocking solution (10% normal goat serum, 1 h) and then incubated overnight at 4°C in primary antibodies. The primary antibodies were goat antibody to CGRP (1720-9007 AbD Serotec, 1:1,000), guinea pig antibody to TRPV1 (AB5566 Chemicon, 1:1,000), and rabbit polyclonal MrgC antibody (1:500), which was custom-made by Proteintech Group, Inc. (Chicago, IL). Slides were incubated in secondary antibody at room temperature for 2 h. The secondary antibodies were diluted 1:100 in blocking solution and included donkey antibody to rabbit (711-295-152, Rhod Red-X-conjugated, Jackson ImmunoResearch, West Grove, PA), donkey antibody to goat (705-096-147, FITC-conjugated, Jackson ImmunoResearch), and donkey antibody to guinea pig (706-096-148, FITC-conjugated, Jackson ImmunoResearch). To detect IB4 binding, we incubated sections with Griffonia simplicifolia isolectin GS-IB4-Alexa 488 (1:200; A11001, Molecular Probes). Preliminary studies were performed to confirm the specificity of the primary antibody (data not shown). Controls included preabsorption of the primary antibody with the corresponding synthetic peptide or omission of the primary antibody. The total number of neurons in each section was determined by counting both labeled and unlabeled cell bodies. For the double-labeling studies, the percent of double-labeled neurons is expressed relative to the total number of labeled neurons. Tissues from different experimental groups were processed together. Data were analyzed by an investigator blinded to experimental group.
Paw withdrawal frequency test in mice
The mouse was placed in a Plexiglas chamber on an elevated mesh screen. Two calibrated von Frey monofilaments (0.07 and 0.45 g) were used (Guan et al., 2010a). Each von Frey filament was applied to the hind paw for approximately 1 s. Each stimulation was repeated 10 times to both hind paws. The occurrence of paw withdrawal is expressed as a percent response frequency. Percent response frequency = (number of paw withdrawals/10 trials) × 100. To minimize variability of the behavioral outcome measures, we trained animals for 3 to 5 days before obtaining baseline data. In addition, animals were habituated to the test environment for ≥30 min before testing was started. All behavioral tests were conducted in the morning. All measurements were made with concurrent controls (Mrg KO vs. wild-type) to blind the investigators.
Data analysis
The method for statistical comparisons in each study is given in the figure legends. The number of animals used in each study was based on our experience with similar studies and power analysis calculation. Representative data are from experiments that were replicated biologically at least three times with similar results. STATISTICA 6.0 software (StatSoft, Inc., Tulsa, OK) was used to conduct all statistical analyses. The Tukey honestly significant difference (HSD) post-hoc test was used to compare specific data points. Two-tailed tests were performed, and data are expressed as mean ± SEM; p<0.05 was considered significant in all tests.
RESULTS
MrgC receptors are expressed in both non-peptidergic and peptidergic DRG neurons in mice
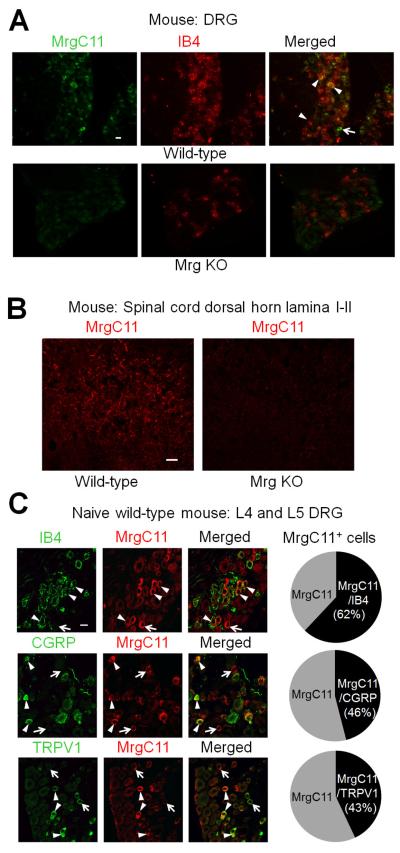
The MrgC-specific antibody selectively labeled a subset of lumbar DRG neurons (Fig. 1A) and was immunoreactive in the superficial dorsal horn (Fig. 1B) in wild-type mice (n=3) but not in Mrg KO mice (n=3). Because the MrgC11 gene is found only in peripheral afferent sensory neurons (Dong et al., 2001), our findings suggest that MrgC11 is expressed in both soma and central terminals of mouse DRG neurons. In immunofluorescence staining study, MrgC11 was labeled in 199 of 1,381 (14.4%) lumbar DRG neurons in wild-type mice (2-4 sections/animal, L4 and L5 levels combined, 3 mice). In separate double-immunofluorescence staining studies (2-4 sections/animal, L4 and L5 levels, 3 mice), MrgC11 immunoreactivity was detected in both non-peptidergic (lectin IB4+) and peptidergic (CGRP+) DRG neurons (Fig. 1C). Specifically, 33 of 53 (62.3%) MrgC11+ neurons also immunostained with IB4, and 47 of 102 (46.1%) MrgC11+ neurons also immunostained with CGRP. In addition, 90 of 211 (42.7%) MrgC11+ neurons also immunostained with TRPV1, another important marker of nociceptive afferent sensory neurons.
Fig. 1. Distribution of MrgC immunoreactivity in different subpopulations of mouse DRG neurons.
(A) Confocal images of double-immunofluorescence–stained DRG sections show that a subset of neurons in lumbar DRGs from wild-type mice are stained with both MrgC11 and lectin IB4 (arrows). The MrgC antibody did not show any positive signal in Mrg-clusterΔ−/− mice (Mrg KO), demonstrating that it is specific for Mrgs. (B) MrgC11 immunofluorescence was also detected in the superficial dorsal horn of wild-type mice, but not in that of Mrg KO mice. (C) Left: Double-immunofluorescence staining shows the distribution of MrgC11 immunoreactivity (+) in different subsets (IB4, CGRP) of DRG neurons in wild-type mice (n=3). MrgC11+ neurons that coexpressed other molecules are indicated by arrowheads, and MrgC11+ neurons negative for other molecules are indicated by arrows. Right: The proportions of double-labeled cells in MrgC11+ neurons at lumbar DRGs of wild-type mice. Scale bar: 20 μm (A-C).
MrgC mRNA level is decreased in injured DRG but increased in adjacent uninjured DRG after an L5 SNL in rats
The temporal changes in gene expression after nerve injury may differ in injured and nearby uninjured DRGs (Hammond et al., 2004; Lee et al., 2011). Therefore, we used the modified “Chung” model (L5 SNL), in which the nerve injury is standardized and limited to L5 (Guan et al., 2010b; He et al., 2013), to investigate the respective effects of SNL on injured and adjacent uninjured DRGs at various post-injury time points. We chose rats for this set of experiments because L5 SNL is easier to perform in rats than in mice, and more DRG tissue can be harvested from rats.
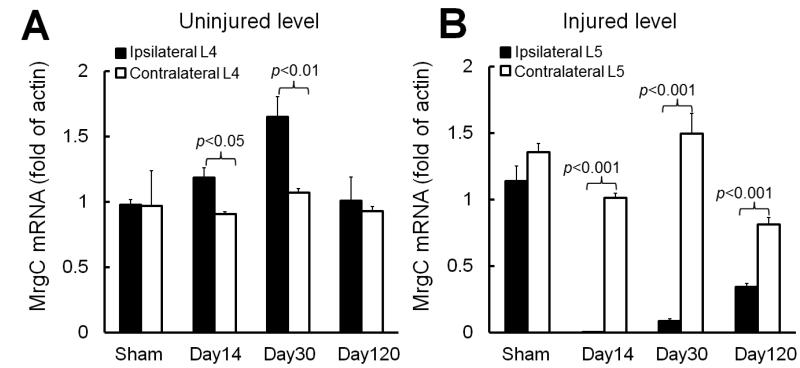
MrgC mRNA levels were significantly greater in the ipsilateral (injured side) L4 DRG (uninjured level) than in the contralateral L4 DRG on days 14 (n=5 rats) and 30 (n=5 rats, Fig. 2A) post-SNL in rats. In this model, these time points generally correspond to the peak and maintenance phases of neuropathic pain, respectively (Kim et al., 1997; Guan et al., 2008; Guan et al., 2010c). The elevated MrgC mRNA level in the ipsilateral L4 DRG had returned to baseline level by day 120 post-SNL (n=4 rats). In contrast, MrgC mRNA levels were significantly decreased in the ipsilateral L5 DRG (injured level) compared to levels on the contralateral side from day 14 through day 120 post-SNL (Fig. 2B). The MrgC mRNA levels in L4 and L5 DRGs did not differ significantly between the ipsilateral and contralateral sides of rats that underwent sham surgery (n=5 rats). Additionally, SNL did not cause a significant change in MrgC mRNA level of the contralateral L4 DRG compared to levels in the sham-operated group.
Fig. 2. L5 spinal nerve injury in rats differentially alters MrgC mRNA levels in injured and uninjured DRGs.
(A) The MrgC mRNA levels in L4 DRGs did not differ significantly between the ipsilateral and contralateral sides of rats that underwent sham surgery (n=5). MrgC mRNA level was significantly increased in the ipsilateral L4 DRG on day 14 (n=5) and day 30 (n=5) post-SNL, as compared to that on the contralateral side. (B) However, MrgC mRNA level was significantly decreased in the ipsilateral L5 DRG for at least 120 days post-SNL (n=4). Unpaired t-test. Data are expressed as mean + SEM.
Spinal nerve injury reduces MrgC immunoreactivity in injured DRG but increases it in adjacent uninjured DRG in rats
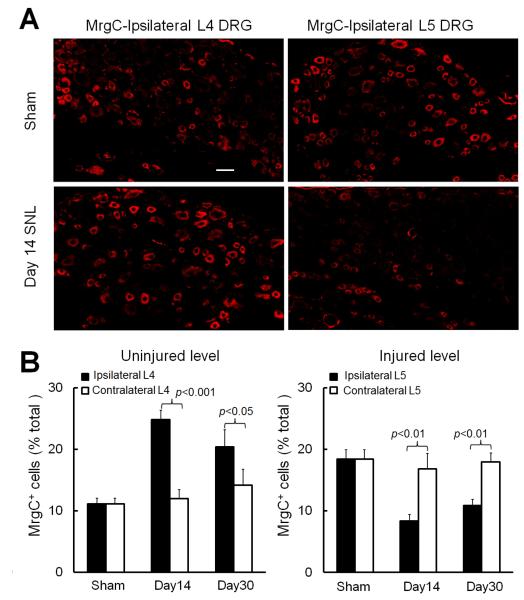
After confirming the specificity of MrgC antibody in Mrg KO mice and showing the time-dependent changes in MrgC mRNA levels, we examined how nerve injury affects MrgC expression at the protein level in injured and uninjured DRGs of SNL rats. Because the MrgC-specific antibody did not work in our Western blot experiment (unpublished observation), we used immunofluorescence staining to examine changes in the distribution of MrgC immunoreactivity in DRG neurons of SNL rats at the peak (day 14) and maintenance (day 30) phases of neuropathic pain (Fig. 3A). The MrgC-specific antibody labeled 833 of 5,515 lumbar DRG neurons (15.1%) in sham-operated rats (4-6 sections/animal, L4 and L5 levels combined, 5 rats), a value similar to that in naïve wild-type mice (14.4%). Compared to MrgC+ DRG neurons on the contralateral side (day 14: 181 of 1,541 neurons [11.7%], 6 rats; day 30: 258 of 1,887 neurons [13.7%], 6 rats), the percentage of MrgC+ DRG neurons was significantly increased in the ipsilateral uninjured L4 DRG on day 14 (547 of 2,231 [24.5%]) and day 30 (415 of 2,054 [20.2%]; Fig. 3B left) post-SNL. In contrast, the percentage of MrgC+ neurons was significantly decreased in the ipsilateral injured L5 DRG (day 14: 106 of 1,433 [7.4%]; day 30: 244 of 2,382 [10.2%]; Fig. 3B right), as compared to that on the contralateral side (day 14: 308 of 1,942 [15.8%]; day 30: 321 of 1,957 [16.4%]). L5 SNL did not cause a significant change in the percentage of MrgC+ neurons in the contralateral L4 and L5 DRGs, as compared to that in the corresponding DRGs of the sham-operated group (n=5). Interestingly, in the sham-operated group (n=5 rats), the percentage of MrgC+ neurons in L4 and L5 DRGs was comparable between the ipsilateral and the contralateral sides of the same level, but it was greater in the L5 DRG (539 of 2,913 neurons [18.5%]) than in the L4 DRG (294 of 2,602 [11.2%], p<0.05, unpaired t-test), indicating segmental differences in MrgC expression in rat DRGs.
Fig. 3. L5 spinal nerve injury increases MrgC immunoreactivity in ipsilateral uninjured rat DRG neurons.
(A) MrgC immunoreactivity was increased in the ipsilateral L4 DRG (uninjured level) but decreased in the ipsilateral L5 DRG (injured level) of rats that underwent L5 SNL compared to that in the corresponding DRGs of sham-operated rats. Scale bar: 50 μm. (B) The percentage of MrgC+ neurons in L4 and L5 DRGs did not differ significantly between the ipsilateral and contralateral sides of rats that underwent sham surgery (n=5). However, the percentage of MrgC+ neurons was significantly increased in the ipsilateral L4 DRGs and decreased in the ipsilateral L5 DRGs on day 14 (n=6) and day 30 (n=6) post-SNL, compared to the percentage of MrgC+ neurons on the corresponding contralateral side. Unpaired t-test. Data are expressed as mean + SEM.
Effects of nerve injury on the proportions of MrgC-positive DRG neurons that colocalize with IB4 and CGRP in rat
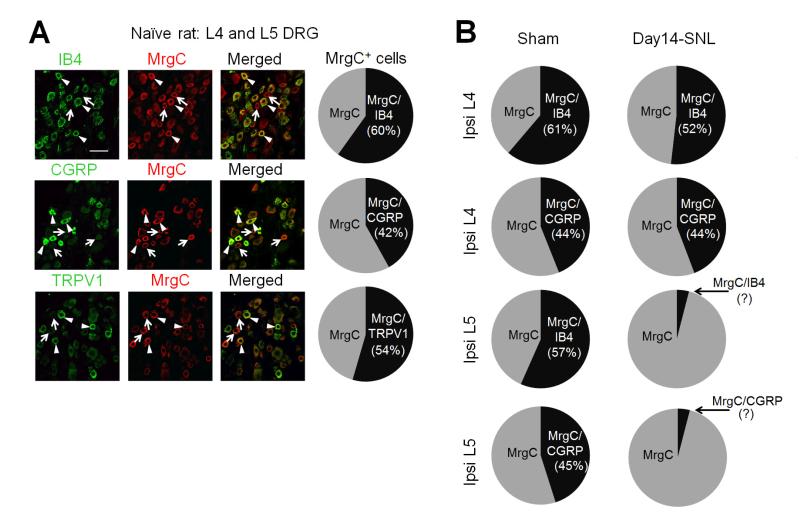
We conducted separate double-immunofluorescence staining studies to examine the proportions of MrgC-immunoreactive DRG neurons that colocalize with IB4 and CGRP in naïve, sham-operated, and nerve-injured rats at day 14 post-SNL. In lumbar DRGs from naïve rats (2-3 sections/animal, L4 and L5 levels combined, 3 rats), 127 of 212 MrgC+ neurons (59.9%) also immunostained for IB4, 57 of 136 (41.9%) immunostained for CGRP, and 121 of 222 (54.5%) immunostained for TRPV1 (Fig. 4A). In sham-operated rats (n=2), the percentages of MrgC+ neurons in ipsilateral DRGs that immunostained for IB4 (L4: 60 of 98 [61.2%]; L5: 141 of 249 [56.6%]) and CGRP (L4: 40 of 91 [43.9%]; L5: 106 of 235 [45.1%], Fig. 4B left) were comparable to those in naïve rats. At day 14 after SNL in rats (n=3), the percentage of MrgC+ neurons in ipsilateral L4 DRGs that immunostained with IB4 was decreased (114 of 220 [51.8%]) and the percentage that immunostained with CGRP was unchanged (119 of 269 [44.2%], Fig. 4B right), as compared to that in sham-operated rats. This decreased and unchanged percentages of MrgC+ neurons in IB4 and CGRP subpopulations of uninjured DRG neurons, respectively, may be due to phenotypic changes and increased expression of these markers in uninjured DRG after nerve injury (Hammond et al., 2004; Chao et al., 2008; Hirose et al., 2010; Nitzan-Luques et al., 2013). The IB4 signal was nearly completely lost, and the CGRP immunoreactivity was very weak and diffusive in the ipsilateral injured L5 DRG; hence their colocalization with MrgC could not be reliably determined.
Fig. 4.
(A) Left: Double-immunofluorescence staining shows the distribution of MrgC immunoreactivity (+) in different subsets (IB4, CGRP) of lumbar DRG neurons in naïve rats. MrgC+ neurons that coexpressed other molecules are indicated by arrowheads, and MrgC+ neurons negative for other molecules are indicated by arrows. Right: The proportions of double-labeled cells in MrgC+ neurons in lumbar DRGs of naïve rats (n=3). Scale bar: 50 μm. (B) The proportions of double-labeled cells in MrgC+ neurons in ipsilateral (Ipsi) L4 and L5 DRGs of rats on day 14 post-SNL (n=3) and after sham-surgery (n=2).
In line with findings from our previous set of experiment, the percentage of MrgC+ neurons among total DRG neurons in this double-immunofluorescence staining study was also significantly higher in the ipsilateral uninjured L4 DRG on day 14 post-SNL (23.7%, n=509 out of 2,485), as compared to that of sham-operated rats of this experiment (11.4%, n=189 out of 1,647, p<0.05, unpaired t-test). In contrast, the percentage of MrgC+ neurons was significantly decreased in the injured L5 DRG (7.0%, n=57 out of 809), as compared to the sham-operated rats (17.2%, n=484 out of 2,822, p<0.05, unpaired t-test).
Nerve injury may activate endogenous Mrgs to counteract the maintenance of neuropathic mechanical hypersensitivity
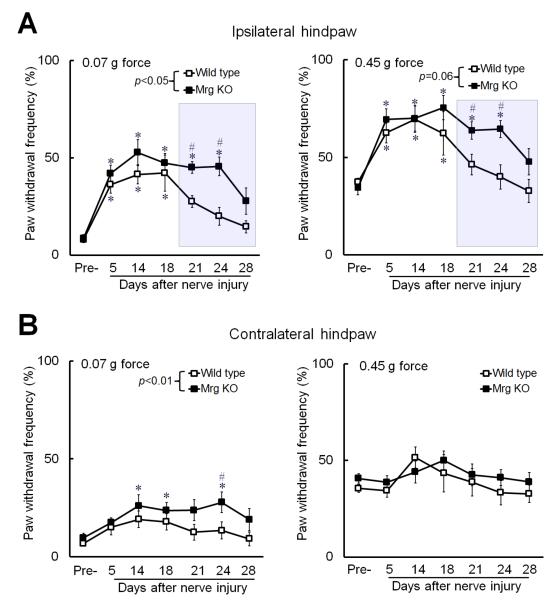
Because mechanical allodynia is an important and common manifestation of neuropathic pain, we further examined how Mrg knockout affects mechanical sensitivity in an experimental model of neuropathic pain. We induced neuropathic pain in mice by CCI of the sciatic nerve because this model usually produces shorter-lasting mechanical hypersensitivity than does SNL (Kim et al., 1997), making CCI more suitable for studying the role of endogenous Mrg signaling in the maintenance and recovery phases of neuropathic pain. In addition, our previous study showed that injection of MrgC agonist BAM8–22 attenuated mechanical pain hypersensitivity in CCI mice (Guan et al., 2010a). Compared to wild-type littermates (n=8), Mrg KO mice (n=12) exhibited prolonged hypersensitivity to both low-force and high-force mechanical stimuli applied to the ipsilateral hind paw after CCI of the sciatic nerve (Fig. 5A). Mrg KO mice also developed hypersensitivity to low-force mechanical stimuli in the contralateral hind paw after nerve injury (Fig. 5B).
Fig. 5. Mrg KO mice display prolonged mechanical hypersensitivity after peripheral nerve injury.
(A) In both Mrg KO (n=12) and wild-type mice (n=8), the ipsilateral paw withdrawal frequency (PWF) to low-force (0.07 g) and high-force (0.45 g) punctuate mechanical stimulation was significantly increased after chronic constriction injury (CCI) of the left sciatic nerve. However, the mechanical hypersensitivity persisted longer in Mrg KO mice than in wild-type mice (shaded area). (B) The PWF of the contralateral hind paw to low-force stimulation also increased in Mrg KO mice after CCI. *p<0.05 vs. pre-injury, #p<0.05 vs. wild-type mice. Two-way mixed model ANOVA. Data are expressed as mean ± SEM.
DISCUSSION
This study provides novel evidence that extends our knowledge about the role of MrgC in neuropathic pain. It is the first study to use an MrgC-selective antibody to examine the distribution of MrgC in mouse and rat DRG, and more importantly, to determine the temporal changes of MrgC immunoreactivity at the peak and maintenance phases of neuropathic pain. By using RT-PCR at multiple post-injury time points, we found time-dependent changes in MrgC mRNA that differ between injured (prolonged decrease) and uninjured (temporary increase) DRG after SNL. Furthermore, studies in Mrg KO mice provide novel evidence that activation of the endogenous Mrg system may inhibit the prolongation of neuropathic pain.
Previous in situ hybridization studies indicated that MrgC is located predominantly in a subgroup of small- and medium-size afferent sensory neurons that can be visualized by IB4 labeling (Dong et al., 2001; Lembo et al., 2002). To date, the distribution of MrgC receptor protein in DRG neurons has not been clearly demonstrated because the specific antibody has not been tested in Mrg-mutant tissue. By using an MrgC-specific antibody(Han et al., 2013), we show here that MrgC immunoreactivity is present in both IB4+ and CGRP+ DRG neurons in mice and rats. A large percentage (>40%) of MrgC+ neurons also coexpressed TRPV1 receptors. These findings support those of Hager et al. (Hager et al., 2008) and suggest a broad pattern of MrgC expression in DRG that covers both peptidergic and non-peptidergic subpopulations. Our findings may differ from those of the previous in situ hybridization studies (Dong et al., 2001; Lembo et al., 2002) because of different experimental conditions and because the MrgC-specific antibody used for immunohistochemistry may have greater sensitivity than the cRNA probes. However, we cannot rule out the possibility that the antibody could react with other members of the Mrg family because several other Mrg subtypes are deleted in the Mrg KO mice. Yet, the likelihood of this cross-reactivity is low because the peptide antigen used to generate the antibody is present only in MrgC11. Nevertheless developing MrgC11 single KO mice in the future will help to validate the antibody specificity. Both peptidergic and non-peptidergic nociceptors are important to the expression of pathological pain (Julius and Basbaum, 2001; Braz et al., 2005; Cavanaugh et al., 2009). The distribution of MrgC in both subsets of nociceptive DRG neurons provides a biological basis for MrgC to modulate their functions and pain transmission.
A previous study showed that ligating both L5 and L6 spinal nerves significantly downregulated MrgC mRNA in the injured DRGs at day 14 post-injury but did not change MrgC mRNA level in uninjured L4 DRG (Gustafson et al., 2005). Additional in situ hybridization experiments demonstrated that the decreased mRNA did not result from neuronal loss. Similarly, using a modified “Chung” model, we found that MrgC mRNA in injured L5 DRG undergoes a prolonged decrease from day 14 to day 120 post-SNL. However, in contrast to the previous study, our results suggest that MrgC mRNA increases in ipsilateral uninjured L4 DRG between days 14 and 30 post-SNL. The reasons for this discrepancy remain unclear, but it may be partially due to different nerve injuries in these studies. In the modified “Chung” model, the L5 spinal nerve is ligated and also cut distally to disrupt the connection between cell body and peripheral tissue and prevent nerve regeneration (Kim et al., 1997; Guan et al., 2008). However in the study by Gustafson et al. (2005), both L5 and L6 spinal nerves were ligated but not cut. It is possible that different amounts (L5 and L6 versus L5 alone) and types (ligation versus ligation and cut) of nerve injuries may differentially affect the magnitude and the time course of changes in gene expression in L4 DRG. Furthermore, the increase of MrgC mRNA in L4 DRG was much greater at day 30 post-SNL than at day 14. It is possible that the authors of the previous study might have detected an increase in MrgC mRNA at later time points.
Due to translational modulation, changes in receptor protein expression may not correlate with changes at the mRNA level. Because it is ultimately the protein that conveys function, we used fluorescent immunocytochemistry to extend our observations to the protein level. The increased MrgC mRNA levels in uninjured L4 DRGs were paralleled by increases in protein expression (e.g., increased percentage of MrgC+ neurons). Similarly, the decreased MrgC mRNA in injured L5 DRGs at days 14 and 30 post-SNL was also associated with a decreased number of MrgC+ neurons. As the action site for MrgC agonist to inhibit pain is likely at central terminals of DRG neurons (Zeng et al., 2004; Chen et al., 2006; Guan et al., 2010a), it remains to be examined if upregulation of MrgC expression in the soma of L4 DRG neurons leads to increased trafficking of receptor into their central projections.
Among MrgC+ neurons, the percentage of cells that expressed IB4 was decreased and the percentage that expressed CGRP was unchanged in ipsilateral L4 DRG at day 14 post-SNL, as compared to percentages in sham-operated rats. This finding suggests that MrgC is also expressed in both subsets of uninjured DRG neurons after SNL. However, consistent with previous reports (Hammond et al., 2004), neurons in injured L5 DRG that were labeled by IB4 were largely absent and those that were labeled by CGRP were very difficult to detect at day 14 after SNL. Phenotypic changes in DRG neurons have been observed after inflammation and peripheral nerve injury (Xiao et al., 2002; Chao et al., 2008; Pernia-Andrade et al., 2009). Since nerve injury may change CGRP expression and IB4 binding in DRG neurons, and these changes are also different in injured and uninjured levels (Hammond et al., 2004; Chao et al., 2008; Hirose et al., 2010; Nitzan-Luques et al., 2013), a triple-immunofluorescence staining study is needed to determine the relative proportion and differential changes of MrgC expression in different subsets of DRG neurons after nerve injury.
The time course of increased MrgC expression in L4 DRG correlates with the peak and maintenance of neuropathic pain in SNL rats (Kim et al., 1997; Guan et al., 2008). Yet, it remains to be determined if MrgC immunoreactivity returns to normal along with MrgC mRNA by day 120 post-SNL, a time point when neuropathic pain manifestations are mostly diminished in SNL rats(Guan et al., 2008; Guan et al., 2010c). The physiological implications of these different changes in MrgC expression in injured and uninjured DRGs after SNL are unclear. Nevertheless, because the transmission of peripheral inputs relies on uninjured DRG neurons, and activation of MrgC at central terminals of DRG neurons attenuates persistent pain (Chen et al., 2006; Chen et al., 2008; Guan et al., 2010a), we postulate that an increase in MrgC expression in uninjured DRGs may represent a compensatory mechanism that inhibits pain exacerbation after nerve injury. Behaviorally, CCI-induced mechanical hypersensitivity persisted longer in Mrg KO mice than in wild-type littermates. Mrg KO mice also showed mechanical hypersensitivity to low-force mechanical stimuli on the contralateral hind paw after nerve injury. These results corroborate our previous findings that Mrg KO mice exhibit greater pain behavior than control mice in different inflammatory pain models (Guan et al., 2010a) and support the idea that Mrg limits pain exacerbation. Although upregulation of MrgC expression after nerve injury might be an essential adaptive component that affects the temporal resolution and maintenance of neuropathic pain, Mrg KO mice showed only slightly more mechanical hypersensitivity than did wild-type mice during the first 2 weeks after nerve injury. This finding suggests that constitutive Mrg activity alone is not sufficient to prevent the development of a neuropathic pain state. However, it is possible that MrgC may work in concert or interactively with other endogenous pain inhibitory mechanisms (e.g., the opioidergic system) after nerve injury. A dynamic balance between excitatory and inhibitory mechanisms may determine the level of neuron excitability and fine-tune the efficiency of spinal pain transmission (Basbaum, 1999). Intense noxious inputs trigger release of endogenous pain inhibitory peptides, such as endomorphins and enkephalins, from multiple sources. Future studies are needed to examine whether nerve injury also increases the expression and release of endogenous MrgC ligands (e.g., BAM22) into the dorsal horn to fine-tune pain transmission, as shown after hind paw inflammation (Cai et al., 2007).
CONCLUSIONS
MrgC may represent a compelling new target for pain therapy. Our study suggests that changes in MrgC expression after nerve injury differ temporally in injured and uninjured DRG neurons and that activation of Mrgs may constitute an endogenous mechanism that inhibits the maintenance of neuropathic pain. Accordingly, upregulation of MrgC expression in uninjured DRG may be an essential adaptive component that affects the temporal resolution of the chronic pain state.
Highlights.
• MrgC is expressed in both IB4+ and CGRP+ DRG neurons in mice and rats.
• An L5 spinal nerve injury decreased MrgC expression in injured L5 DRG.
• However, L5 nerve injury increased MrgC expression in adjacent uninjured L4 DRG.
• Mrg KO mice exhibited prolonged mechanical hypersensitivity after nerve injury.
Acknowledgments
The authors thank Claire F. Levine, MS (scientific editor, Department of Anesthesiology/CCM, Johns Hopkins University), for editing the manuscript and Yixun Geng and Alene Carteret for mouse genotyping and maintenance. This study was supported by grants from the NIH: NS70814 (Y.G.), NS58481 (X.D.), GM087369 (X.D.), NS26363 (S.N.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The work was conducted in the Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD, USA.
Author contributions
S-Q.H. and L.H. performed most of the experiments and were involved in writing a draft manuscript. Z.L., V.T., Q.X. X.G. and Y.F. conducted some of the molecular and behavioral experiments. Y.W. and S.N.R. were involved in experimental design and data analysis. Y.G. and X.D. designed and directed the project and wrote the final manuscript.
REFERENCES
- Basbaum AI. Distinct neurochemical features of acute and persistent pain. Proc Natl Acad Sci U S A. 1999;96:7739–7743. doi: 10.1073/pnas.96.14.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel "pain" pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Cai M, Chen T, Quirion R, Hong Y. The involvement of spinal bovine adrenal medulla 22-like peptide, the proenkephalin derivative, in modulation of nociceptive processing. Eur J Neurosci. 2007;26:1128–1138. doi: 10.1111/j.1460-9568.2007.05755.x. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao T, Pham K, Steward O, Gupta R. Chronic nerve compression injury induces a phenotypic switch of neurons within the dorsal root ganglia. J Comp Neurol. 2008;506:180–193. doi: 10.1002/cne.21537. [DOI] [PubMed] [Google Scholar]
- Chen T, Cai Q, Hong Y. Intrathecal sensory neuron-specific receptor agonists bovine adrenal medulla 8-22 and (Tyr6)-gamma2-MSH-6-12 inhibit formalin-evoked nociception and neuronal Fos-like immunoreactivity in the spinal cord of the rat. Neuroscience. 2006;141:965–975. doi: 10.1016/j.neuroscience.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Chen T, Hu Z, Quirion R, Hong Y. Modulation of NMDA receptors by intrathecal administration of the sensory neuron-specific receptor agonist BAM8-22. Neuropharmacology. 2008;54:796–803. doi: 10.1016/j.neuropharm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, Meyer RA, Raja SN. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008;138:318–329. doi: 10.1016/j.pain.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Liu Q, Tang Z, Raja SN, Anderson DJ, Dong X. Mas-related G-protein-coupled receptors inhibit pathological pain in mice. Proc Natl Acad Sci U S A. 2010a;107:15933–15938. doi: 10.1073/pnas.1011221107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Wacnik PW, Yang F, Carteret AF, Chung CY, Meyer RA, Raja SN. Spinal cord stimulation-induced analgesia: electrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology. 2010b;113:1392–1405. doi: 10.1097/ALN.0b013e3181fcd95c. [DOI] [PubMed] [Google Scholar]
- Guan Y, Yuan F, Carteret AF, Raja SN. A partial L5 spinal nerve ligation induces a limited prolongation of mechanical allodynia in rats: an efficient model for studying mechanisms of neuropathic pain. Neurosci Lett. 2010c;471:43–47. doi: 10.1016/j.neulet.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson EL, Maguire M, Campanella M, Tarozzo G, Jia Y, Dong XW, Laverty M, Murgolo N, Priestley T, Reggiani A, Monsma F, Beltramo M. Regulation of two rat mas-related genes in a model of neuropathic pain. Brain Res Mol Brain Res. 2005;142:58–64. doi: 10.1016/j.molbrainres.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Hager UA, Hein A, Lennerz JK, Zimmermann K, Neuhuber WL, Reeh PW. Morphological characterization of rat Mas-related G-protein-coupled receptor C and functional analysis of agonists. Neuroscience. 2008;151:242–254. doi: 10.1016/j.neuroscience.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Ackerman L, Holdsworth R, Elzey B. Effects of spinal nerve ligation on immunohistochemically identified neurons in the L4 and L5 dorsal root ganglia of the rat. J Comp Neurol. 2004;475:575–589. doi: 10.1002/cne.20209. [DOI] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, LaMotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SQ, Yang F, Perez FM, Xu Q, Shechter R, Cheong YK, Carteret AF, Dong X, Sweitzer SM, Raja SN, Guan Y. Tolerance develops to the antiallodynic effects of the peripherally acting opioid loperamide hydrochloride in nerve-injured rats. Pain. 2013;154:2477–2486. doi: 10.1016/j.pain.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Iwakura N, Orita S, Yamashita M, Inoue G, Yamauchi K, Eguchi Y, Ochiai N, Kishida S, Nakamura J, Takaso M, Ishikawa T, Arai G, Miyagi M, Kamoda H, Aoki Y, Hiwatari R, Kakizaki J, Kunishi T, Kono M, Suzuki T, Toyone T, Takahashi K, Kuniyoshi K, Ohtori S. Evaluation of behavior and neuropeptide markers of pain in a simple, sciatic nerve-pinch pain model in rats. Eur Spine J. 2010;19:1746–1752. doi: 10.1007/s00586-010-1428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Wang D, Zhou X, Huo Y, Chen T, Hu F, Quirion R, Hong Y. Effect of Mas-related gene (Mrg) receptors on hyperalgesia in rats with CFA-induced inflammation via direct and indirect mechanisms. Br J Pharmacol. 2013 doi: 10.1111/bph.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- Lee CY, Perez FM, Wang W, Guan X, Zhao X, Fisher JL, Guan Y, Sweitzer SM, Raja SN, Tao YX. Dynamic temporal and spatial regulation of mu opioid receptor expression in primary afferent neurons following spinal nerve injury. Eur J Pain. 2011;15:669–675. doi: 10.1016/j.ejpain.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Groblewski T, O'Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, Labarre M, Gosselin M, Fortin Y, Banville D, Shen SH, Strom P, Payza K, Dray A, Walker P, Ahmad S. Proenkephalin A gene products activate a new family of sensory neuron--specific GPCRs. Nat Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzan-Luques A, Minert A, Devor M, Tal M. Dynamic genotype-selective "phenotypic switching" of CGRP expression contributes to differential neuropathic pain phenotype. Exp Neurol. 2013;250C:194–204. doi: 10.1016/j.expneurol.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schuttler J, Ji G, Neugebauer V, Marsicano G, Lutz B, Vanegas H, Zeilhofer HU. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science. 2009;325:760–764. doi: 10.1126/science.1171870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Yamada H, Moriyama T, Honda K, Takano Y, Kamiya HO, Katsuragi T. Measurement of dorsal root ganglion P2X mRNA by SYBR Green fluorescence. Brain Res Brain Res Protoc. 2002;10:95–101. doi: 10.1016/s1385-299x(02)00187-3. [DOI] [PubMed] [Google Scholar]
- Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Huang H, Hong Y. Effects of intrathecal BAM22 on noxious stimulus-evoked c-fos expression in the rat spinal dorsal horn. Brain Res. 2004;1028:170–179. doi: 10.1016/j.brainres.2004.09.020. [DOI] [PubMed] [Google Scholar]