Abstract
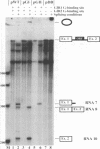
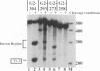
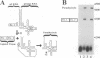
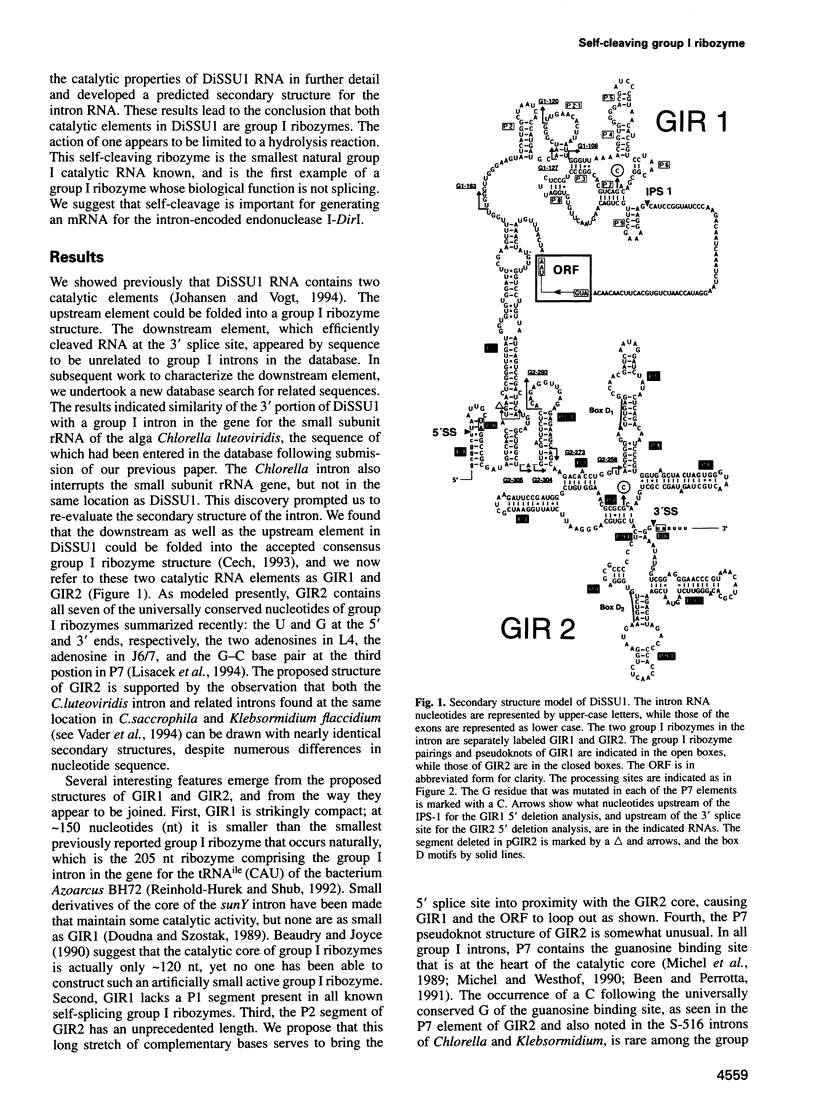
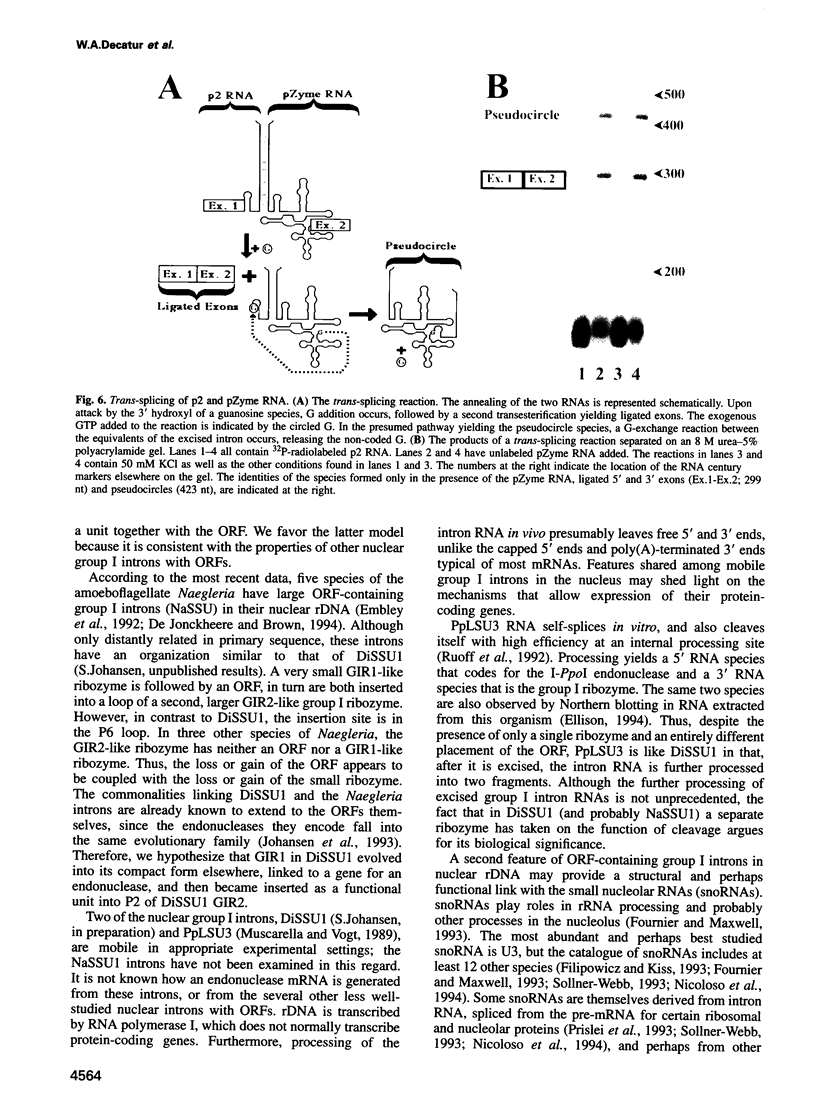
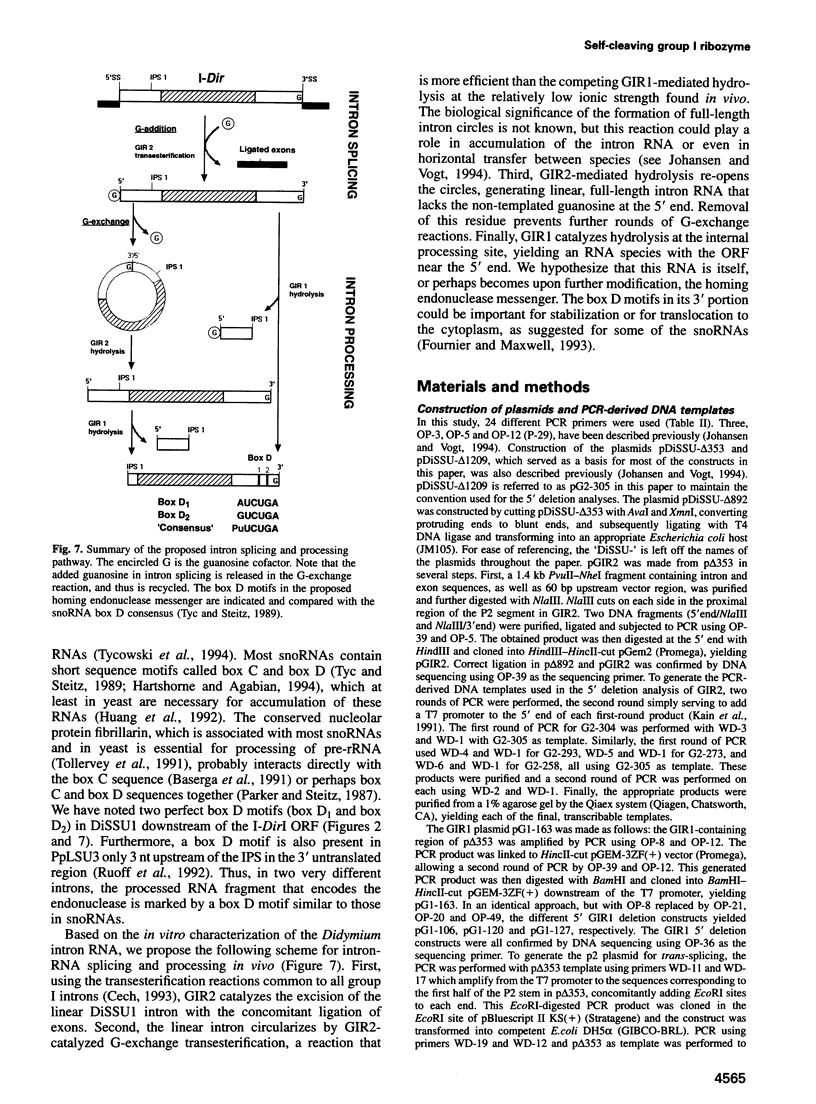
DiSSU1, a mobile intron in the nuclear rRNA gene of Didymium iridis, was previously reported to contain two independent catalytic RNA elements. We have found that both catalytic elements, renamed GIR1 and GIR2, are group I ribozymes, but with differing functionality. GIR2 carries out the several reactions associated with self-splicing. GIR1 carries out a hydrolysis reaction at an internal processing site (IPS-1). These conclusions are based on the catalytic properties of RNAs transcribed in vitro. Mutation of the P7 pairing segment of GIR2 abrogated self-splicing, while mutation of P7 in GIR1 abrogated hydrolysis at the IPS-1. Much of the P2 stem and all of the associated loop could be deleted without effect on self-splicing. These results are accounted for by a secondary structure model, in which a long P2 pairing segment brings the 5' splice site to the GIR2 catalytic core. GIR1 is the smallest natural group I ribozyme yet reported and is the first example of a group I ribozyme whose presumptive biological function is hydrolysis. We hypothesize that GIR1-mediated cleavage of the excised intron RNA functions in the generation and expression of the mRNA for the intron-encoded endonuclease I-DirI.
Full text
PDF
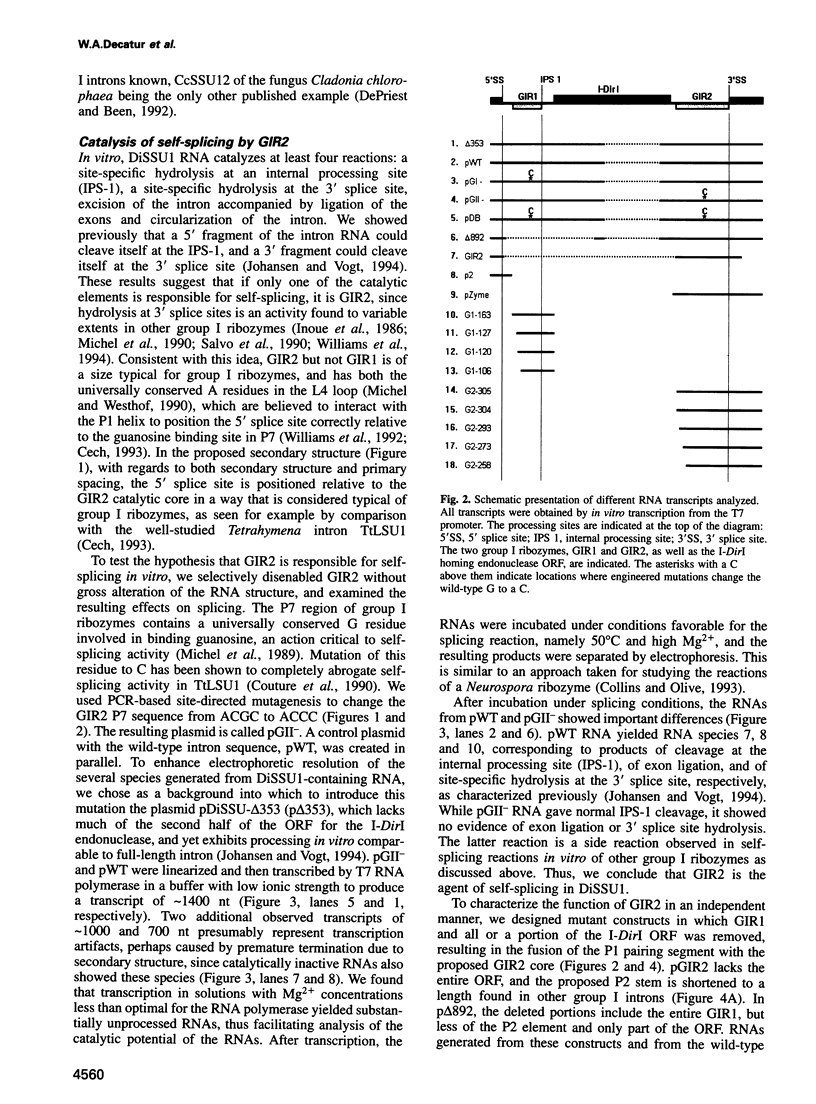
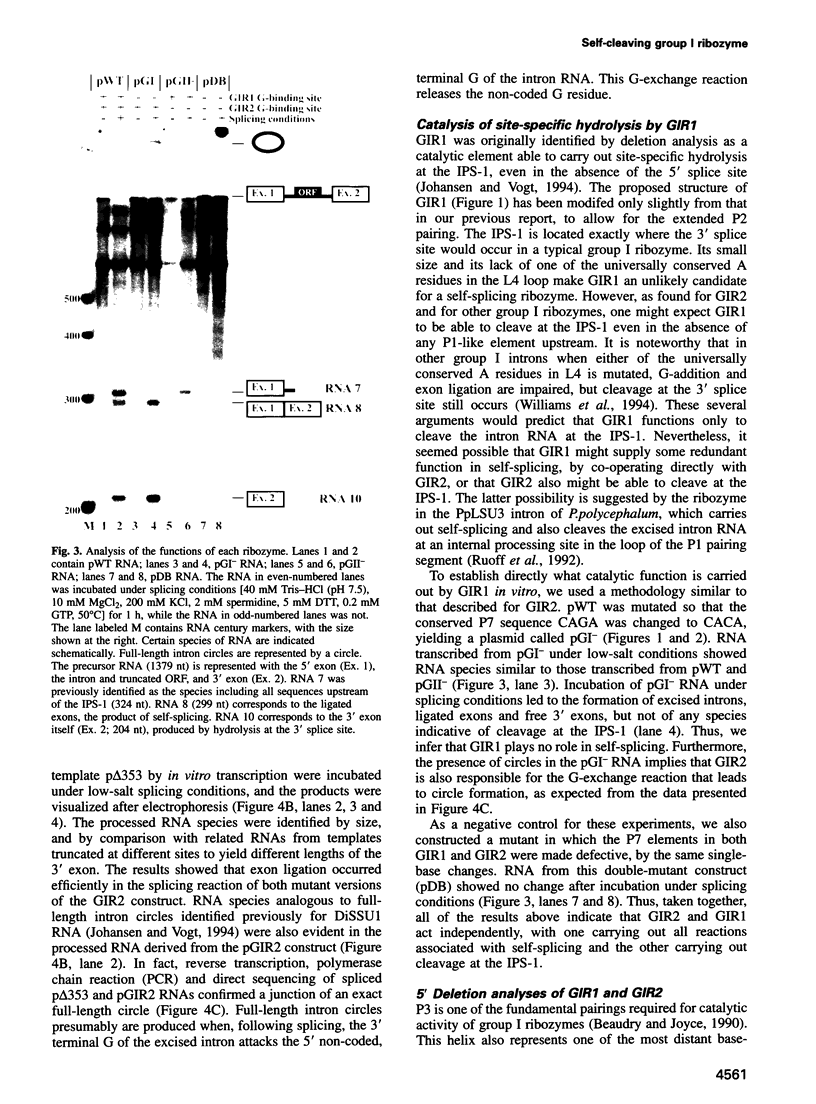
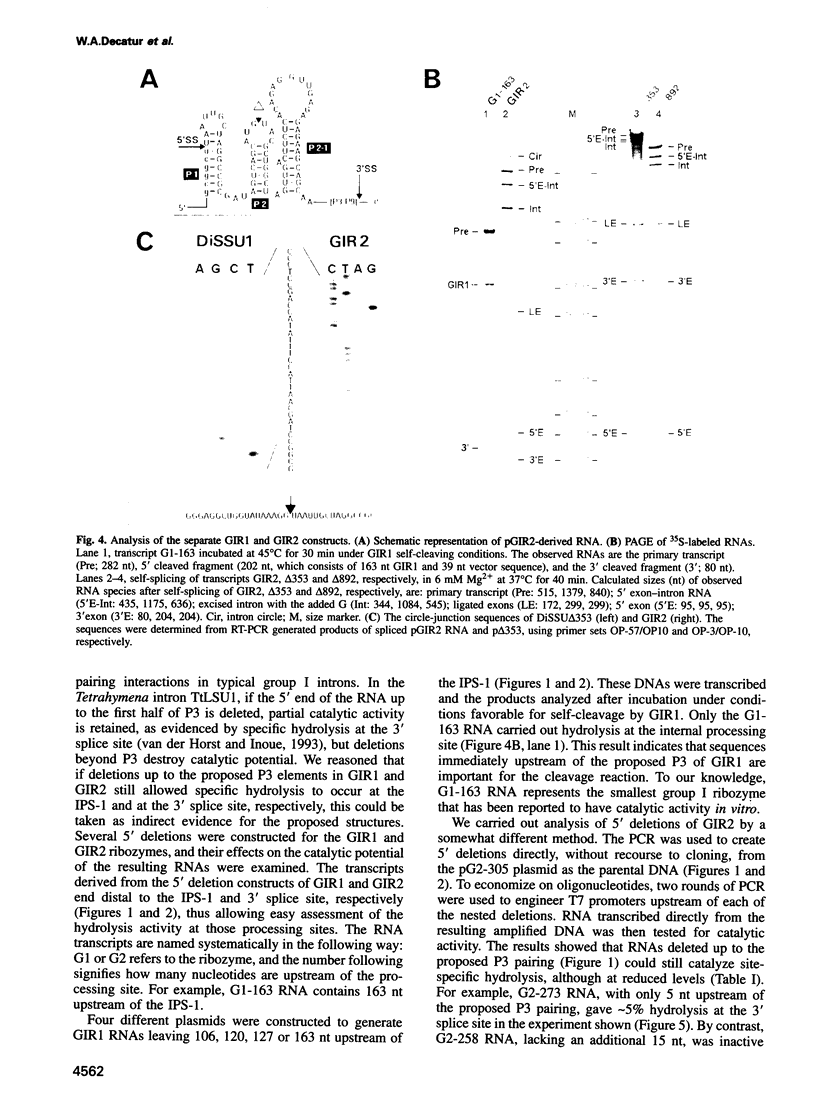
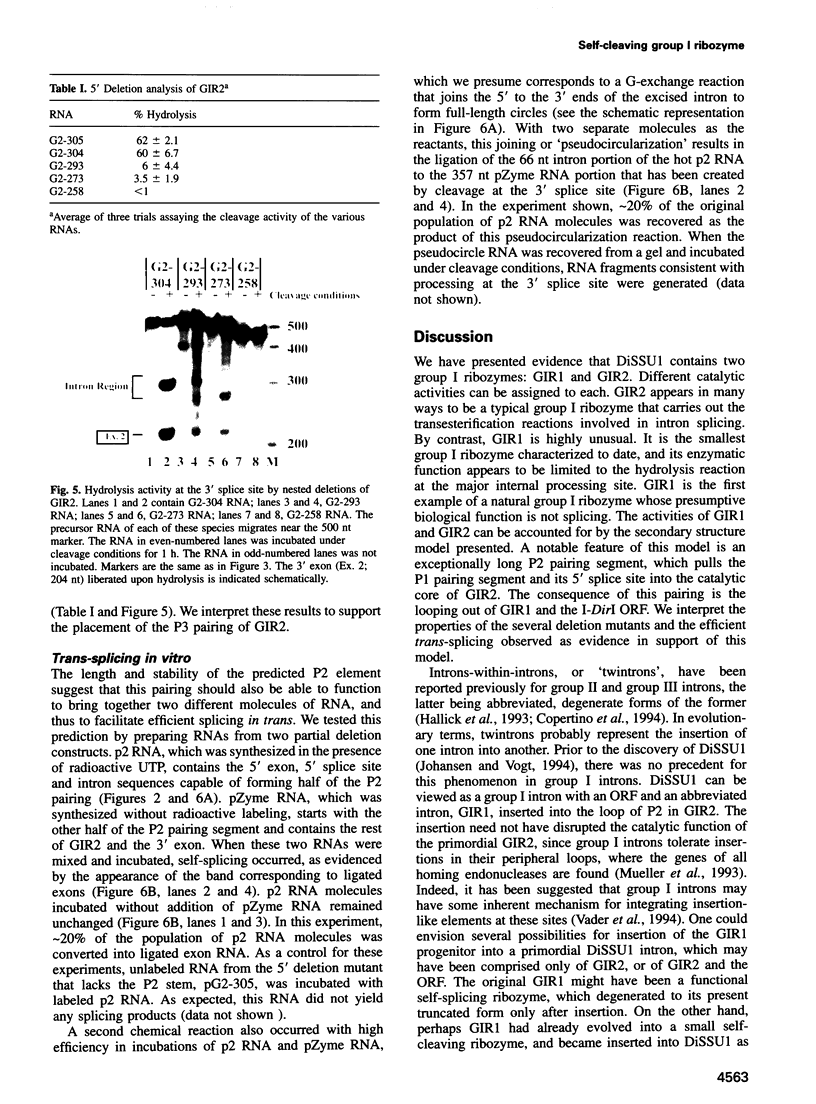



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baserga S. J., Yang X. D., Steitz J. A. An intact Box C sequence in the U3 snRNA is required for binding of fibrillarin, the protein common to the major family of nucleolar snRNPs. EMBO J. 1991 Sep;10(9):2645–2651. doi: 10.1002/j.1460-2075.1991.tb07807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudry A. A., Joyce G. F. Minimum secondary structure requirements for catalytic activity of a self-splicing group I intron. Biochemistry. 1990 Jul 10;29(27):6534–6539. doi: 10.1021/bi00479a027. [DOI] [PubMed] [Google Scholar]
- Been M. D., Perrotta A. T. Group I intron self-splicing with adenosine: evidence for a single nucleoside-binding site. Science. 1991 Apr 19;252(5004):434–437. doi: 10.1126/science.2017681. [DOI] [PubMed] [Google Scholar]
- Bhattacharya D., Surek B., Rüsing M., Damberger S., Melkonian M. Group I introns are inherited through common ancestry in the nuclear-encoded rRNA of Zygnematales (Charophyceae). Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9916–9920. doi: 10.1073/pnas.91.21.9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R. A., Olive J. E. Reaction conditions and kinetics of self-cleavage of a ribozyme derived from Neurospora VS RNA. Biochemistry. 1993 Mar 23;32(11):2795–2799. doi: 10.1021/bi00062a009. [DOI] [PubMed] [Google Scholar]
- Copertino D. W., Hall E. T., Van Hook F. W., Jenkins K. P., Hallick R. B. A group III twintron encoding a maturase-like gene excises through lariat intermediates. Nucleic Acids Res. 1994 Mar 25;22(6):1029–1036. doi: 10.1093/nar/22.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture S., Ellington A. D., Gerber A. S., Cherry J. M., Doudna J. A., Green R., Hanna M., Pace U., Rajagopal J., Szostak J. W. Mutational analysis of conserved nucleotides in a self-splicing group I intron. J Mol Biol. 1990 Oct 5;215(3):345–358. doi: 10.1016/s0022-2836(05)80356-0. [DOI] [PubMed] [Google Scholar]
- De Jonckheere J. F., Brown S. Loss of the ORF in the SSUrDNA group I intron of one Naegleria lineage. Nucleic Acids Res. 1994 Sep 25;22(19):3925–3927. doi: 10.1093/nar/22.19.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePriest P. T., Been M. D. Numerous group I introns with variable distributions in the ribosomal DNA of a lichen fungus. J Mol Biol. 1992 Nov 20;228(2):315–321. doi: 10.1016/0022-2836(92)90819-6. [DOI] [PubMed] [Google Scholar]
- Embley T. M., Dyal P., Kilvington S. A group I intron in the small subunit ribosomal RNA gene from Naegleria andersoni ssp. andersoni strain PPMFB-6. Nucleic Acids Res. 1992 Dec 11;20(23):6411–6411. doi: 10.1093/nar/20.23.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W., Kiss T. Structure and function of nucleolar snRNPs. Mol Biol Rep. 1993 Aug;18(2):149–156. doi: 10.1007/BF00986770. [DOI] [PubMed] [Google Scholar]
- Fournier M. J., Maxwell E. S. The nucleolar snRNAs: catching up with the spliceosomal snRNAs. Trends Biochem Sci. 1993 Apr;18(4):131–135. doi: 10.1016/0968-0004(93)90020-n. [DOI] [PubMed] [Google Scholar]
- Galloway Salvo J. L., Coetzee T., Belfort M. Deletion-tolerance and trans-splicing of the bacteriophage T4 td intron. Analysis of the P6-L6a region. J Mol Biol. 1990 Feb 5;211(3):537–549. doi: 10.1016/0022-2836(90)90264-m. [DOI] [PubMed] [Google Scholar]
- Guo Q., Lambowitz A. M. A tyrosyl-tRNA synthetase binds specifically to the group I intron catalytic core. Genes Dev. 1992 Aug;6(8):1357–1372. doi: 10.1101/gad.6.8.1357. [DOI] [PubMed] [Google Scholar]
- Hallick R. B., Hong L., Drager R. G., Favreau M. R., Monfort A., Orsat B., Spielmann A., Stutz E. Complete sequence of Euglena gracilis chloroplast DNA. Nucleic Acids Res. 1993 Jul 25;21(15):3537–3544. doi: 10.1093/nar/21.15.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne T., Agabian N. A common core structure for U3 small nucleolar RNAs. Nucleic Acids Res. 1994 Aug 25;22(16):3354–3364. doi: 10.1093/nar/22.16.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G. M., Jarmolowski A., Struck J. C., Fournier M. J. Accumulation of U14 small nuclear RNA in Saccharomyces cerevisiae requires box C, box D, and a 5', 3' terminal stem. Mol Cell Biol. 1992 Oct;12(10):4456–4463. doi: 10.1128/mcb.12.10.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Sullivan F. X., Cech T. R. New reactions of the ribosomal RNA precursor of Tetrahymena and the mechanism of self-splicing. J Mol Biol. 1986 May 5;189(1):143–165. doi: 10.1016/0022-2836(86)90387-6. [DOI] [PubMed] [Google Scholar]
- Johansen S., Embley T. M., Willassen N. P. A family of nuclear homing endonucleases. Nucleic Acids Res. 1993 Sep 11;21(18):4405–4405. doi: 10.1093/nar/21.18.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen S., Vogt V. M. An intron in the nuclear ribosomal DNA of Didymium iridis codes for a group I ribozyme and a novel ribozyme that cooperate in self-splicing. Cell. 1994 Feb 25;76(4):725–734. doi: 10.1016/0092-8674(94)90511-8. [DOI] [PubMed] [Google Scholar]
- Kain K. C., Orlandi P. A., Lanar D. E. Universal promoter for gene expression without cloning: expression-PCR. Biotechniques. 1991 Mar;10(3):366–374. [PubMed] [Google Scholar]
- Lambowitz A. M., Belfort M. Introns as mobile genetic elements. Annu Rev Biochem. 1993;62:587–622. doi: 10.1146/annurev.bi.62.070193.003103. [DOI] [PubMed] [Google Scholar]
- Lisacek F., Diaz Y., Michel F. Automatic identification of group I intron cores in genomic DNA sequences. J Mol Biol. 1994 Jan 28;235(4):1206–1217. doi: 10.1006/jmbi.1994.1074. [DOI] [PubMed] [Google Scholar]
- Michel F., Hanna M., Green R., Bartel D. P., Szostak J. W. The guanosine binding site of the Tetrahymena ribozyme. Nature. 1989 Nov 23;342(6248):391–395. doi: 10.1038/342391a0. [DOI] [PubMed] [Google Scholar]
- Michel F., Netter P., Xu M. Q., Shub D. A. Mechanism of 3' splice site selection by the catalytic core of the sunY intron of bacteriophage T4: the role of a novel base-pairing interaction in group I introns. Genes Dev. 1990 May;4(5):777–788. doi: 10.1101/gad.4.5.777. [DOI] [PubMed] [Google Scholar]
- Michel F., Westhof E. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol. 1990 Dec 5;216(3):585–610. doi: 10.1016/0022-2836(90)90386-Z. [DOI] [PubMed] [Google Scholar]
- Muscarella D. E., Ellison E. L., Ruoff B. M., Vogt V. M. Characterization of I-Ppo, an intron-encoded endonuclease that mediates homing of a group I intron in the ribosomal DNA of Physarum polycephalum. Mol Cell Biol. 1990 Jul;10(7):3386–3396. doi: 10.1128/mcb.10.7.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscarella D. E., Vogt V. M. A mobile group I intron in the nuclear rDNA of Physarum polycephalum. Cell. 1989 Feb 10;56(3):443–454. doi: 10.1016/0092-8674(89)90247-x. [DOI] [PubMed] [Google Scholar]
- Nicoloso M., Caizergues-Ferrer M., Michot B., Azum M. C., Bachellerie J. P. U20, a novel small nucleolar RNA, is encoded in an intron of the nucleolin gene in mammals. Mol Cell Biol. 1994 Sep;14(9):5766–5776. doi: 10.1128/mcb.14.9.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. A., Steitz J. A. Structural analysis of the human U3 ribonucleoprotein particle reveal a conserved sequence available for base pairing with pre-rRNA. Mol Cell Biol. 1987 Aug;7(8):2899–2913. doi: 10.1128/mcb.7.8.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prislei S., Michienzi A., Presutti C., Fragapane P., Bozzoni I. Two different snoRNAs are encoded in introns of amphibian and human L1 ribosomal protein genes. Nucleic Acids Res. 1993 Dec 25;21(25):5824–5830. doi: 10.1093/nar/21.25.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold-Hurek B., Shub D. A. Self-splicing introns in tRNA genes of widely divergent bacteria. Nature. 1992 May 14;357(6374):173–176. doi: 10.1038/357173a0. [DOI] [PubMed] [Google Scholar]
- Rocheleau G. A., Woodson S. A. Requirements for self-splicing of a group I intron from Physarum polycephalum. Nucleic Acids Res. 1994 Oct 11;22(20):4315–4320. doi: 10.1093/nar/22.20.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoff B., Johansen S., Vogt V. M. Characterization of the self-splicing products of a mobile intron from the nuclear rDNA of Physarum polycephalum. Nucleic Acids Res. 1992 Nov 25;20(22):5899–5906. doi: 10.1093/nar/20.22.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha R., Mohr G., Belfort M., Lambowitz A. M. Group I and group II introns. FASEB J. 1993 Jan;7(1):15–24. doi: 10.1096/fasebj.7.1.8422962. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B. Novel intron-encoded small nucleolar RNAs. Cell. 1993 Nov 5;75(3):403–405. doi: 10.1016/0092-8674(93)90374-y. [DOI] [PubMed] [Google Scholar]
- Tollervey D., Lehtonen H., Carmo-Fonseca M., Hurt E. C. The small nucleolar RNP protein NOP1 (fibrillarin) is required for pre-rRNA processing in yeast. EMBO J. 1991 Mar;10(3):573–583. doi: 10.1002/j.1460-2075.1991.tb07984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyc K., Steitz J. A. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 1989 Oct;8(10):3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycowski K. T., Shu M. D., Steitz J. A. Requirement for intron-encoded U22 small nucleolar RNA in 18S ribosomal RNA maturation. Science. 1994 Dec 2;266(5190):1558–1561. doi: 10.1126/science.7985025. [DOI] [PubMed] [Google Scholar]
- Vader A., Naess J., Haugli K., Haugli F., Johansen S. Nucleolar introns from Physarum flavicomum contain insertion elements that may explain how mobile group I introns gained their open reading frames. Nucleic Acids Res. 1994 Nov 11;22(22):4553–4559. doi: 10.1093/nar/22.22.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. P., Fujimoto D. N., Inoue T. A region of group I introns that contains universally conserved residues but is not essential for self-splicing. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10400–10404. doi: 10.1073/pnas.89.21.10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. P., Fujimoto D. N., Inoue T. Two universally conserved adenosines of the group I intron that are important for self-splicing but not for core catalytic activity. J Biochem. 1994 Jan;115(1):126–130. doi: 10.1093/oxfordjournals.jbchem.a124286. [DOI] [PubMed] [Google Scholar]
- Winter A. J., Groot Koerkamp M. J., Tabak H. F. The mechanism of group I self-splicing: an internal guide sequence can be provided in trans. EMBO J. 1990 Jun;9(6):1923–1928. doi: 10.1002/j.1460-2075.1990.tb08319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Tamura K., Aimi T., Songsri P. Self-splicing group I introns in eukaryotic viruses. Nucleic Acids Res. 1994 Jul 11;22(13):2532–2537. doi: 10.1093/nar/22.13.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaug A. J., Grabowski P. J., Cech T. R. Autocatalytic cyclization of an excised intervening sequence RNA is a cleavage-ligation reaction. Nature. 1983 Feb 17;301(5901):578–583. doi: 10.1038/301578a0. [DOI] [PubMed] [Google Scholar]
- van der Horst G., Inoue T. Requirements of a group I intron for reactions at the 3' splice site. J Mol Biol. 1993 Feb 5;229(3):685–694. doi: 10.1006/jmbi.1993.1072. [DOI] [PubMed] [Google Scholar]