Abstract
The purpose of this study was to assess the concentration of C-reactive protein (CRP) in obese type 2 diabetes mellitus (DM2) patients and its association with macrovascular and microvascular complications. The study group consisted of 80 obese DM2 patients, including 20 macrovascular, 20 microvascular, 20 both macrovascular and microvascular, and 20 with no complications patients. The control group comprised 40 normoglycemic subjects—20 obese and 20 of normal body weight. Highly sensitive CRP and metabolic control parameters were assessed. CRP levels in obese diabetes subgroups and normoglycemic obese were similar and significantly higher than those in nonobese controls. No correlation was found between CRP and diabetes control parameters. There was a strong positive correlation between CRP level and body mass index in all groups. A multivariate analysis showed that DM2 and obesity are independent factors increasing CRP levels. Increased concentration of CRP in obese DM2 patients is related to obesity and diabetes itself. The lack of association between CRP and vascular complications remains unclear.
KEY WORDS: CRP, type 2 diabetes, obesity, microvascular and macrovascular complications
INTRODUCTION
Obesity and type 2 diabetes mellitus (DM2) are considered to be associated with a low-grade inflammation, which may play a significant role in the development of cardiovascular complications [1, 2]. C-reactive protein (CRP) is an extremely sensitive marker of systemic inflammation produced mainly by the liver under the stimulation of adipocyte-derived proinflammatory cytokines [3]. CRP has also emerged as a powerful predictor of cardiovascular diseases [4]. Elevated levels of CRP are described in DM2 subjects; however, it is not clear if they are related to the presence of obesity, diabetes, or both [5–9]. Some studies indicate a relationship between CRP and macrovascular complications of diabetes [10–14]. It has not yet been shown whether the CRP level increases due to the metabolic effects of obesity and diabetes and plays a direct role in promoting the inflammatory component of atherosclerosis or whether it is merely a marker of the ongoing inflammation in the vessels affected by diabetes [15, 16]. Data on elevated CRP levels in diabetic patients with nephropathy or retinopathy providing a link between inflammation and the development of microvascular complications are very scarce [17–21]. None of the performed studies conducted in DM2 patients examined CRP levels in connection with both macro- and microangiopathy. The purpose of this study was to evaluate the concentration of CRP in obese patients with long-standing DM2 (>10 years) and its association with the presence of diabetes macrovascular and microvascular complications.
MATERIAL AND METHODS
Material
The study group consisted of 80 obese individuals (46 men and 34 women) aged 43–82 years (65.1 ± 8.6 years) with DM2 of 10–35 years of duration (16.4 ± 5.6 years), with a body mass index (BMI) of 30.0–49.7 kg/m2 (34.0 ± 3.7 kg/m2), and treated with diet and/or oral hypoglycemic drugs and/or insulin. The study group was constituted in such a way as to include 20 subjects with macrovascular complications (MA), 20 subjects with microvascular complications (MI), 20 subjects with macrovascular and microvascular complications (MA + MI), and 20 patients without chronic organ complications of diabetes (COMP (−)). The MA group consisted of 20 patients with ischemic heart disease; in 4 of them, chronic limb ischemia was found. In the MI group, retinopathy was present in 11 subjects (9 with simplex retinopathy and 2 with preproliferative retinopathy associated with maculopathy), nephropathy in 12 (11 defined by increased albuminuria and 1 by proteinuria), and neuropathy in 7. The MA + MI group included 20 patients diagnosed ischemic heart disease with chronic limb ischemia in 6 and history of stroke in 2 of them; coexisting microangiopathy in this group was as follows: 11 subjects—presence of simplex retinopathy (2 cases associated with maculopathy), 12—presence of nephropathy (10 defined by increased albuminuria and 2 by overt proteinuria), and 11—neuropathy.
The control group DM (−) comprised 40 persons, including 20 obese subjects (15 women and 5 men) aged 40–76 years (57.0 ± 9.5 years), with BMI of 30.3–44.8 kg/m2 (37.0 ± 4.3 kg/m2), and 20 subjects with normal body weight (lean) (16 women and 4 men) aged 40–67 years (54.3 ± 6.5 years), with BMI of 19.3–24.8 kg/m2 (22.6 ± 1.4 kg/m2). In both groups, carbohydrate metabolism disorders were excluded by means of an oral glucose tolerance test (75 g). Persons with a diagnosis of ischemic heart disease, atherosclerosis, and occlusive arterial disease, those after stroke, and those with suspected ischemic heart disease on the basis of their medical history and/or standard ECG were not included in the control group. The study was performed after approval by the Ethics Committee of the Pomeranian Medical University in Szczecin, Poland.
Methods
After giving informed written consent to participate in the study, each subject was inquired about age, diabetes duration, coexisting diseases (hypertension, ischemic heart disease, coronary artery disease, lipid disorders, stroke), and therapy, including the use of drugs modifying the CRP blood concentration (thiazolidinediones, statins, fibrates, hormone replacement therapy (HRT)) and stimulants (cigarettes, alcohol). All subjects underwent physical examination and measurement of BMI and waist/hip ratio (WHR).
The diagnosis of ischemic heart disease was based on a medical history of stenocardial pain, myocardial infarction, previous tests used in diagnosing ischemic heart disease, and ischemic changes detected in the standard electrocardiogram. If necessary, additional tests, such as treadmill stress testing, cardiac ultrasound, and angiography, were performed. The presence of atherosclerosis of the lower extremities was assessed on the basis of a history of claudication and the measurement of pulse in the lower limb arteries. Cerebral atherosclerosis was diagnosed on the basis of a history of dizziness and previous stroke and by carotid auscultation. Additional tests, such as carotid Doppler ultrasound, Doppler ultrasound of the lower limb arteries, and angiography, were performed if necessary.
Diabetic retinopathy was diagnosed by fundoscopic examination by an ophthalmologist. The presence of diabetic nephropathy was evaluated on the basis of proteinuria present in urinalysis or diurnal urinary albumin excretion rate (UAER) ≥30 mg/24 h (measured twice). The diagnosis of diabetic neuropathy was based on detailed clinical assessment including diabetic neuropathy symptoms (typical neuropathic pain, orthostatic hypotension), loss of vibration perception with a 128-Hz tuning fork (scale value <6/8), or loss of sensation found with a 10-g Semmes–Weinstein monofilament examination. Both mentioned tests were performed in all patients. Subjects were not included in the study if there was an increased serum creatinine level and a suspicion of inflammation, cancer, or other diseases likely to affect the CRP level on the basis of their medical history, physical examination, and additional laboratory tests, such as blood count, erythrocyte sedimentation rate (ESR), or urinalysis. The levels of fasting plasma glucose (FPG); total, LDL, and HDL cholesterol; triglycerides; CRP; and glycated hemoglobin A1c (HbA1c) were measured, and 24-h blood pressure monitoring (SpaceLabs 9027) was conducted in all subjects.
Laboratory Tests
Glucose concentration was determined by an enzymatic method (Cormay kit with hexokinase, PZ Cormay SA, Poland). Cholesterol and triglycerides were determined by an enzymatic colorimetric method (COBAS INTEGRA kit, Roche, Switzerland). The concentration of CRP was evaluated by immunonephelometry (CardioPhase* hsCRP set, Dade Behring, Germany). HbA1c was determined by high-pressure liquid chromatography (HPLC; Variant™ HemoglobinA1c, BIO-RAD, USA). Creatinine concentration was determined with the Jaffe method (COBAS INTEGRA kit, Roche, Switzerland). Urinalysis was performed using test strips (Lab U11 Strip Plus Analyticon, Germany). Albuminuria was determined by the immunoradiometric assay (Albumin RIA kit, Immunotech, Czech Republic). Leukocytosis was evaluated using a Pentra DX 120 analyzer (Horiba ABX Diagnostics, Japan). ESR was determined by sedimentation (Westergren method).
Statistical Analysis
The relationships between nominal variables (e.g., gender) were analyzed using the chi-square test or with Fisher's exact test for dichotomous variables. All measurable variables (e.g., CRP) were assessed for normal distribution with the Shapiro–Wilk test. In case of normal distribution, the means of the measurable variables were compared across groups with the use of Student's t test and in case of deviation from the normal distribution, with the use of nonparametric tests. In the latter case, in order to compare the values of variables between the two groups, the Mann–Whitney U test was used, and in case of a larger number of compared groups, the ANOVA Kruskal–Wallis test was used. The relationship between two measurable variables was analyzed with Spearman's rank correlation coefficient (Rs). Variables with normal distribution were described as mean ± standard deviation (SD). Variables with distributions significantly deviating from normal (where p for the Shapiro–Wilk test was below 0.05 and the SD was higher than the average) were described as median (interquartile range). Multivariate analysis was based on the linear regression model. The threshold p value for statistical significance was set to p < 0.05. The analysis was performed using Statistica 6.1 Pl.
RESULTS
Group Comparison
The characteristics of patients in the studied subgroups are summarized in Table 1. The 24-h blood pressure, heart rate, and laboratory test results are shown in Table 2.
Table 1.
Characteristics of Patients in Studied Subgroups
| Parameter | DM2 | DM (−) | ||||
|---|---|---|---|---|---|---|
| MA n = 20 | MI n = 20 | MA + MI n = 20 | COMP (−) n = 20 | OBESE n = 20 | LEAN n = 20 | |
| Age (years) | 67.0 ± 7.5 | 60.7 ± 10.3 ~ - | 67.6 ± 6.7 | 63.1 ± 7.3 ~ - | 57.0 ± 9.5 ~~ -- * | 54.3 ± 6.5 ~~ -- ** |
| Duration of DM (years) | 16.9 ± 7.0 | 15.7 ± 4.6 | 19.0 ± 5.5* | 14.2 ± 3.9 | – | – |
| Sex (F/M) | 14/6 | 11/9 | 9/11 | 12/8 | 15/5 | 16/4 |
| BMI (kg/m2) | 33.1 ± 2.8 | 34.7 ± 5.2 | 34.0 ± 2.7 | 34.2 ± 3.6 | 37.0 ± 4.3 ~~ - | 22.6 ± 1.4 ~~ ++ -- ** ^^ |
| WHR | 0.92 ± 0.07 | 0.94 ± 0.07 | 0.95 ± 0.06 | 0.92 ± 0.06 | 0.90 ± 0.07 - | 0.82 ± 0.07 ~~ ++ -- ** ^^ |
| Hypertension (n) | 20 | 18 | 20 | 15 ~ - | 12 ~ - | 2 ~~ ++ -- ** ^ |
| Smoking (n) | 0 | 5 | 1 | 1 | 2 | 6 |
| Alcohol abuse (n) | 0 | 0 | 0 | 0 | 0 | 0 |
| HRT (n) | 3 | 0 | 0 | 1 | 0 | 0 |
| Statins (n) | 11 | 5 | 12 | 3 ~ - | 1 ~ - | 0 ~ + - |
| Fibrates (n) | 2 | 1 | 0 | 0 | 0 | 0 |
| PPAR-γ agonists (n) | 0 | 0 | 0 | 0 | 0 | 0 |
~p < 0.05, ~~p < 0.001 vs. MA; +p < 0.05, ++p < 0.001 vs. MI; -p < 0.05, --p < 0.001 vs. MA + MI; *p < 0.05, **p < 0.001 vs. COMP (−); ^p < 0.05, ^^p < 0.001 vs. OBESE
Table 2.
The 24-h Blood Pressure, Heart Rate Values, and Laboratory Test Results in Studied Subgroups
| Parameter | DM2 | DM (−) | ||||
|---|---|---|---|---|---|---|
| MA n = 20 | MI n = 20 | MA + MI n = 20 | COMP (−) n = 20 | OBESE n = 20 | LEAN n = 20 | |
| 24-h sBP (mm Hg) | 126.2 ± 6.7 | 127.8 ± 121 | 136.9 ± 9.8 ~~ + | 130.3 ± 10.0 | 125.7 ± 87 -- | 115.9 ± 8.6 ~~ ++ -- ** ^^ |
| 24-h dBP (mm Hg) | 66.6 ± 4.0 + -- * | 71.4 ± 6.2 | 71.0 ± 5.9 | 70.7 ± 4.7 | 75.6 ± 8.4 ~~ - * | 70.9 ± 7.4 ~ ^ |
| 24-h HR (t/min) | 69.5 ± 8.1 | 74.4 ± 8.8 | 73.6 ± 12.0 | 74.6 ± 7.3 | 71.1 ± 5.7 | 72.0 ± 6.9 |
| Fasting glucose (mg/dL) | 153.6 ± 37.6 | 147.1 ± 42.9 | 168.2 ± 42.4 | 149.1 ± 32.9 | 98.6 ± 6.5 ~~ ++ -- ** | 95.4 ± 6.7 ~~ ++ -- ** |
| HbA1c (%) | 6.8 ± 0.7 | 7.3 ± 1.2 | 7.8 ± 1.1 ~~ * | 6.9 ± 0.8 | 5.6 ± 0.4 ~~ ++ -- ** | 5.8 ± 0.3 ~~ ++ -- ** |
| Total cholesterol (mg/dL) | 171.9 ± 41.9 + | 200.7 ± 44.5 | 200.8 ± 57.4 | 199.8 ± 44.1 | 223.4 ± 48.5 ~~ | 209.8 ± 25.3 ~ |
| LDL cholesterol (mg/dL) | 96.7 ± 32.4 | 113.5 ± 38.7 | 114.8 ± 52.3 | 116.4 ± 40.4 | 133.8 ± 44.9 ~ | 123.2 ± 29.7 ~ |
| HDL cholesterol (mg/dL) | 49.7 ± 8.8 | 58.5 ± 19.2 | 56.2 ± 16.0 | 55.6 ± 16.3 | 61.7 ± 16.6 ~ | 70.4 ± 15.7 ~~ - * |
| Triglycerides (mg/dL) | 128.4 ± 57.7 | 142.5 ± 92.6 | 152.6 ± 70.6 | 139.2 ± 115 | 140.0 ± 52.8 | 76.5 ± 20.2 ~ + -- * ^^ |
| Creatinine (mg/dL) | 0.83 ± 0.23 | 0.71 ± 0.15 | 0.84 ± 0.24 | 0.73 ± 0.14 | 0.67 ± 0.14 ~~ -- | 0.71 ± 0.11 |
| UAER (mg/24 h) | 16.6 (9.2–25.8) + - | 36.1 (8.8–430.5) | 37.1 (10.8–1,670) | 12.7 (8.3–26.3) ++ -- | 12.6 (7.8–23.8) ~ ++ -- | 12.5 (3.2–84) + -- |
| ESR (mm/h) | 18.3 ± 10.3 | 16.8 ± 9.3 | 19.2 ± 8.8 | 13.7 ± 7.2 | 15.4 ± 10.5 | 10.2 ± 4.3 ~ + -- |
| Leukocytes (G/L) | 7.2 ± 1.9 | 7.1 ± 2.0 | 7.8 ± 1.8 | 7.0 ± 1.1 | 6.0 ± 1.3 ~ - * | 6.2 ± 1.4 ~ - |
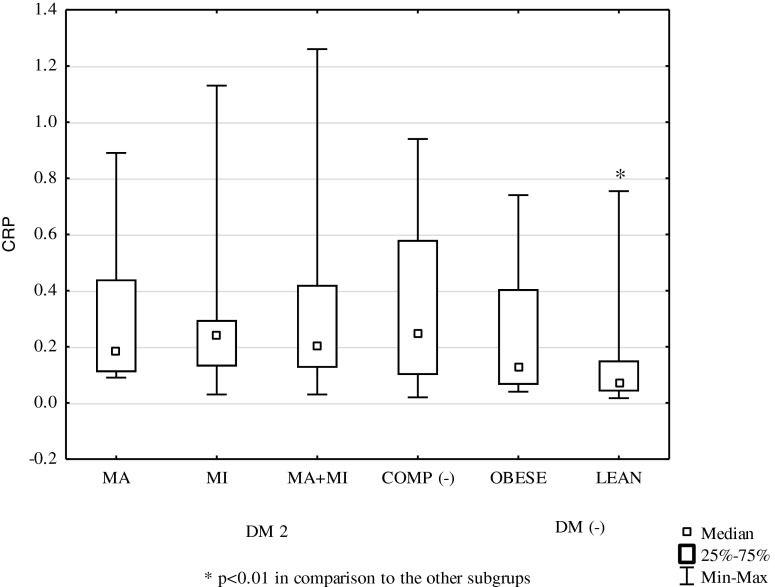
| CRP (mg/dL) | 0.19 (0.09–0.89) | 0.24 (0.03–1.13) | 0.21 (0.03–1.26) | 0.25 (0.02–0.94) | 0.13 (0.04–0.74) | 0.07 (0.02–0.75) ~ - + * ^ |
UAER and CRP are presented as median
~p < 0.05, ~~p < 0.001 vs. MA; +p < 0.05, ++p < 0.001 vs. MI; -p < 0.05, --p < 0.001 vs. MA + MI; *p < 0.05, **p < 0.001 vs. COMP (−); ^p < 0.05, ^^p < 0.001 vs. OBESE
CRP Concentrations in the Studied Subgroups
CRP concentration in the group of obese DM2 patients was 0.22 mg/dL (0.02–1.26) and did not differ significantly from that in the group of obese subjects without diabetes: 0.13 mg/dL (0.04–0.74). There were no differences between CRP concentrations in different subgroups of the DM2 group (Fig. 1). In the subgroup with macrovascular complications, they measured 0.19 mg/dL (0.09–0.89), in the subgroup with microvascular complications 0.24 mg/dL (0.03–1.13), in the subgroup with both macrovascular and microvascular complications 0.21 mg/dL (0.03–1.26), and in the subgroup without chronic vascular complications 0.25 mg/dL (0.02–0.94). The above CRP concentrations were not different from those observed in the obese controls, while they were significantly higher when compared to those in the normal body weight subjects: 0.07 mg/dL (0.02–0.75, p < 0.01).
Fig. 1.
Median concentrations of CRP in studied subgroups.
The Relationship Between Serum CRP Concentration and the Studied Parameters
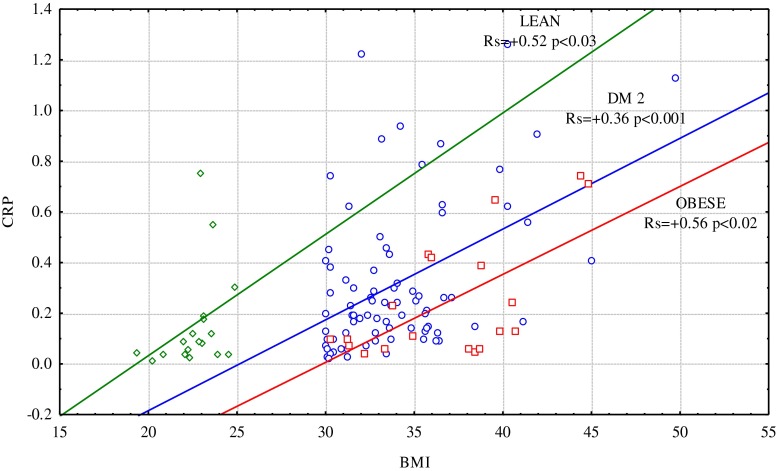
There was a positive correlation between CRP concentration and BMI in the obese subjects with DM2 (Rs = 0.36, p = 0.001), obese without diabetes (Rs = 0.56, p = 0.012), and those with normal body weight without diabetes (Rs = 0.52, p = 0.028), but with no correlation with WHR (Fig. 2). A very strong correlation between CRP and BMI was observed in DM2 subjects without vascular complications (Rs=0.79, p < 0.0001), but was not present in subgroups with different diabetic complications and was not related to patients' age, duration of diabetes, hypertension, or cigarette smoking.
Fig. 2.
The relationship between CRP concentration and BMI in studied subgroups.
The relationships between CRP and the parameters analyzed in the different subgroups of the study group are summarized in Table 3. The subgroup with macrovascular complications of diabetes showed a positive correlation between CRP and HbA1c levels, and the subgroup with macrovascular and microvascular complications showed a negative correlation between CRP and FPG. There was no statistically significant correlation between CRP concentration and the parameters analyzed in the subgroup with microvascular complications. A positive correlation between CRP and total cholesterol, and ESR was observed in the subgroup of patients without chronic vascular complications of diabetes.
Table 3.
The Relationship Between CRP Concentration and Parameters Analyzed in Studied Subgroups
| Parameter | DM2 | DM (−) | ||||
|---|---|---|---|---|---|---|
| MA n = 20 | MI n = 20 | MI + MA n = 20 | COMP (−) n = 20 | OBESE n = 20 | LEAN n = 20 | |
| Data from the medical interview | ||||||
| Age (years) | Rs = −0.2 | Rs = −0.42 | Rs = +0.14 | Rs = −0.32 | Rs = −0.06 | Rs = +0.36 |
| p = 0.4 | p = 0.06 | p = 0.57 | p = 0.17 | p = 0.79 | p = 0.12 | |
| Duration of DM (years) | Rs = −0.12 | Rs = −0.42 | Rs = +0.13 | Rs = −0.05 | – | – |
| p = 0.61 | p = 0.06 | p = 0.59 | p = 0.83 | |||
| Anthropometric measurements | ||||||
| BMI (kg/m2) | Rs = −0.27 | Rs = +0.19 | Rs = +0.34 | Rs = +0.79 | Rs = +0.56 | Rs = +0.52 |
| p = 0.25 | p = 0.42 | p = 0.14 | p < 0.0001 | p < 0.02 | p < 0.03 | |
| WHR | Rs = −0.2 | Rs = +0.14 | Rs = −0.09 | Rs = −0.3 | Rs = −0.02 | Rs = +0.22 |
| p = 0.43 | p = 0.58 | p = 0.7 | p = 0.2 | p = 0.94 | p = 0.38 | |
| 24-h blood pressure and heart rate values | ||||||
| 24-h sBP (mm Hg) | Rs = −0.18 | Rs = +0.09 | Rs = +0.33 | Rs = −0.16 | Rs = −0.07 | Rs = +0.16 |
| p = 0.45 | p = 0.71 | p = 0.15 | p = 0.51 | p = 0.76 | p = 0.51 | |
| 24-h dBP (mm Hg) | Rs = +0.09 | Rs = +0.19 | Rs = −0.21 | Rs = −0.39 | Rs = −0.37 | Rs = −0.2 |
| p = 0.72 | p = 0.42 | p = 0.39 | p = 0.09 | p = 0.12 | p = 0.41 | |
| 24-h HR (t/min) | Rs = −0.01 | Rs = +0.16 | Rs = −0.25 | Rs = −0.37 | Rs = +0.17 | Rs = +0.13 |
| p = 0.95 | p = 0.5 | p = 0.29 | p = 0.12 | p = 0.49 | p = 0.6 | |
| Laboratory tests | ||||||
| Fasting glucose (mg/dL) | Rs = +0.01 | Rs = −0.11 | Rs = −0.49 | Rs = −0.16 | Rs = −0.23 | Rs = +0.06 |
| p = 0.96 | p = 0.63 | p = 0.03 | p = 0.51 | p = 0.33 | p = 0.8 | |
| HbA1c (%) | Rs = +0.53 | Rs = +0.14 | Rs = +0.26 | Rs = +0.08 | Rs = −0.02 | Rs = +0.13 |
| p < 0.02 | p = 0.56 | p = 0.26 | p = 0.73 | p = 0.92 | p = 0.59 | |
| Total cholesterol (mg/dL) | Rs = +0.16 | Rs = −0.1 | Rs = +0.12 | Rs = +0.52 | Rs = +0.07 | Rs = +0.08 |
| p = 0.5 | p = 0.69 | p = 0.6 | p < 0.02 | p = 0.77 | p = 0.74 | |
| LDL cholesterol (mg/dL) | Rs = +0.12 | Rs = +0.02 | Rs = +0.12 | Rs = +0.32 | Rs = +0.04 | Rs = +0.39 |
| p = 0.62 | p = 0.9 | p = 0.62 | p = 0.17 | p = 0.87 | p = 0.09 | |
| HDL cholesterol (mg/dL) | Rs = −0.01 | Rs = −0.37 | Rs = +0.09 | Rs = +0.06 | Rs = +0.03 | Rs = −0.49 |
| p = 0.96 | p = 0.1 | p = 0.7 | p = 0.8 | p = 0.9 | p < 0.03 | |
| Triglycerides (mg/dL) | Rs = +0.1 | Rs = +0.27 | Rs = +0.18 | Rs = +0.26 | Rs = +0.03 | Rs = +0.12 |
| p = 0.66 | p = 0.26 | p = 0.5 | p = 0.27 | p = 0.89 | p = 0.6 | |
| Creatinine (mg/dL) | Rs = −0.23 | Rs = −0.25 | Rs = +0.06 | Rs = −0.1 | Rs = −0.11 | Rs = +0.28 |
| p = 0.24 | p = 0.92 | p = 0.8 | p = 0.69 | p = 0.67 | p = 0.23 | |
| UAER (mg/24 h) | Rs = −0.22 | Rs = +0.37 | Rs = +0.02 | Rs = −0.31 | Rs = +0.24 | Rs = +0.4 |
| p = 0.35 | p = 0.11 | p = 0.9 | p = 0.18 | p = 0.29 | p = 0.09 | |
| ESR (mm/h) | Rs = 0.39 | Rs = +0.34 | Rs = +0.4 | Rs = +0.59 | Rs = +0.73 | Rs = +0.26 |
| p = 0.14 | p = 0.19 | p = 0.11 | p < 0.02 | p < 0.001 | p = 0.29 | |
| Leukocytes (G/L) | Rs = +0.4 | Rs = +0.2 | Rs = +0.12 | Rs = +0.23 | Rs = +0.43 | Rs = +0.15 |
| p = 0.08 | p = 0.4 | p = 0.63 | p = 0.33 | p = 0.06 | p = 0.52 | |
A multivariate analysis of the cumulative effect of age, gender, BMI, and presence of diabetes on the concentration of CRP was carried out, and the resulting linear model (R 2 = 0.25) showed that both BMI (Beta = 0.52, p < 0.000001) and the presence of diabetes (Beta = 0.26, p = 0.011) are significant independent factors affecting the CRP level.
DISCUSSION
The present study compared the concentration of CRP in obese DM2 patients, obese without diabetes, and normal body weight subjects without diabetes and assessed the relationship between CRP concentration and the presence of macrovascular and microvascular complications, and glycemic control. The selection of the study group was not random. Chronic inflammation plays a specific role in people with DM2 and coexisting obesity. The sources of inflammatory cytokines that modulate inflammatory reactions in these patients are both immune cells, activated by hyperglycemia and associated metabolic disorders, and adipocytes. The study group was split into four subgroups according to vascular complications in order to assess the relationship between inflammatory markers and the presence of diabetes complications. The purpose of the two control groups: obese and normal body weight nondiabetic patients, was to enable the assessment of the extent to which CRP is determined by the presence of diabetes and obesity itself.
The very clear relationship that we observed between CRP concentration and BMI in obese DM2 patients, as well as in the group of obese people without diabetes and normal-weight subjects without diabetes, confirms the role of adipose tissue in initiating and sustaining inflammation. Significantly higher CRP levels in obese subjects, both diabetic and nondiabetic, vs. those in slim individuals, as well as a strong positive correlation between CRP and ESR values in obese subjects, both diabetic and nondiabetic, which was not observed in normal body weight persons, suggest that obesity is a state corresponding to subminimal inflammation. It should be emphasized that in all patients included in the study, the presence of any medical condition with features of inflammation was excluded, and ESR and leukocyte count were within normal limits. These results are consistent with data reported by other authors [5, 22–26].
It is surprising that despite the very clear relationship between CRP and BMI, there was no relationship between CRP and WHR ratio, which is an indicator of visceral obesity since, according to current knowledge, the principal place of production of inflammatory cytokines and proteins is visceral tissue [27, 28]. However, it is worth remembering that in order to directly determine the amount of visceral fat, it is necessary to use radiological techniques (CT, MRI), which would allow to distinguish the two types of abdominal fat: visceral and subcutaneous [29]. In addition, recent reports suggest that apart from visceral tissue, the perivascular adipose tissue, surrounding almost all blood vessels, may also be a source of inflammatory cytokines [30]. In the present study, CRP levels in obese diabetic and nondiabetic subjects did not differ significantly. Since the obese nondiabetic group was characterized by significantly higher BMI in comparison to obese patients with DM2, with a strong relationship between CRP and BMI, one could have expected that the CRP concentration in the group of obese nondiabetic subjects would be higher than that in obese diabetic patients. Contrary to expectations, CRP levels were, in fact, slightly lower in that group. The explanation may be the presence of diabetes itself; as shown in the multivariate analysis, DM2 is an independent factor increasing the value of CRP. It thus seems that elevated CRP levels in subjects with DM2 and obesity are the result of the simultaneous impact of obesity and diabetes. The mechanism of the effect of diabetes on the CRP concentration remains unclear, even more so since there was no correlation between CRP and glycemic control measured by HbA1c and glucose levels. CRP values also showed no relation to lipid disorders and hypertension, which accompany diabetes.
The results indicate no relationship between CRP concentration and diabetic macro- and microangiopathy. This relationship certainly does not exist for microvascular complications, which were assessed very precisely. In the group with evidence of microangiopathy, the CRP level was 0.24 mg/dL and in subjects with macro- and microangiopathy 0.21 mg/dL. These values were almost identical to those observed in patients without microvascular complications (0.25 mg/dL). The results reported by other authors indicating the existence of such a relationship could be due to the fact that they did not consider body weight and the presence of obesity in their assessments [11]. This assumption is confirmed by a study which showed higher levels of CRP and fibrinogen in a group with DM2 and microalbuminuria compared to a group of patients with DM2 and normoalbuminuria, but in which, this correlation was significantly weakened after adjustment for BMI [18]. In our study, the diagnosis of diabetic macrovascular disease was not precise, for it was based largely on physical examination and ECG while imaging studies (ultrasound, angiography) were performed only in part of the patients. Therefore, it cannot be definitely excluded that among the diabetic patients assigned to the group with microvascular complications or to the group without complications, some individuals with asymptomatic coronary artery disease or other macroangiopathy could be found. Even if this happened, subjects with diagnosed macrovascular disease had to have changes much more advanced compared to those in other groups. Nevertheless, CRP levels were not higher in this group than in the others. This delivered arguments against the concept that CRP is a marker of inflammatory changes associated with the presence of atherosclerosis in the vessels, as suggested by some authors [11, 31]. The lack of association between elevated CRP levels and vascular complications observed in our study remains unclear. It is possible that in the group of patients with diabetic macroangiopathy (both with and without concurrent microangiopathy), the CRP level might have been slightly lowered due to drugs used for treatment of cardiovascular complications. Some of them, including aspirin, beta-blockers, convertase inhibitors, and sartans, have been shown to lower the CRP concentration [32, 33]. Statins, which can also have such an effect [34], were used with similar frequency in the groups of patients with different complications.
The present study showed no relationship between CRP and the degree of glycemic control, as assessed by the level of HbA1c. Similar results were obtained by the authors of other studies [5, 23, 25]. However, there are counter-reports indicating a correlation between increased levels of CRP and worse glycemic control [26, 35, 36]. It cannot be ruled out that this discrepancy of results may be due to different degrees of metabolic control since one of the studies showed that the CRP concentration had a tendency to increase in parallel with HbA1c, but this relationship was only observed in patients with HbA1c above 9 % [36]. It should be emphasized that the group of patients evaluated in our study presented significantly better glycemic control (HbA1c = 7.2 %). The relationship between CRP concentration and glycemic control parameters indicated by some authors can stem from not taking into account the influence of BMI on the results obtained. After BMI values were incorporated into the statistical analysis, the correlations were importantly disvalued and became statistically insignificant [25, 31]. The lack of relationship between CRP concentration and glycemic control was also confirmed by a study conducted by Pradhan et al., who, having introduced hypoglycemic therapy that resulted in an improvement of glycemic control, did not observe a decrease of CRP, IL-6, or TNF-α levels [37].
CONCLUSIONS
The obtained results indicate that the most important factor determining an increase in the concentration of CRP in obese DM2 patients is excess body fat and the presence of diabetes itself, while the vascular complications and the degree of glycemic control do not show any significant correlation with this inflammatory parameter. Further research is required to elucidate the role of inflammation in the development of diabetic vascular complications.
Contributor Information
Aneta Fronczyk, Phone: +48-91-4253858, FAX: +48-91-4253858, Email: aneta.fronczyk@wp.pl.
Piotr Molęda, Email: pmoleda@wp.pl.
Krzysztof Safranow, Email: chrissaf@mp.pl.
Wiesław Piechota, Email: wpiechota@wim.mil.pl.
Lilianna Majkowska, Email: majkaend@pum.edu.pl.
References
- 1.Bray GA, Clearfield MB, Fintel DJ, Nelinson DS. Overweight and obesity: The pathogenesis of cardiometabolic risk. Clinical Cornerstone. 2009;9(4):30–40. doi: 10.1016/S1098-3597(09)80003-3. [DOI] [PubMed] [Google Scholar]
- 2.Garcia C, Feve B, Ferré P, et al. Diabetes and inflammation: Fundamental aspects and clinical implications. Diabetes & Metabolism. 2010;36(5):327–338. doi: 10.1016/j.diabet.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Pepys MB, Hirschfield GM. C-reactive protein: A critical update. Journal of Clinical Investigation. 2003;111:1805–1812. doi: 10.1172/JCI200318921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yosef-Levi IM, Grad E, Danenberg HD. C-reactive protein and atherothrombosis—a prognostic factor or a risk factor? Harefuah. 2007;146(12):970–974. [PubMed] [Google Scholar]
- 5.Kahn SE, Zinman B, Haffner SM, et al. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006;55(8):2357–2364. doi: 10.2337/db06-0116. [DOI] [PubMed] [Google Scholar]
- 6.Park JS, Cho MH, Nam JS, et al. Visceral adiposity and leptin are independently associated with C-reactive protein in Korean type 2 diabetic patients. Acta Diabetologica. 2010;47(2):113–118. doi: 10.1007/s00592-009-0125-4. [DOI] [PubMed] [Google Scholar]
- 7.Belfki H, Ben Ali S, Bougatef S, et al. Association between C-reactive protein and type 2 diabetes in a Tunisian population. Inflammation. 2012;35(2):684–689. doi: 10.1007/s10753-011-9361-1. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan A, Tabassum R, Chavali S, et al. High-sensitivity C-reactive protein levels and type 2 diabetes in urban North Indians. Journal of Clinical Endocrinology and Metabolism. 2009;94(6):2123–2127. doi: 10.1210/jc.2008-2754. [DOI] [PubMed] [Google Scholar]
- 9.Lu B, Yang Y, Yang Z, et al. Insulin resistance in Chinese patients with type 2 diabetes is associated with C-reactive protein independent of abdominal obesity. Cardiovascular Diabetology. 2010;9:92. doi: 10.1186/1475-2840-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanai A, Kawamura T, Umemura T, et al. Association between future events of brain infarction and soluble levels of intercellular adhesion molecule-1 and C-reactive protein in patients with type 2 diabetes mellitus. Diabetes Research and Clinical Practice. 2008;82(2):157–164. doi: 10.1016/j.diabres.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Pu LJ, Lu L, Xu XW, et al. Value of serum glycated albumin and high-sensitivity C-reactive protein levels in the prediction of presence of coronary artery disease in patients with type 2 diabetes. Cardiovascular Diabetology. 2006;5:27. doi: 10.1186/1475-2840-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox AJ, Agarwal S, M Herrington D, Carr JJ, Freedman BI, Bowden DW. C-reactive protein concentration predicts mortality in type 2 diabetes: The Diabetes Heart Study. Diabetic Medicine. 2012;29(6):767–770. doi: 10.1111/j.1464-5491.2011.03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su G, Mi S, Tao H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovascular Diabetology. 2011;10:19. doi: 10.1186/1475-2840-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin C, Lu L, Zhang RY, et al. Association of serum glycated albumin, C-reactive protein and ICAM-1 levels with diffuse coronary artery disease in patients with type 2 diabetes mellitus. Clinica Chimica Acta. 2009;408(1–2):45–49. doi: 10.1016/j.cca.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: Molecular links between obesity and atherosclerosis. American Journal of Physiology. Heart and Circulatory Physiology. 2005;288(5):2031–2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 16.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165–2168. doi: 10.1161/01.CIR.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 17.Abrahamian H, Endler G, Exner M, et al. Association of low-grade inflammation with nephropathy in type 2 diabetic patients: Role of elevated CRP-levels and 2 different gene-polymorphisms of proinflammatory cytokines. Experimental and Clinical Endocrinology and Diabetes. 2007;115(1):38–41. doi: 10.1055/s-2007-948213. [DOI] [PubMed] [Google Scholar]
- 18.Festa A, D'Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The Insulin Resistance Atherosclerosis Study. Kidney International. 2000;58(4):1703–1710. doi: 10.1046/j.1523-1755.2000.00331.x. [DOI] [PubMed] [Google Scholar]
- 19.Navarro JF, Mora C, Maca M, Garca J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. American Journal of Kidney Diseases. 2003;42(1):53–61. doi: 10.1016/S0272-6386(03)00408-6. [DOI] [PubMed] [Google Scholar]
- 20.Del Cañizo Gómez FJ, Fernández Pérez C, Moreno Ruiz I, et al. Microvascular complications and the risk factors in patients with type 2 diabetes. Endocrinología y Nutrición. 2011;58(4):163–168. doi: 10.1016/j.endonu.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Nowak M, Wielkoszyński T, Marek B, et al. Antioxidant potential, paraoxonase 1, ceruloplasmin activity and C-reactive protein concentration in diabetic retinopathy. Clinical and Experimental Medicine. 2010;10(3):185–192. doi: 10.1007/s10238-009-0084-7. [DOI] [PubMed] [Google Scholar]
- 22.Anan F, Masaki T, Umeno Y, et al. Correlations of high-sensitivity C-reactive protein and atherosclerosis in Japanese type 2 diabetes patients. European Journal of Endocrinology. 2007;157(3):311–317. doi: 10.1530/EJE-07-0388. [DOI] [PubMed] [Google Scholar]
- 23.Gustavsson CG, Agardh CD. Markers of inflammation in patients with coronary artery disease are also associated with glycosylated haemoglobin A1c within the normal range. European Heart Journal. 2004;25(23):2120–2124. doi: 10.1016/j.ehj.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Huffman F, Whisner S, Zarini GG, Nath S. Waist circumference and BMI in relation to serum high sensitivity C-reactive protein (hs-CRP) in Cuban Americans with and without type 2 diabetes. International Journal of Environmental Research and Public Health. 2010;7(3):842–852. doi: 10.3390/ijerph7030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfützner A, Standl E, Strotmann HJ, et al. Association of high-sensitive C-reactive protein with advanced stage beta-cell dysfunction and insulin resistance in patients with type 2 diabetes mellitus. Clinical Chemistry and Laboratory Medicine. 2006;44(5):556–560. doi: 10.1515/CCLM.2006.108. [DOI] [PubMed] [Google Scholar]
- 26.Streja D, Cressey P, Rabkin SW. Associations between inflammatory markers, traditional risk factors, and complications in patients with type 2 diabetes mellitus. Journal of Diabetes and its Complications. 2003;17(3):120–127. doi: 10.1016/S1056-8727(02)00204-0. [DOI] [PubMed] [Google Scholar]
- 27.Trayhurn P, Wood IS. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. British Journal of Nutrition. 2004;92(3):347–355. doi: 10.1079/BJN20041213. [DOI] [PubMed] [Google Scholar]
- 28.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sam S, Haffner S, Davidson MH, et al. Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes Care. 2009;32(5):932–937. doi: 10.2337/dc08-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao CY, Li ZY. The role of perivascular adipose tissue in vascular smooth muscle cell growth. British Journal of Pharmacology. 2012;165(3):643–658. doi: 10.1111/j.1476-5381.2011.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mojiminiyi OA, Abdella N, Moussa MA, Akanji AO, Mohammedi H, Zaki M. Association of C-reactive protein with coronary heart disease risk factors in patients with type 2 diabetes mellitus. Diabetes Research and Clinical Practice. 2002;58(1):37–44. doi: 10.1016/S0168-8227(02)00101-8. [DOI] [PubMed] [Google Scholar]
- 32.Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovascular Drug Reviews. 2006;24(1):33–50. doi: 10.1111/j.1527-3466.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 33.Palmas W, Ma S, Psaty B, Goff DC, Jr, Darwin C, Barr RG. Antihypertensive medications and C-reactive protein in the multi-ethnic study of atherosclerosis. American Journal of Hypertension. 2007;20(3):233–241. doi: 10.1016/j.amjhyper.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Sathyapalan T, Atkin SL, Kilpatrick ES. Disparate effects of atorvastatin compared with simvastatin on C-reactive protein concentrations in patients with type 2 diabetes. Diabetes Care. 2010;33(9):1948–1950. doi: 10.2337/dc10-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bahceci M, Tuzcu A, Ogun C, Canoruc N, Iltimur K, Aslan C. Is serum CRP concentration correlated with HbA1c and insulin resistance in type 2 diabetic men with or without coronary heart disease? Journal of Endocrinological Investigation. 2005;28(2):145–150. doi: 10.1007/BF03345357. [DOI] [PubMed] [Google Scholar]
- 36.King DE, Mainous AG, 3rd, Buchanan TA, Pearson WS. C-reactive protein and glycemic control in adults with diabetes. Diabetes Care. 2003;26(5):1535–1539. doi: 10.2337/diacare.26.5.1535. [DOI] [PubMed] [Google Scholar]
- 37.Pradhan AD, Everett BM, Cook NR, Rifai N, Ridker PM. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: The LANCET randomized trial. JAMA. 2009;302(11):1186–1194. doi: 10.1001/jama.2009.1347. [DOI] [PubMed] [Google Scholar]