Abstract
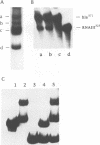
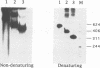
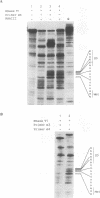
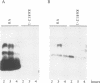
The synthesis of virulence factors in Staphylococcus aureus is controlled by a regulatory RNA molecule, RNAIII, encoded by the agr locus. Transcription of genes coding for secreted toxins and enzymes is stimulated, while transcription of cell-surface protein genes is repressed by RNAIII. In the case of staphylococcal alpha-toxin, RNAIII also seems to stimulate translation by an independent mechanism. In this report we show that in a mutant lacking RNAIII the rate of alpha-toxin (hla) production relative to the cellular concentration of hla mRNA was reduced 10-fold as compared with the wild-type strain. A 75% complementarity between the 5' end of RNAIII and the 5' untranslated region of the hla transcript suggests a direct interaction between the RNAs. A complex of RNAIII and hla mRNA was demonstrated in extracts of total RNA from the wild-type strain, and also with in vitro synthesized RNAs. Ribonuclease T1 digestion experiments revealed that the ribosome binding site of the hla transcript is blocked by intramolecular base-pairing. Hybridization with RNAIII prevents this intramolecular base-pairing and makes the hla mRNA accessible for translation initiation. This is, to our knowledge, the first example of an 'antisense RNA' that stimulates translation of the target mRNA.
Full text
PDF


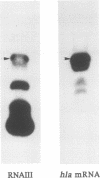
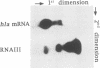
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaban N., Novick R. P. Autocrine regulation of toxin synthesis by Staphylococcus aureus. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1619–1623. doi: 10.1073/pnas.92.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg P., Wagner E. G., Nordström K. Control of replication of plasmid R1: the duplex between the antisense RNA, CopA, and its target, CopT, is processed specifically in vivo and in vitro by RNase III. EMBO J. 1990 Jul;9(7):2331–2340. doi: 10.1002/j.1460-2075.1990.tb07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Cheung A. L., Koomey J. M., Butler C. A., Projan S. J., Fischetti V. A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L., Projan S. J. Cloning and sequencing of sarA of Staphylococcus aureus, a gene required for the expression of agr. J Bacteriol. 1994 Jul;176(13):4168–4172. doi: 10.1128/jb.176.13.4168-4172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delihas N. Regulation of gene expression by trans-encoded antisense RNAs. Mol Microbiol. 1995 Feb;15(3):411–414. doi: 10.1111/j.1365-2958.1995.tb02254.x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y., Itoh T., Tomizawa J. Antisense RNA. Annu Rev Biochem. 1991;60:631–652. doi: 10.1146/annurev.bi.60.070191.003215. [DOI] [PubMed] [Google Scholar]
- Gendron N., Putzer H., Grunberg-Manago M. Expression of both Bacillus subtilis threonyl-tRNA synthetase genes is autogenously regulated. J Bacteriol. 1994 Jan;176(2):486–494. doi: 10.1128/jb.176.2.486-494.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. R., Hatfull G. F., Salmond G. P. A new cell division operon in Escherichia coli. Mol Gen Genet. 1986 Oct;205(1):134–145. doi: 10.1007/BF02428043. [DOI] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Gray G. S., Kehoe M. Primary sequence of the alpha-toxin gene from Staphylococcus aureus wood 46. Infect Immun. 1984 Nov;46(2):615–618. doi: 10.1128/iai.46.2.615-618.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L., Lubon H. Interaction of protein with DNA in vitro. Methods Enzymol. 1987;152:721–735. doi: 10.1016/0076-6879(87)52076-6. [DOI] [PubMed] [Google Scholar]
- Janzon L., Arvidson S. The role of the delta-lysin gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 1990 May;9(5):1391–1399. doi: 10.1002/j.1460-2075.1990.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzon L., Löfdahl S., Arvidson S. Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol Gen Genet. 1989 Nov;219(3):480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- Kanclerski K., Möllby R. A simple and exact two-point interpolation method for determination of haemolytic activity in microtiter plates. Acta Pathol Microbiol Immunol Scand B. 1987 Jun;95(3):175–179. doi: 10.1111/j.1699-0463.1987.tb03108.x. [DOI] [PubMed] [Google Scholar]
- Kornblum J. S., Projan S. J., Moghazeh S. L., Novick R. P. A rapid method to quantitate non-labeled RNA species in bacterial cells. Gene. 1988;63(1):75–85. doi: 10.1016/0378-1119(88)90547-1. [DOI] [PubMed] [Google Scholar]
- McCarthy J. E., Gualerzi C. Translational control of prokaryotic gene expression. Trends Genet. 1990 Mar;6(3):78–85. doi: 10.1016/0168-9525(90)90098-q. [DOI] [PubMed] [Google Scholar]
- Morfeldt E., Janzon L., Arvidson S., Löfdahl S. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol Gen Genet. 1988 Mar;211(3):435–440. doi: 10.1007/BF00425697. [DOI] [PubMed] [Google Scholar]
- Nellen W., Lichtenstein C. What makes an mRNA anti-sense-itive? Trends Biochem Sci. 1993 Nov;18(11):419–423. doi: 10.1016/0968-0004(93)90137-c. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Ross H. F., Projan S. J., Kornblum J., Kreiswirth B., Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993 Oct;12(10):3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Ohman M., Wagner E. G. Secondary structure analysis of the RepA mRNA leader transcript involved in control of replication of plasmid R1. Nucleic Acids Res. 1989 Apr 11;17(7):2557–2579. doi: 10.1093/nar/17.7.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Kofoid E. C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- Patel A. H., Kornblum J., Kreiswirth B., Novick R., Foster T. J. Regulation of the protein A-encoding gene in Staphylococcus aureus. Gene. 1992 May 1;114(1):25–34. doi: 10.1016/0378-1119(92)90703-r. [DOI] [PubMed] [Google Scholar]
- Putzer H., Gendron N., Grunberg-Manago M. Co-ordinate expression of the two threonyl-tRNA synthetase genes in Bacillus subtilis: control by transcriptional antitermination involving a conserved regulatory sequence. EMBO J. 1992 Aug;11(8):3117–3127. doi: 10.1002/j.1460-2075.1992.tb05384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P., Kreiswirth B., O'Reilly M., Schlievert P., Gruss A., Novick R. P. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986 Jan;202(1):58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- Regassa L. B., Betley M. J. Alkaline pH decreases expression of the accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1992 Aug;174(15):5095–5100. doi: 10.1128/jb.174.15.5095-5100.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regassa L. B., Novick R. P., Betley M. J. Glucose and nonmaintained pH decrease expression of the accessory gene regulator (agr) in Staphylococcus aureus. Infect Immun. 1992 Aug;60(8):3381–3388. doi: 10.1128/iai.60.8.3381-3388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Biological regulation by antisense RNA in prokaryotes. Annu Rev Genet. 1988;22:567–600. doi: 10.1146/annurev.ge.22.120188.003031. [DOI] [PubMed] [Google Scholar]
- Smeltzer M. S., Hart M. E., Iandolo J. J. Phenotypic characterization of xpr, a global regulator of extracellular virulence factors in Staphylococcus aureus. Infect Immun. 1993 Mar;61(3):919–925. doi: 10.1128/iai.61.3.919-925.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg B., Lundberg U., Hartl F. U., von Gabain A. Functional interaction of heat shock protein GroEL with an RNase E-like activity in Escherichia coli. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):277–281. doi: 10.1073/pnas.90.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenesch F., Kornblum J., Novick R. P. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J Bacteriol. 1991 Oct;173(20):6313–6320. doi: 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]