Abstract
Skin scarification (s.s.) with Vaccinia virus (VACV) is essential for generation of an optimal protective T cell memory immune response. Dendritic Cells (DC), which are professional antigen presenting cells, are required for naïve T cell priming and activation. At least three subsets of skin resident DC have been identified: Langerhans Cells (LC), Dermal Langerin+ DC (Lang+dDC) and Dermal Langerin− DC (Lang−dDC). Using Langerin-diphtheria toxin receptor mice and established mouse model of VACV delivered by s.s., we demonstrated that Lang+dDC, but not LC, are absolutely required for the induction of a rapid and robust antigen-specific CD8+ T cell response after s.s. with VACV. The depletion of Lang+dDC led to a significant delay in the priming and proliferation of antigen-specific CD8+ T cells. Moreover CD8+ T cells generated after VACV s.s. in the absence of Lang+dDC lacked effector cytotoxic functions both in vitro and in vivo. While s.s.-immunized WT and LC depleted mice controlled the progression of OVA257–264 expressing T cell lymphoma EG7 (injected intradermally), the depletion of Lang+dDC led to rapid lymphoma progression and mortality. These data indicate that of all skin DC subsets, Lang+dDC the most critical for the generation of robust CD8+ T cell immunity after s.s. with VACV.
Introductory Paragraph
Vaccinia virus (VACV) immunization provides complete protection against Variola major, the causative agent of smallpox, and a vaccination campaign based on this strategy led to the worldwide eradication of smallpox disease (Hammarlund et al., 2003; Stewart and Devlin, 2006). We have observed that protection against VACV is more closely associated with a protective T cell immune response, rather than neutralizing antibody production (Liu et al., 2010). More specifically, CD8+ T cells, many of them tissue resident memory cells (TRM) play a pivotal role in controlling VACV infections(Jiang et al., 2012). In mice and human, immunization with VACV results in an acute infection that elicits strong and efficient development of cytotoxic CD8+ T cells response. Moreover we have previously shown that the mode of vaccine delivery is very important in the induction of a robust immune response against VACV. We have demonstrated that immunization through the skin via skin scarification (s.s.), rather than conventional vaccine injection routes as subcutaneous (s.c.) or intramuscular (i.m.), is most effective for the generation of the strong T cell mediated immune response (Liu et al., 2010).
T cell priming, activation and proliferation are dependent upon professional antigen presenting cells such as dendritic cells (DC). In mice, blood-derived conventional DC can be divided in two subsets: CD8α+ DC and CD8α− DC. The CD8α− DC were then classified into CD4+ and CD4−DC populations (Heath and Carbone, 2009; Vremec et al., 2000). The lymph nodes contain also a subset of migrating DC. In skin draining lymph nodes (LN), three subsets of migrating DC can be defined: Langerhans cells (LC), dermal Langerin+ DC (Lang+dDC), and dermal Langerin− DC (Lang−dDC) (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007). It is now clear that in mice models, LC are unable to generate CD8+ T cell immunity to viral infection, especially to herpes simplex virus type 1 (HSV-1) after infection of the skin epidermis (Allan et al., 2003; Bedoui et al., 2009). Moreover, in constitutive and inducible LC deficient mice models, LC appeared to be largely dispensable for contact hypersensitivity sensitization, and may actually generate tolerance and dampen inflammation (Bobr et al., 2010; Igyarto et al., 2009; Kaplan et al., 2005). Since the discovery of bone marrow derived skin Lang+dDC, extensive studies evaluated the role of this DC subset for antigen presentation and for the generation of immune response during infection or inflammation. It has been suggested that Lang+dDC contribute to immune responses against HSV (Bedoui et al., 2009), leishmania major (Brewig et al., 2009), or those generated after contact hypersensitivity reactions (Kaplan et al., 2008). However, the relative role of these subsets of skin resident DC in the induction of immune response after s.s. with VACV remains unknown.
In this study, we investigated the role of LC and Lang+dDC in cross-priming responses to VACV inoculated by s.s. in transgenic mice expressing the Diphtheria toxin receptor under the control of the murine Langerin promoter (Lang-DTR) mouse model (Kissenpfennig et al., 2005). As shown previously, the systemic administration of DT depletes LC, and repopulation is delayed for several weeks, whereas Lang+dDC are replenished within a few days. Taking advantage of this model, we selectively depleted either LC alone or both LC and Lang+DC. Then, using recombinant VACV that expressed the ovalbumin peptide OVA257–264 (rVACV-ova) and adoptive transfer of OVA257–264-specific CD8+ T cells from OT-I TCR transgenic mice, we demonstrated that Lang+dDC are required for the induction of a rapid and robust antigen-specific CD8+ T cells immunity after s.s. against VV. The depletion of LC alone had no discernable effect on immunity after s.s. However, the depletion of Lang+DC as well led to a significant delay in the priming and proliferation of antigen-specific CD8+ T cells. Moreover, CD8+ T cells generated after VACV s.s. in the absence of Lang+dDC lacked effector cytotoxic functions both in in vitro and in vivo. While s.s.-immunized WT and LC depleted mice control the progression of the OVA257–264 expressing T cell lymphoma EG7 (injected intradermally), the depletion of Lang+dDC led to more rapid lymphoma progression and mortality. These data suggest of all skin DC subsets, only Lang+dDC are critical for the generation of robust protective CD8+ T cell immunity after s.s. with VACV.
RESULTS
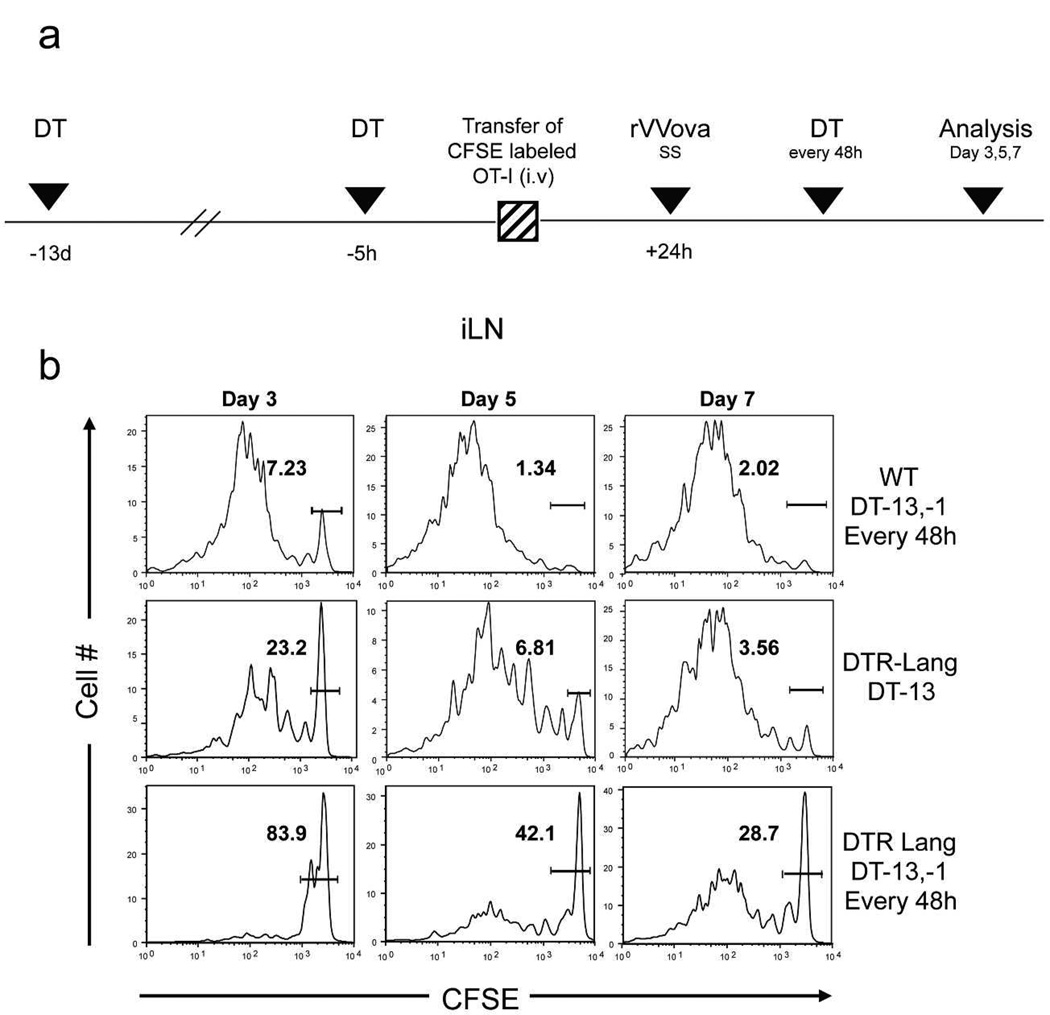
Lang+dDC are required for efficient activation and proliferation of antigen-specific CD8+ T cells in draining LN after VACV immunization via s.s
To compare the activation and proliferation of antigen-specific CD8+ T cells after VACV immunization via s.s., we first treated lang-DTR mice with systemic DT 13 days before immunization (LC depleted group) or 13 days, 1 day before immunization and then every 48h (Lang +DC depleted group). WT C57Bl/6 recipient mice were also treated with systemic administration of DT as a control (Figure 1a). As shown in Supplementary Figure S1, Lang+DC are not affected by DT injection in WT mice. After one single injection of DT 13 days before immunization, LC are still absent of the epidermis and Lang+dDC are replenished but at a lower level compared to WT group, while Lang+DC are totally absent when DT injection is injected 13 days and 1 day before immunization. We then adoptively transferred carboxy-fluorescein diacetate succinimidyl ester (CFSE) labeled Th1.1+ naïve CD8+ T cells isolated from OT-I T cell receptor (TCR) transgenic mice 24h prior rVACV-ova immunization via s.s. At day 3, 5 and 7, skin draining inguinal lymph nodes (ILN), were harvested and analyzed for OT-I cell proliferation (Figure 1b). As previously shown(Liu et al., 2006), high proliferation of OT-I harvested from WT mice group was detected in ILN that drained the scarified infected site. In LC depleted group, proliferation of OT-I cells was comparable to WT, while in Lang+DC depleted group, proliferation of OT-I mice was significantly reduced at day 3, 5 and 7. However, some activated OT-I cells were detected at day 7 in ILN from Lang +DC depleted mice group. Moreover, no significant proliferation were found in LN draining others tissues in all mice groups, although a small number of dividing OT-I cells were also found in spleen in control group (Supplementary Figure S2). These data suggest that Lang+dDC are required for a rapid and robust priming and proliferation of antigen-specific CD8+ T cells in skin draining lymph nodes after s.s. immunization against VACV. In additional experiments, to analyze the capacity to cross-present rVACV-ova of each subsets of DC early after immunization, we used a sorting scheme of DC previously described(Henri et al., 2010), allowing the isolation of populations of CD207 (EGFP)+ CD103+ dDC, CD207 (EGFP)+ CD103− fraction that comprises the majority of mLCs and a small fraction of CD207 (EGFP)+ CD103− dDC and CD207 (EGFP)− CD103− DC and LN resident DC in cutaneous LN, 48h after immunization with rVACV-ova. As shown in Supplementary Figuer S3, each subset were then incubated in vitro with purified CFSE-labeled OT-I cells and OT-I proliferation was then assessed by flow cytometry, showing that CD207(EGFP)+ CD103+ dDC were the major DC population capable of cross-presentation, in an early phase of immunization compared to others skin DC populations showing minimal or absence of ability for cross-presentation.
Figure 1. CD8+ T cells activation was delayed in the absence of Lang+dDC after tail s.s. with rVACV-ova.
CFSE-labeled naïve Thy1.1+ OT-I cells were transferred into Thy1.2+ B6 or in Thy1.2+ Lang-DTR mice. (a) Recipient Lang-DTR mice were treated with DT 13 days only (LC depleted group) or 13 days, 1 day before rVV-ova immunization and then every 48h (Lang+DC depleted group). WT C57BL/6 mice were also treated with DT as the Lang+DC depleted group. Recipient mice were then infected with rVACV-ova by s.s. (b) Proliferation of OT-I cells in ILN was analyzed at day 3, 5 and 7 by flow cytometry. Histograms were gated on Thy1.1+CD8+ OT-I donor and are representative of 4 independent experiments.
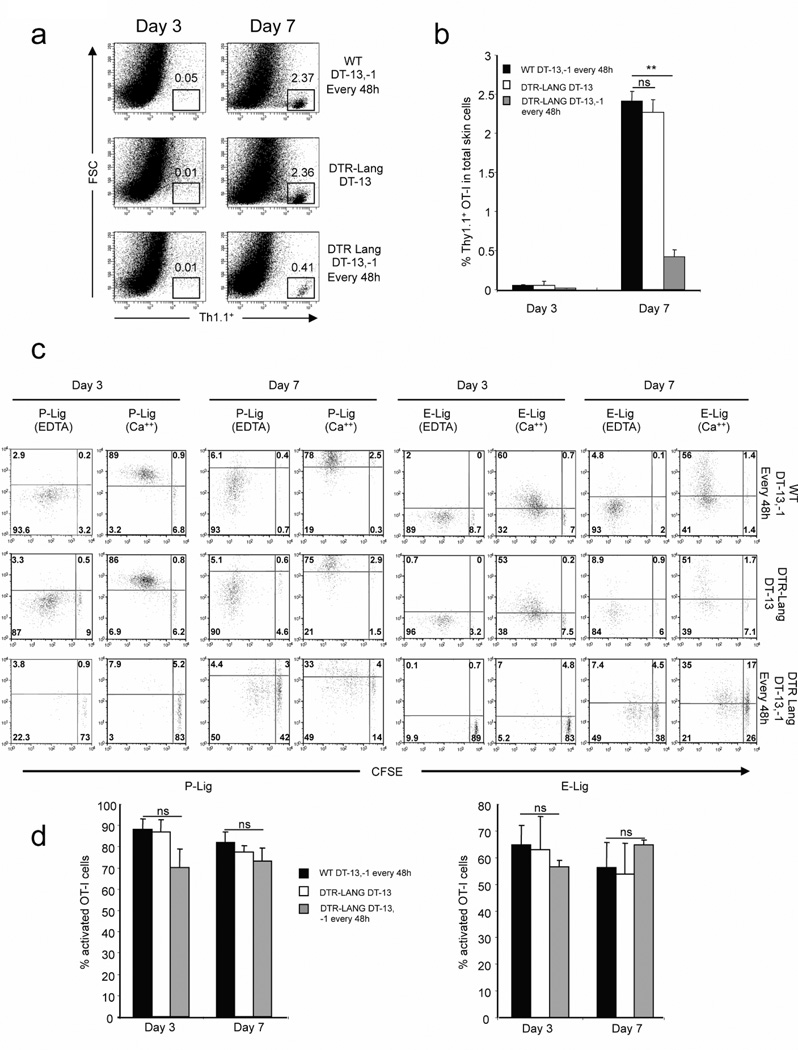
The absence of Lang+DC delays and reduces the infiltration of antigen-specific CD8+ T cells in infected skin
Having shown that Lang+dDC are required for early activation and proliferation of antigen-specific CD8+ T cells in skin draining LN after s.s. infection with VACV, we asked whether the absence of Lang+dDC can also affect the infiltration of T cells in infected skin at different time point (days 3 and 7). Skin s.s. infected tails from WT group, LC depleted group and Lang+DC depleted group, were harvested. T cells were extracted from tail skin and analyzed by flow cytometry. Activated OT-I cells were significantly reduced in Lang+DC depleted group when compared to WT and LC depleted group (Figure 2a,b). We next analyzed the expression of homing molecules: E- and P-Lig on T cells using E selectin/Fc or P selectin/Fc chimeric molecules, on OT-I cells in ILN after s.s. In both WT and the LC depleted group proliferating OT-I cells showed an upregulation of skin-homing molecules in ILN of s.s. infected mice (Figure 2c,d). In the absence of Lang+DC, the activation and proliferation of OT-I cells decreased dramatically, but the small fraction of T cells undergoing proliferation in the Lang+DC depleted group expressed E- and P-Lig at the same level than WT and LC depleted groups. Altogether our data demonstrate that Lang+dDC are required for the effective early activation and proliferation of antigen-specific T cells in skin draining LN after immunization via s.s., they do not appear to affect the expression of skin homing receptors once antigen-specific CD8+ T cells become activated.
Figure 2. Recruitment of OT-I cells to the infected skin site is less efficient in the absence of Lang+dDC after s.s. with VACV.
CFSE-labeled naïve Thy1.1+ OT-I cells were transferred into Thy1.2+ C57BL/6 or in Thy1.2+ lang-DTR mice. Recipient lang-DTR mice were treated with DT 13 days only (LC depleted group) or 13 days, 1 day before rVACV-ova immunization and then every 48h (Lang+DC depleted group). WT C57BL/6 mice were also treated with DT as the Lang+DC depleted group. 24h after OT-I transfer, recipient mice were infected with rVACV-ova by s.s. OT-I cells were isolated from infected skin at various time points (day 3 and 7). (a) Numbers in quadrant indicate percent of Thy1.1+ for one representative mouse. (b) Percentage of Thy1.1+ cells in infected skin. The graph shows means +/− SD. Data are representative of 3 independent experiments. (c) E- and P-lig expression was examined by incubating cells from ILN with rmCD62E/Fc or rmCD62P/Fc chimera in HBSS buffer containing 2 mM calcium at day 3 and 7. HBSS buffer supplemented with 5 mM EDTA was used for the controls. Data are representative of 3 independent experiments. (d) Percentage of E- and P-lig+ cells in the proliferating cells. The graph shows means +/− SD. Data are representative of 3 independent experiments.
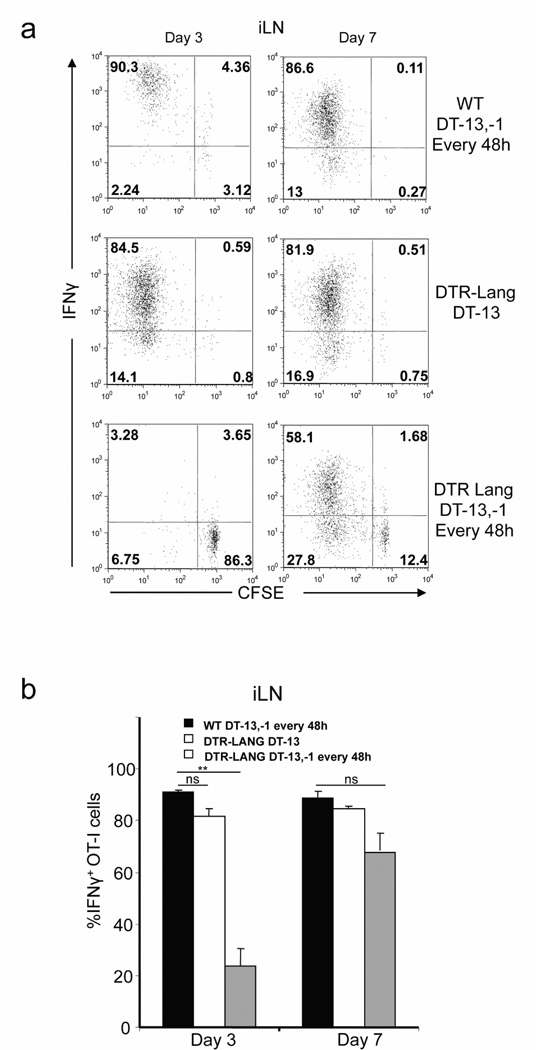
In the absence of Lang+dDC, OT-I cells do not acquire effector functions
Having shown that absence of Lang+dDC decreases and delays the activation and proliferation of antigen-specific CD8+ T cells after immunization via s.s., we wished to determine whether OT-I cells could acquire effector functions despite of the depletion of Lang+dDC. WT mice group (DT -13,-1 and every 48h), LC depleted group (DT-13) and Lang+DC depleted group (DT-13,-1 and every 48h) were immunized with rVACV-ova via s.s. 1 day after adoptive transfer of CFSE labeled OT-I cells. On day 3 and 7 after immunization, skin draining ILN and spleen cells were harvested and re-stimulated in vitro with 1µM OVA257–264 in the presence of Brefeldin. IFNγ production was then analyzed using flow cytometry. Proliferating OT-I T cells in WT and LC depleted group produced high amount of IFNγ in skin draining ILN and spleen at day 3 and 7. In contrast, in the absence of Lang+DC, the percentage of OT-I producing IFNγ was significantly reduced in the early phase of T cells activation in contrast to latter activation. (Figure 3a,b).
Figure 3. OT-I effector response is impaired in the absence of Lang+dDC after s.s. withrVACV-ova.
CFSE-labeled naïve Thy1.1+ OT-I cells were transferred into Thy1.2+ C57BL/6 or in Thy1.2+ Lang-DTR mice. Recipient Lang-DTR mice were treated with DT 13 days before immunization (LC depleted group), or 13 days, 1 day before immunization and then every 48h (Lang+DC depleted group). WT C57BL/6 mice were also treated as the Lang+DC depleted group. Recipient mice were then s.s. immunized with rVACV-ova. (a) At day 3 and 7, cells from ILN and spleen were isolated and re-stimulated in vitro with OVA257–264 peptide for 6h and analyzed for intracellular interferon-γ (IFN-γ). Cells were gated on Thy1.1+CD8+ OT-I cells. Numbers in quadrant indicate percent of cells. (b) Percentage of IFNγ+ OT-I cells in ILN and spleen. The graph shows means +/− SD. Data are representative of 3 independent experiments.
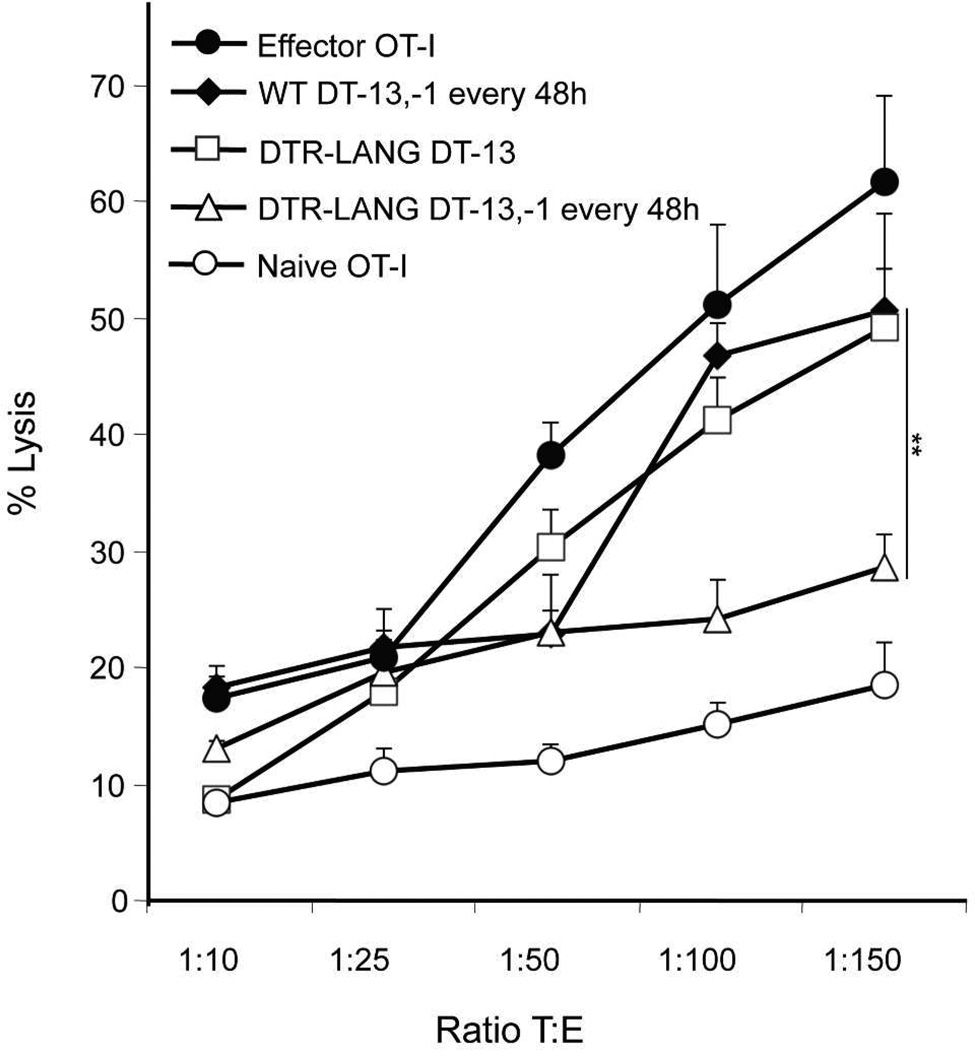
CD8+ T cells effector functions are not only measured by the production of IFNγ, but also by their cytotoxic activity in vitro. In order to test this function, we set up a cytolysis assay. Mice from the WT group, LC depleted group and Lang+DC depleted group were immunized with rVACV-ova after adoptive transfer of OT-I cells. On day 5, OT-I cells from skin draining ILN were harvested by positive selection. EG7 cells were used as target cells. Target cells were labeled with PKH-26 according to the manufacturer’s instructions and then with CFSE and dispensed into plates. OT-I cells isolated from lymph node at day 5 after s.s.-immunization, were added at different ratios and mixed with target cells for 5h before analysis. In vitro generated effector OT-I cells and naïve OT-I cells were used as positive and negative control respectively. After 5h of incubation, dilution of CFSE on PKH-26+ target cells was analyzed by flow cytometry. Cytotoxic activity was lower in the presence of OT-I isolated from Lang+DC depleted group whereas cytotoxic activity was high in the presence of OT-I harvested from WT and LC depleted groups (Figure 4). These data suggest that Lang+dDC are required for the induction of a strong effector cytotoxic CD8+ T cell immune response after immunization via s.s.
Figure 4. Lang+dDC are required to induce a strong specific cytotoxic activity in vitro.
Naïve Thy1.1+ OT-I cells were transferred into Thy1.2+ C57BL/6 or in Thy1.2+ lang-DTR mice. Recipient Lang-DTR mice were treated with DT 13 days only (LC depleted group) or 13 days, 1 day before rVACV-ova immunization and then every 48h (Lang+DC depleted group). WT C57BL/6 mice were also treated with DT as the Lang+DC depleted group. Recipient mice were then s.s. immunized with rVACV-ova. At day 5 post immunization, spleen and skin draining lymph nodes were harvested and OT-I cells were isolated. Effector OT-I were generated in vitro and used as positive control. Naïve OT-I cells were used as a negative control. OT-I cells were then cultured with target cells (EG7) stained with PKH-26 and CFSE during 4h at various ratio in triplicate. Cytolysis was analyzed by the dilution of CFSE on PKH-26+ cells using flow cytometry. Each data point represent means +/− SD. Data are representative of 2 independent experiments.
Lang+dDC modulate tumor immunity induced by immunization via s.s
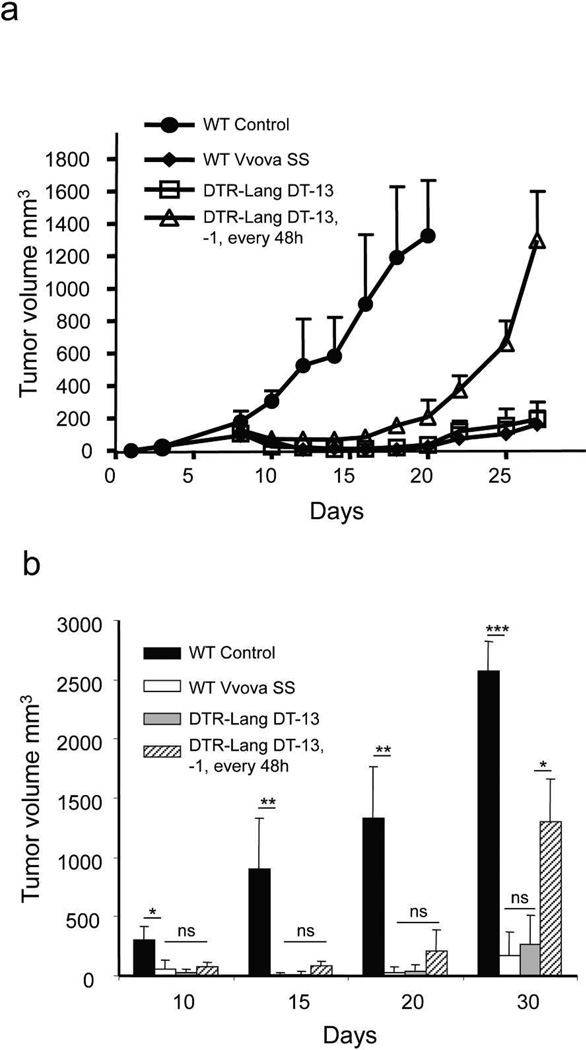
Our data demonstrate that Lang+dDC modulate the development of the strong effector/cytotoxic CD8+ T cells immune response against VACV after s.s. that we have observed previously (Jiang et al, 2012). Our previous study showed that immunization with VACV via s.s. is more effective than other routes of immunization to protect mice against tumor challenge (Liu et al., 2010). We asked whether Lang+dDC could impair the effective protection of s.s. route in terms of tumor protection. WT group, LC depleted (DT-13) and Lang+DC depleted group (DT-13,-1, every 72h) were immunized with rVV-ova via s.s. and then challenged with EG7 tumor cells intradermally on the same day. In WT mice, tumors became palpable 5–8 days after challenge in the untreated group, and subsequent tumor growth was rapid (Figure 5 a,b). In WT mice immunized with VACV-ova, tumor growth was significantly retarded and was negligible even at 25 days. The LC depleted group showed results indistinguishable from the WT group. In contrast, Lang+DC depleted group developed early palpable tumors which, over time, grew to a large size at day 18 (Supplementary Figure S4) and which grew rapidly thereafter and experienced rapid mortality (Supplementary Figure S5). Thus, all immunized mice developed some tumor immunity, althoughdepleted of both LC and Lang+dDC developed tumor immunity that was less robust. These data indicate that Lang+dDC represent a subset of DC important for an efficient tumor immune response after s.s. immunization, but also suggest that other Lang-DC play a significant role in tumor immunity.
Figure 5. Lang+dDC are required for efficient protection provided by s.s. with rVACV against tumor challenge.
Lang-DTR mice were treated with DT 13 days (LC depleted group) or 13 days, 1 day before immunization and then every 72h (Lang+DC depleted group). C57BL/6 WT mice were treated with DT as the Lang+DC depleted group. Mice were then s.s. immunized with rVACV-ova. The same day mice were challenged with EG7 (5×106 cells/mouse) intradermally, and monitored for tumor growth (a,b). Each data point represents the mean tumor volume of different groups (n=8). Data is the pooled results of 2 independent experiments.
DISCUSSION
Immunization through the skin using VACV delivered by s.s. effectively eradicated Variola major infection worldwide. We have recently observed that this remarkable efficacy was more closely associated with the delivery mode of immunization using superficially injured skin (i.e., s.s.), rather than immunogenicity of the virus per se (Liu et al., 2010). We have demonstrated that immunization via s.s. using VACV is highly effective to induce antigen-specific T cell response, protecting mice against VACV infection independently of neutralizing antibody production. Moreover, mice previously immunized via s.s. with a recombinant VV loaded with tumor antigen were better protected against tumor challenge in skin than mice immunized via conventional routes. However, the precise mechanism by which immunization through epidermis is so remarkably effective remains unknown. The present study rules out epidermal LC as contributing to this efficacy. Immunization using skin disruption mobilizes a large number of skin resident DC (Romani et al., 2010; Sparber et al., 2010; Steinman, 2008). Migrating DC to skin draining LN are required to prime the specific T cell immune response. It is now well accepted that poxvirus infection through skin scarification results in a response highly dependent on cross-presentation(Shen et al., 2002) and our present data suggest that a specific subset of skin resident DC, the Lang+dDC are required for the induction of an early activation and proliferation of antigen specific effector CD8 T cell after VACV immunization through the skin. Cross-priming has been studied in systems where DC are not theirself infected. In the case of VACV, infection of DC can occur but is known to be abortive, leading to expression of a subset of early genes but absolutely no late viral genes, particularly ones required to viral assembly and replication (Chahroudi et al., 2006; Jenne et al., 2000). Infected DC are subject to apoptosis, supporting an immune response against VACV through cross-presentation of exogenous viral antigens acquired by uninfected DC from dying cells, as well as from ambient late gene products released by apoptotic keratinocytes. Moreover It has been recently shown in a mouse model of VACV infection that cross-priming of virus specific T cells was mediated through the expression of a DC-restricted receptor DNGR-1(Iborra et al., 2012).
Mouse skin resident DC subpopulations consist at least of three different DC subsets: LC, Lang−dDC and the Lang+dDC. Lang+dDC were recently discovered in 2007 as a Lang+DC population distinct from LC (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007). Since this time, research focused to define the role of these different skin resident DC subpopulations in the generation of immune response.
In our present study, we combined the advantages of two different models: the murine VACV vaccination model developed in our laboratory(Liu et al., 2006) and the lang-DTR mice allowing the selective depletion of LC and/or Lang+DC, in order to define the precise role of resident skin DC in the induction of the early phase of T cell immune response after VACV immunization. We first observed that the depletion of Lang+dDC inhibited the early activation and proliferation of antigen-specific CD8+ T cell in the skin draining LN and Lang+dDC appear as the only DC population to cross-present virus in the early phase of immunization. Moreover, in the absence of Lang+dDC, the generation of CD8+ T cell effector function was also impaired as measured by production of IFNγ and in their functional in vitro cytotoxic activity. These data are consistent with findings observed in the literature for other antigens. Recently, after subcutaneous infection with leishmania major, Lang+ non-LC were found to be the major subset of skin migratory DC to induce CD8+ T cell activation (Brewig et al., 2009). Later studies using HSV-1 model have found that Lang+dDC contribute to the generation of HSV-1-specific CTL immunity during the second phase of HSV infection (Bedoui et al., 2009). However, in our present study, we still observed a late activation and proliferation of antigen-specific T cells despite the total absence of Lang+DC. It is well documented that the LN resident CD8α+ DC can also play an active role in CD8+ T immunity. LN resident CD8α+ DC can capture antigen from migratory DC or from material draining directly through the lymphatic conduits (Heath and Carbone, 2009). Another hypothesis could be the influx of blood-derived DC that can migrate to tissue anytime there is inflammation whether sterile or infectious. We have previously shown that blood-derived DC constitutively express high level of E-and P-selctin ligand leading to their ability for skin migration(Robert et al., 1999). Moreover It has been previously shown(Eidsmo et al., 2009) in HSV skin infection model that blood-derived DC can migrate to infected skin at a latter time by day 5. These newly recruited DC might serve as new antigen presenting cells to continue T cell activation.
Another point of our observation is that antigen-specific CD8+ T cell migration to skin is reduced in the absence of Lang+dDC. At day 7 post immunization, we found in the Lang+DC depleted mice, reduced percentage of CD8+ T cells in infected skin when compared to the WT group and LC depleted group. However, despite the late activation of effector CD8+ T cells occurring in the absence of Lang+dDC, these CD8+ T cells still expressed skin homing receptors when they undergo proliferation in the skin draining LN at the same level as control groups. The absence of Lang+dDC delays the generation of a specific effector T cell response but does not impair skin homing imprinting when CD8+ T cells become activated. The defect of migration observed in our study results from a defect of T cells proliferation but is not due to a defect of skin imprinting. These findings are consistent with our previous results suggesting that homing-imprinting program can be acquired by activated T cells circulating through the tissue-draining LN microenvironments (stromal cells, tissue-specific DC) independent of antigen presentation (Liu et al., 2006).
A persistent question is the precise role of LC in the generation of immune response. LC have long been accepted in the literature to be the most powerful DC subset for T cell priming (Merad et al., 2008). This postulate has been challenged since the discovery of Lang+dDC as a distinct DC subset from LC (Bursch et al., 2007; Poulin et al., 2007). Data using LC- deficient mouse models indicated that LC mediate immune tolerance, especially in contact hypersensitivity reactions or more generally are not required for the generation of effector T cells response against a variety of antigens (Igyarto et al., 2009; Kaplan, 2010; Kaplan et al., 2005; Zahner et al., 2011). Other recent data from human samples suggest that LC function to induce proliferation of regulatory T cells, or Treg cells(Seneschal et al., 2012). However, recent observations indicate that LC are capable to induce Th17 responses especially against extracellular pathogens in a infection model(Igyarto et al., 2011).
However it is less clear that LC could induce cross-presentation. Our present data did not show significant differences between the LC depleted groups and the WT groups in any of the in vitro or in vivo assays we performed. We can conclude that LC are dispensable for CD8+ T cell priming against VACV, compared to Lang+dDC. However, it is also notable that eliminating the LC population did not enhance the activation and the proliferation of CD8+ T cells, and we could not demonstrate in the VACV model any immunoregulatory role of LC. These results may be due to technical issues: LC depleted mice group were i.p. injected with DT 13 days before the beginning of the experiment. While epidermal LC completely absent from skin after 13 days, the Lang+dDC population has partially but not completely been restored in the dermis. However, the diminished number of Lang+dDC in our Lang-DTR mice 13 days after DT injection could explain the absence of exaggerated immune response in the LC-depleted mice group. Another hypothesis could be the functional impairment that occurs during early repopulation of Lang+dDC, leading to the reduction of their pro-inflammatory capacity as previously suggested (Kaplan, 2010). Our study does not distinguish between these possibilities. Finally, our in vitro was extended in vivo in an antigen specific tumor challenge model. While s.s.-immunized WT and LC depleted mice controlled the progression of OVA expressing T cell lymphoma injected intradermally, the depletion of Lang+DC led to eventual lymphoma progression and mortality. However, tumor growth was still significantly delayed in these mice, suggesting a role for Lang−DC and lymph node resident DC. In conclusion, our present data indicate that of all skin resident DC subsets, Ln+dDC are critical for the generation of robust CD8+ T cell immunity to infection after s.s. with VACV.
METHODS
Mice, viruses, and viral infection
All animal work in compliance with the guidelines set out by the Center for Animal Resources and Comparative Medicine at Harvard Medical School (HMS). Six-to eight week-old female wt C57BL/6, Lang-DTR, Lang-EGFP and Thy1.1+ OT-I Rag1-/- mice were bred in a biosafety level 1 facility at HMS. rVACV-ova were kind gifts from Dr. Bernard Moss (National Institutes of Health, Bethesda, MD). The virus stocks were expanded and titered in Hela cells and CV-1 cells (American Tissue Culture Company) by standard procedures. Mice were infected with the virus by skin scarification (2×106 pfu in 5µl of PBS). For scarification, mice were anesthetized i.p. with 2, 2, 2 tribromoethanol (250mg/kg, Sigma). 5µl of diluted virus was applied to tail skin 1 cm from the base of the tail. The skin area was gently scratched 25 times with a 28 ½ G needle.
Chimeras, antibodies and Flow Cytometry
Directly conjugated mAbs Thy1.1-PerCP (OX7), CD8α-PECy7 (53-6.7), IFNγ-APC (XMG1.2), MHC class II-Biotin (AF6-120.1), CD11b-APC (M1/70), CD19-PE (1D3), PerCP streptavidin were purchased from BD Pharmingen. rmE-Selectin/Fc chimera and rmP-Selectin/Fc chimera were purchased from R&D Systems. Data were acquired on 6-color flow cytometer and analyzed.
Adoptive Transfer of OT-I Cells
Spleens and LNs were harvested from three-to four week-old Thy1.1+ OT-I Rag1-/-mice. Red blood cells (RBC) were lysed and single-cell suspension was prepared. CD8+ T cells were isolated with the mouse CD8α+ T cell isolation kit according to the manufacturer’s protocol (Miltenyi Biotec). The purity of the isolated cells was >95% measured by flow cytometry. Before CFSE staining, cells were washed twice with cold PBS and incubated at 10×106 cells/ml in PBS with 2 µM CFSE (Molecular Probes) at 37C for 3 min. Cells were then washed twice with cold DMEM/10% FCS. 2× 106 OT-I cells were injected i.v. in Thy1.2+ recipient mice.
In vivo depletion of LC or of Lang+ dDC
For systemic in vivo depletion of Lang+ dDC, Lang-DTR mice were injected i.p. with 1µg DT (Sigma-Aldrich) using the specified timing of administration. For some in vivo experiments mice were injected i.p. with 20ng/g DT.
Intracellular Staining
Ex vivo intracellular cytokine staining, cells were cultured at 37°C for 6h in complete medium supplemented with 1.0 µg/ml of Monensin (GolgiStop BD Pharmingen), in either the presence or the absence of OVA257–264 peptide at 1.0 µg/ml. Cells were then labeled for surface epitopes and then were fixed and permeabilized using Cytofix/Cytoperm kit (BD Biosciences) before staining for intracellular markers according to the manufacturer’s instructions.
Detection of T cells in skin tissue
Skin samples were harvested from the base of the tails at different times after rVACV-ova vaccinia challenge. Skin tail was incubated in HBSS containing 1mg/ml collagenase A and 40 µg/ml DNase I (Roche Diagnostics) at 37°C for 2 h. HBSS containing 2mM EDTA and 5% FCS was then added to stop the digestion. Single cell suspension was prepared by passing cells through 70-micron cell strainers. Cells were then stained with appropriate antibodies and analyzed by Flow Cytometry.
CD62E and P Analysis
To detect E-lig and P-lig expression, non specific binding to Fc receptors was blocked by anti-mouse CD16/CD32. Cells then were incubated with 5µg/ml of rmCD62E/Fc chimera or rmCD62P/Fc chimera in HBSS supplemented with 2mM calcium, 5% FCS and 1 mM Hepes buffer at 4°C fro 30 mn. After washes, cells were incubated with APC anti-hFc-IgG at 4°C for an additional 30 mib. HBSS buffer supplemented with 5 mM EDTA instead of calcium was used as control.
Cytotoxicity Assay
OT-I CTL used as a positive control were generated as described previously. Briefly, OT-I splenocytes (1×106/well) in 24-well plates containing 1 ml of RPMI 1640, 10%FCS and Penicillin/streptomycin solution (CM), containing OVA257–264 (1nM), IL-4 (175 U/ml R&D System), IL-2 (50 U/ml). After 3 days in culture, the cells were washed and re-cultured at 0.5×106 cells/well in 24-well plates containing 1 ml of CM without OVA257–264, but with the same cytokines that were present during initial culture. After an additional 2 days in culture, the cells were harvested and used as effector cells. Naïve OT-I were used as a negative control. At day 5 post immunization with rVACV-ova, cells were isolated from spleen and skin draining lymph nodes (LN). Single cell suspension was prepared by passing cells through 70-micron cell strainers. RBC were lysed and then OT-I were isolated by using CD90.1 microbeads isolation kit (Miltenyi Biotec) according to the manufacturer’s instructions.
2×103 Target cells (EG7: an OVA gene transfected EL4 thymoma cell line ATCC) were successively stained with PKH-26 (Sigma; final concentration 2.5×10−6 M) and then with 2µM CFSE as previously described. Target cells were then co-cultured with Effector Cells at various T:E ratio (1:25, 1:50, 1:100, 1:150) during 4h in a 96-well U-bottom plates (Becton Dickinson) at 37°C, 5% CO2. Then cells were fixed and analyzed by Flow Cytometry within 24h for CFSE dilution.
Tumor challenge
EG7 cells were maintained in DMEM supplemented with 10% heat-inactivated FCS, Penicillin/Streptomycin solution, and 400µg/ml G-418 (Invitrogen Life Technologies). C57BL/6 or Lang-DTREGFP mice were intradermally challenged in the lower back with 5× 106 EG7 cells. The longest diameter (L) and the perpendicular diameter (W) of the local tumor were measured with calipers at the indicated time points. Tumor volumes were calculated using the formula Volume =L2× W / 2.
Statistics
The differences in the means between groups were compared using a one-tailed student’s test. P ≤ 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (5R01-AI-041707-15). J.S. was supported by grants from Fondation René Touraine (European Fellowships 2008), Ministère Français des Affaire Etrangères, the Centre Hospitalier Universitaire de Bordeaux, France. J.S. designed the experiments, analyzed and interpreted the data, and drafted the paper; T.S.K. conceived the project, helped plan the experiments, analyzed and interpreted the data, and contributed to drafting and editing the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by zLangerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nature immunology. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- Bobr A, Olvera-Gomez I, Igyarto BZ, Haley KM, Hogquist KA, Kaplan DH. Acute ablation of Langerhans cells enhances skin immune responses. J Immunol. 2010;185:4724–4728. doi: 10.4049/jimmunol.1001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewig N, Kissenpfennig A, Malissen B, Veit A, Bickert T, Fleischer B, Mostbock S, Ritter U. Priming of CD8+ and CD4+ T cells in experimental leishmaniasis is initiated by different dendritic cell subtypes. J Immunol. 2009;182:774–783. doi: 10.4049/jimmunol.182.2.774. [DOI] [PubMed] [Google Scholar]
- Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahroudi A, Garber DA, Reeves P, Liu L, Kalman D, Feinberg MB. Differences and similarities in viral life cycle progression and host cell physiology after infection of human dendritic cells with modified vaccinia virus Ankara and vaccinia virus. J Virol. 2006;80:8469–8481. doi: 10.1128/JVI.02749-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidsmo L, Allan R, Caminschi I, van Rooijen N, Heath WR, Carbone FR. Differential migration of epidermal and dermal dendritic cells during skin infection. J Immunol. 2009;182:3165–3172. doi: 10.4049/jimmunol.0802950. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nature immunology. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, Devilard E, Viret C, Azukizawa H, Kissenpfennig A, Malissen B. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med. 2010;207:189–206. doi: 10.1084/jem.20091964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra S, Izquierdo HM, Martinez-Lopez M, Blanco-Menendez N, Reis e Sousa C, Sancho D. The DC receptor DNGR-1 mediates cross-priming of CTLs during vaccinia virus infection in mice. J Clin Invest. 2012;122:1628–1643. doi: 10.1172/JCI60660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyarto BZ, Jenison MC, Dudda JC, Roers A, Muller W, Koni PA, Campbell DJ, Shlomchik MJ, Kaplan DH. Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10. J Immunol. 2009;183:5085–5093. doi: 10.4049/jimmunol.0901884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne L, Hauser C, Arrighi JF, Saurat JH, Hugin AW. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 2000;7:1575–1583. doi: 10.1038/sj.gt.3301287. [DOI] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010 doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nature reviews. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Fuhlbrigge RC, Kieffer JD, Ayehunie S, Hynes RO, Cheng G, Grabbe S, von Andrian UH, Kupper TS. Interaction of dendritic cells with skin endothelium: A new perspective on immunosurveillance. J Exp Med. 1999;189:627–636. doi: 10.1084/jem.189.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N, Thurnher M, Idoyaga J, Steinman RM, Flacher V. Targeting of antigens to skin dendritic cells: possibilities to enhance vaccine efficacy. Immunol Cell Biol. 2010;88:424–430. doi: 10.1038/icb.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Wong SB, Buck CB, Zhang J, Siliciano RF. Direct priming and cross-priming contribute differentially to the induction of CD8+ CTL following exposure to vaccinia virus via different routes. J Immunol. 2002;169:4222–4229. doi: 10.4049/jimmunol.169.8.4222. [DOI] [PubMed] [Google Scholar]
- Sparber F, Tripp CH, Hermann M, Romani N, Stoitzner P. Langerhans cells and dermal dendritic cells capture protein antigens in the skin: possible targets for vaccination through the skin. Immunobiology. 2010;215:770–779. doi: 10.1016/j.imbio.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Stewart AJ, Devlin PM. The history of the smallpox vaccine. J Infect. 2006;52:329–334. doi: 10.1016/j.jinf.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- Zahner SP, Kel JM, Martina CA, Brouwers-Haspels I, van Roon MA, Clausen BE. Conditional deletion of TGF-betaR1 using Langerin-Cre mice results in Langerhans cell deficiency and reduced contact hypersensitivity. J Immunol. 2011;187:5069–5076. doi: 10.4049/jimmunol.1101880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.