Abstract
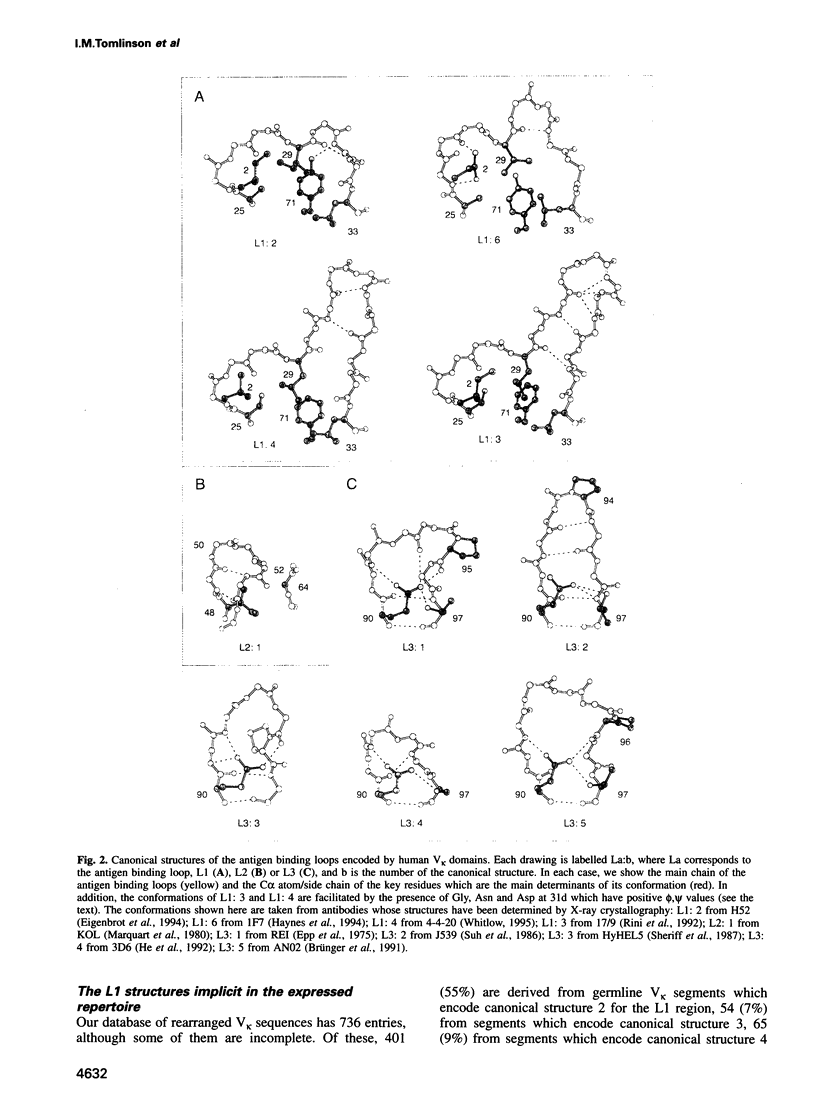
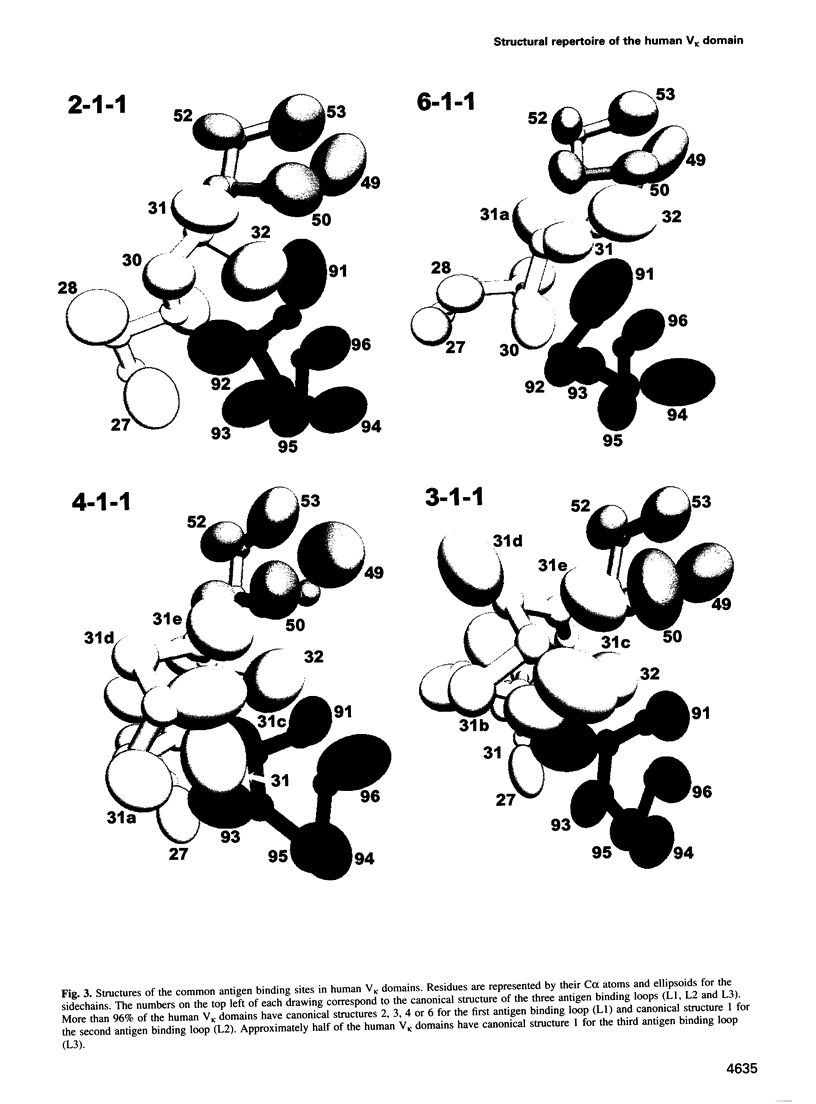
In humans, the gene for the V kappa domain is produced by the recombination of one of 40 functional V kappa segments and one of five functional J kappa segments. We have analysed the sequences of these germline segments and of 736 rearranged V kappa genes to determine the repertoire of main chain conformations, or canonical structures, they encode. Over 96% of the sequences correspond to one of four canonical structures for the first antigen binding loop (L1) and one canonical structure for the second antigen binding loop (L2). Junctional diversity produces some variation in the length of the third antigen binding loop (L3) and in the identity of residues at the V kappa-J kappa join. However, this is limited and 70% of the rearranged sequences correspond to one of three known canonical structures for the L3 region. Furthermore, we show that the canonical structures selected during the primary response are conserved during affinity maturation: the key residues that determine the conformations of the antigen binding loops are unmutated or undergo conservative mutation. The implications of these results for immune recognition are discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akamatsu Y., Tsurushita N., Nagawa F., Matsuoka M., Okazaki K., Imai M., Sakano H. Essential residues in V(D)J recombination signals. J Immunol. 1994 Nov 15;153(10):4520–4529. [PubMed] [Google Scholar]
- Alzari P. M., Spinelli S., Mariuzza R. A., Boulot G., Poljak R. J., Jarvis J. M., Milstein C. Three-dimensional structure determination of an anti-2-phenyloxazolone antibody: the role of somatic mutation and heavy/light chain pairing in the maturation of an immune response. EMBO J. 1990 Dec;9(12):3807–3814. doi: 10.1002/j.1460-2075.1990.tb07598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Evolution of immunoglobulin V genes: evidence indicating that recently duplicated human V kappa sequences have diverged by gene conversion. Cell. 1983 Jan;32(1):181–189. doi: 10.1016/0092-8674(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Human immunoglobulin variable region genes--DNA sequences of two V kappa genes and a pseudogene. Nature. 1980 Dec 25;288(5792):730–733. doi: 10.1038/288730a0. [DOI] [PubMed] [Google Scholar]
- Berek C., Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987 Apr;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Bhat T. N., Bentley G. A., Fischmann T. O., Boulot G., Poljak R. J. Small rearrangements in structures of Fv and Fab fragments of antibody D1.3 on antigen binding. Nature. 1990 Oct 4;347(6292):483–485. doi: 10.1038/347483a0. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Leahy D. J., Hynes T. R., Fox R. O. 2.9 A resolution structure of an anti-dinitrophenyl-spin-label monoclonal antibody Fab fragment with bound hapten. J Mol Biol. 1991 Sep 5;221(1):239–256. doi: 10.1016/0022-2836(91)80217-i. [DOI] [PubMed] [Google Scholar]
- Chacko S., Silverton E., Kam-Morgan L., Smith-Gill S., Cohen G., Davies D. Structure of an antibody-lysozyme complex unexpected effect of conservative mutation. J Mol Biol. 1995 Jan 20;245(3):261–274. doi: 10.1006/jmbi.1994.0022. [DOI] [PubMed] [Google Scholar]
- Chen C., Roberts V. A., Stevens S., Brown M., Stenzel-Poore M. P., Rittenberg M. B. Enhancement and destruction of antibody function by somatic mutation: unequal occurrence is controlled by V gene combinatorial associations. EMBO J. 1995 Jun 15;14(12):2784–2794. doi: 10.1002/j.1460-2075.1995.tb07278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. P., Albrandt K., Orida N. K., Radoux V., Chen E. Y., Schrantz R., Liu F. T., Carson D. A. Genetic basis for the cross-reactive idiotypes on the light chains of human IgM anti-IgG autoantibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8318–8322. doi: 10.1073/pnas.83.21.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. P., Robbins D. L., Jirik F. R., Kipps T. J., Carson D. A. Isolation and characterization of a light chain variable region gene for human rheumatoid factors. J Exp Med. 1987 Dec 1;166(6):1900–1905. doi: 10.1084/jem.166.6.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987 Aug 20;196(4):901–917. doi: 10.1016/0022-2836(87)90412-8. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Gherardi E., Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. Structural repertoire of the human VH segments. J Mol Biol. 1992 Oct 5;227(3):799–817. doi: 10.1016/0022-2836(92)90224-8. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R. Conformations of immunoglobulin hypervariable regions. Nature. 1989 Dec 21;342(6252):877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Laver W. G., Varghese J. N., Baker A. T., Tulloch P. A., Air G. M., Webster R. G. Three-dimensional structure of a complex of antibody with influenza virus neuraminidase. 1987 Mar 26-Apr 1Nature. 326(6111):358–363. doi: 10.1038/326358a0. [DOI] [PubMed] [Google Scholar]
- Cox J. P., Tomlinson I. M., Winter G. A directory of human germ-line V kappa segments reveals a strong bias in their usage. Eur J Immunol. 1994 Apr;24(4):827–836. doi: 10.1002/eji.1830240409. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Sheriff S. Antibody-antigen complexes. Annu Rev Biochem. 1990;59:439–473. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- Denzin L. K., Whitlow M., Voss E. W., Jr Single-chain site-specific mutations of fluorescein-amino acid contact residues in high affinity monoclonal antibody 4-4-20. J Biol Chem. 1991 Jul 25;266(21):14095–14103. [PubMed] [Google Scholar]
- Eigenbrot C., Gonzalez T., Mayeda J., Carter P., Werther W., Hotaling T., Fox J., Kessler J. X-ray structures of fragments from binding and nonbinding versions of a humanized anti-CD18 antibody: structural indications of the key role of VH residues 59 to 65. Proteins. 1994 Jan;18(1):49–62. doi: 10.1002/prot.340180107. [DOI] [PubMed] [Google Scholar]
- Epp O., Colman P., Fehlhammer H., Bode W., Schiffer M., Huber R., Palm W. Crystal and molecular structure of a dimer composed of the variable portions of the Bence-Jones protein REI. Eur J Biochem. 1974 Jun 15;45(2):513–524. doi: 10.1111/j.1432-1033.1974.tb03576.x. [DOI] [PubMed] [Google Scholar]
- Foote J., Winter G. Antibody framework residues affecting the conformation of the hypervariable loops. J Mol Biol. 1992 Mar 20;224(2):487–499. doi: 10.1016/0022-2836(92)91010-m. [DOI] [PubMed] [Google Scholar]
- Gerstein M., Lesk A. M., Chothia C. Structural mechanisms for domain movements in proteins. Biochemistry. 1994 Jun 7;33(22):6739–6749. doi: 10.1021/bi00188a001. [DOI] [PubMed] [Google Scholar]
- Hawkins R. E., Russell S. J., Baier M., Winter G. The contribution of contact and non-contact residues of antibody in the affinity of binding to antigen. The interaction of mutant D1.3 antibodies with lysozyme. J Mol Biol. 1993 Dec 20;234(4):958–964. doi: 10.1006/jmbi.1993.1650. [DOI] [PubMed] [Google Scholar]
- Hawkins R. E., Russell S. J., Winter G. Selection of phage antibodies by binding affinity. Mimicking affinity maturation. J Mol Biol. 1992 Aug 5;226(3):889–896. doi: 10.1016/0022-2836(92)90639-2. [DOI] [PubMed] [Google Scholar]
- Haynes M. R., Stura E. A., Hilvert D., Wilson I. A. Routes to catalysis: structure of a catalytic antibody and comparison with its natural counterpart. Science. 1994 Feb 4;263(5147):646–652. doi: 10.1126/science.8303271. [DOI] [PubMed] [Google Scholar]
- He X. M., Rüker F., Casale E., Carter D. C. Structure of a human monoclonal antibody Fab fragment against gp41 of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7154–7158. doi: 10.1073/pnas.89.15.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P. A., Maizel J. V., Jr, Leder P. Evolution of human immunoglobulin kappa J region genes. J Biol Chem. 1982 Feb 10;257(3):1516–1522. [PubMed] [Google Scholar]
- Huber C., Schäble K. F., Huber E., Klein R., Meindl A., Thiebe R., Lamm R., Zachau H. G. The V kappa genes of the L regions and the repertoire of V kappa gene sequences in the human germ line. Eur J Immunol. 1993 Nov;23(11):2868–2875. doi: 10.1002/eji.1830231121. [DOI] [PubMed] [Google Scholar]
- Jaenichen H. R., Pech M., Lindenmaier W., Wildgruber N., Zachau H. G. Composite human VK genes and a model of their evolution. Nucleic Acids Res. 1984 Jul 11;12(13):5249–5263. doi: 10.1093/nar/12.13.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Chothia C. The structure of protein-protein recognition sites. J Biol Chem. 1990 Sep 25;265(27):16027–16030. [PubMed] [Google Scholar]
- Kabat E. A., Wu T. T. Attempts to locate complementarity-determining residues in the variable positions of light and heavy chains. Ann N Y Acad Sci. 1971 Dec 31;190:382–393. doi: 10.1111/j.1749-6632.1971.tb13550.x. [DOI] [PubMed] [Google Scholar]
- Klein R., Jaenichen R., Zachau H. G. Expressed human immunoglobulin kappa genes and their hypermutation. Eur J Immunol. 1993 Dec;23(12):3248–3262. doi: 10.1002/eji.1830231231. [DOI] [PubMed] [Google Scholar]
- Klobeck H. G., Bornkamm G. W., Combriato G., Mocikat R., Pohlenz H. D., Zachau H. G. Subgroup IV of human immunoglobulin K light chains is encoded by a single germline gene. Nucleic Acids Res. 1985 Sep 25;13(18):6515–6529. doi: 10.1093/nar/13.18.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascombe M. B., Alzari P. M., Boulot G., Saludjian P., Tougard P., Berek C., Haba S., Rosen E. M., Nisonoff A., Poljak R. J. Three-dimensional structure of Fab R19.9, a monoclonal murine antibody specific for the p-azobenzenearsonate group. Proc Natl Acad Sci U S A. 1989 Jan;86(2):607–611. doi: 10.1073/pnas.86.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautner-Rieske A., Huber C., Meindl A., Pargent W., Schäble K. F., Thiebe R., Zocher I., Zachau H. G. The human immunoglobulin kappa locus. Characterization of the duplicated A regions. Eur J Immunol. 1992 Apr;22(4):1023–1029. doi: 10.1002/eji.1830220422. [DOI] [PubMed] [Google Scholar]
- Liu M. F., Robbins D. L., Crowley J. J., Sinha S., Kozin F., Kipps T. J., Carson D. A., Chen P. P. Characterization of four homologous L chain variable region genes that are related to 6B6.6 idiotype positive human rheumatoid factor L chains. J Immunol. 1989 Jan 15;142(2):688–694. [PubMed] [Google Scholar]
- Lorenz W., Schäble K. F., Thiebe R., Stavnezer J., Zachau H. G. The J kappa proximal region of the human K locus contains three uncommon V kappa genes which are arranged in opposite transcriptional polarities. Mol Immunol. 1988 May;25(5):479–484. doi: 10.1016/0161-5890(88)90168-x. [DOI] [PubMed] [Google Scholar]
- Marquart M., Deisenhofer J., Huber R., Palm W. Crystallographic refinement and atomic models of the intact immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 A and 1.0 A resolution. J Mol Biol. 1980 Aug 25;141(4):369–391. doi: 10.1016/0022-2836(80)90252-1. [DOI] [PubMed] [Google Scholar]
- Milstein C., Even J., Jarvis J. M., Gonzalez-Fernandez A., Gherardi E. Non-random features of the repertoire expressed by the members of one V kappa gene family and of the V-J recombination. Eur J Immunol. 1992 Jun;22(6):1627–1634. doi: 10.1002/eji.1830220642. [DOI] [PubMed] [Google Scholar]
- Pargent W., Meindl A., Thiebe R., Mitzel S., Zachau H. G. The human immunoglobulin kappa locus. Characterization of the duplicated O regions. Eur J Immunol. 1991 Aug;21(8):1821–1827. doi: 10.1002/eji.1830210807. [DOI] [PubMed] [Google Scholar]
- Pech M., Jaenichen H. R., Pohlenz H. D., Neumaier P. S., Klobeck H. G., Zachau H. G. Organization and evolution of a gene cluster for human immunoglobulin variable regions of the kappa type. J Mol Biol. 1984 Jun 25;176(2):189–204. doi: 10.1016/0022-2836(84)90420-0. [DOI] [PubMed] [Google Scholar]
- Pech M., Smola H., Pohlenz H. D., Straubinger B., Gerl R., Zachau H. G. A large section of the gene locus encoding human immunoglobulin variable regions of the kappa type is duplicated. J Mol Biol. 1985 Jun 5;183(3):291–299. doi: 10.1016/0022-2836(85)90001-4. [DOI] [PubMed] [Google Scholar]
- Pech M., Zachau H. G. Immunoglobulin genes of different subgroups are interdigitated within the VK locus. Nucleic Acids Res. 1984 Dec 21;12(24):9229–9236. doi: 10.1093/nar/12.24.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoux V., Chen P. P., Sorge J. A., Carson D. A. A conserved human germline V kappa gene directly encodes rheumatoid factor light chains. J Exp Med. 1986 Dec 1;164(6):2119–2124. doi: 10.1084/jem.164.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini J. M., Schulze-Gahmen U., Wilson I. A. Structural evidence for induced fit as a mechanism for antibody-antigen recognition. Science. 1992 Feb 21;255(5047):959–965. doi: 10.1126/science.1546293. [DOI] [PubMed] [Google Scholar]
- Schillbach J. F., Near R. I., Bruccoleri R. E., Haber E., Jeffrey P. D., Novotny J., Sheriff S., Margolies M. N. Modulation of antibody affinity by a non-contact residue. Protein Sci. 1993 Feb;2(2):206–214. doi: 10.1002/pro.5560020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäble K. F., Zachau H. G. The variable genes of the human immunoglobulin kappa locus. Biol Chem Hoppe Seyler. 1993 Nov;374(11):1001–1022. [PubMed] [Google Scholar]
- Schäble K., Thiebe R., Flügel A., Meindl A., Zachau H. G. The human immunoglobulin kappa locus: pseudogenes, unique and repetitive sequences. Biol Chem Hoppe Seyler. 1994 Mar;375(3):189–199. doi: 10.1515/bchm3.1994.375.3.189. [DOI] [PubMed] [Google Scholar]
- Scott M. G., Crimmins D. L., McCourt D. W., Chung G., Schäble K. F., Thiebe R., Quenzel E. M., Zachau H. G., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. IV. The less frequently expressed VL are heterogeneous. J Immunol. 1991 Dec 1;147(11):4007–4013. [PubMed] [Google Scholar]
- Scott M. G., Crimmins D. L., McCourt D. W., Zocher I., Thiebe R., Zachau H. G., Nahm M. H. Clonal characterization of the human IgG antibody repertoire to Haemophilus influenzae type b polysaccharide. III. A single VKII gene and one of several JK genes are joined by an invariant arginine to form the most common L chain V region. J Immunol. 1989 Dec 15;143(12):4110–4116. [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon J. Structural correlates of high antibody affinity: three engineered amino acid substitutions can increase the affinity of an anti-p-azophenylarsonate antibody 200-fold. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4814–4817. doi: 10.1073/pnas.87.12.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff S., Silverton E. W., Padlan E. A., Cohen G. H., Smith-Gill S. J., Finzel B. C., Davies D. R. Three-dimensional structure of an antibody-antigen complex. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8075–8079. doi: 10.1073/pnas.84.22.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield R. L., Takimoto-Kamimura M., Rini J. M., Profy A. T., Wilson I. A. Major antigen-induced domain rearrangements in an antibody. Structure. 1993 Oct 15;1(2):83–93. doi: 10.1016/0969-2126(93)90024-b. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Kekish O., Batter D., Grenier J., Balazs I., Henderson E., Zegers B. J. Aberrant recombination events in B cell lines derived from a kappa-deficient human. Nucleic Acids Res. 1985 May 24;13(10):3495–3514. doi: 10.1093/nar/13.10.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steipe B., Plückthun A., Huber R. Refined crystal structure of a recombinant immunoglobulin domain and a complementarity-determining region 1-grafted mutant. J Mol Biol. 1992 Jun 5;225(3):739–753. doi: 10.1016/0022-2836(92)90398-4. [DOI] [PubMed] [Google Scholar]
- Stiernholm N. B., Berinstein N. L. A mutated promoter of a human Ig V lambda gene segment is associated with reduced germ-line transcription and a low frequency of rearrangement. J Immunol. 1995 Feb 15;154(4):1748–1761. [PubMed] [Google Scholar]
- Straubinger B., Huber E., Lorenz W., Osterholzer E., Pargent W., Pech M., Pohlenz H. D., Zimmer F. J., Zachau H. G. The human VK locus. Characterization of a duplicated region encoding 28 different immunoglobulin genes. J Mol Biol. 1988 Jan 5;199(1):23–34. doi: 10.1016/0022-2836(88)90376-2. [DOI] [PubMed] [Google Scholar]
- Straubinger B., Thiebe R., Huber C., Osterholzer E., Zachau H. G. Two unusual human immunoglobulin V kappa genes. Biol Chem Hoppe Seyler. 1988 Jul;369(7):601–607. doi: 10.1515/bchm3.1988.369.2.601. [DOI] [PubMed] [Google Scholar]
- Suh S. W., Bhat T. N., Navia M. A., Cohen G. H., Rao D. N., Rudikoff S., Davies D. R. The galactan-binding immunoglobulin Fab J539: an X-ray diffraction study at 2.6-A resolution. Proteins. 1986 Sep;1(1):74–80. doi: 10.1002/prot.340010112. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Stanfield R. L. Antibody-antigen interactions: new structures and new conformational changes. Curr Opin Struct Biol. 1994 Dec;4(6):857–867. doi: 10.1016/0959-440x(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Zachau H. G. The immunoglobulin kappa locus-or-what has been learned from looking closely at one-tenth of a percent of the human genome. Gene. 1993 Dec 15;135(1-2):167–173. doi: 10.1016/0378-1119(93)90062-8. [DOI] [PubMed] [Google Scholar]