Abstract
Purpose We evaluated the safety, tolerability, and pharmacokinetics (PK) of EMD 525797 (DI17E6), a humanized monoclonal antibody targeting αv-integrins, in healthy subjects. Methods In this first-in-human, double-blind, placebo-controlled, randomized Phase 1 study, healthy male volunteers were consecutively assigned to 6 ascending single-dose cohorts of 35, 100, 250, 500, 1000, or 1500 mg. Per dose cohort, EMD 525797 or placebo was administered over 1 h as an intravenous 250-mL infusion to 6 and 3 volunteers, respectively. Escalation to the next dose level was based on evaluation of safety, tolerability, and PK data. Results Fifty-five subjects (aged 18–45 years) were randomized. Twenty-seven of 37 (73 %) subjects receiving EMD 525797 reported a total of 61 adverse events (AEs), including 38 events (in 17 subjects) considered by the investigator to be treatment related. A total of 35 AEs were reported by 14 of 18 (78 %) placebo-treated subjects. The most commonly occurring AEs were gastrointestinal disorders, abnormal laboratory values, and increased or decreased biochemistry and/or hematology values, as well as headaches, which occurred at a slightly higher frequency in the EMD 525797 group compared with placebo. There were no serious AEs or deaths. EMD 525797 PK appeared to be dose dependent, especially at lower doses. Conclusion Ascending single doses of EMD 525797 were shown to be safe and well tolerated. No safety concerns were identified. This study supports the ongoing investigation of EMD 525797.
Keywords: αv integrins overexpression, EMD 525797 (DI17E6)
Introduction
Integrins are a large family of heterodimeric transmembrane glycoproteins consisting of an alpha (α) and a beta (ß) subunit. They mediate a wide range of cell-to-extracellular matrix (ECM) and cell-to-cell adhesive interactions that occur as part of normal tissue function and in diverse human pathologies [1]. Integrins have been shown to play a role in cellular proliferation and the regulation of cell-cycle progression [2], cellular invasion and migration [3, 4], cell signaling [3, 4], and as regulators of gene transcription [5]. In cancer, integrins have demonstrated a direct role in tumor progression via their effects on tumor cell survival, angiogenesis, and metastasis [6, 7]. As a result, agents that specifically target integrin function have potential as anticancer therapies. Thus far, many therapeutics targeting integrins are in clinical development in and outside of oncology, including 5 that have been approved for clinical use in areas such as multiple sclerosis or thrombosis [1].
The αv-integrins are a subfamily of integrins composed of 5 members whose functions include regulation of cell adhesion to ECM, and cellular proliferation and migration [5, 6]. These αv-integrins are highly expressed on tumor cells and the tumor vasculature of many human cancers [8–10]. Because of the nature and pathologic functions of integrins, a lasting integrin inhibition would be desirable. Therefore, antibodies like EMD 525797 were designed to inhibit cell-cell interactions mediated by one or more of these integrins [11].
EMD 525797 (DI17E6) is a novel, humanized monoclonal IgG2 antibody specifically directed against the αv-subunit of human integrin receptors [1]. This antibody inhibits ligand binding to all αv heterodimers (αvß1, αvß3, αvß5, αvß6, αvß8) without cross-reacting with other members of the integrin family. The binding of EMD 525797 to the αv heterodimers antagonizes their interaction with cognate ligands in the ECM [1], preventing cell attachment and motility, which can trigger apoptosis. As preclinical data have shown that EMD 525797 targets tumor cells and the microenvironment including angiogenic blood vessels, and inhibits tumor growth in mouse xenograft human tumor models (unpublished data), further clinical development was warranted. Results from a Phase 1 trial in patients with progressive castration-resistant prostate cancer with bone metastases after chemotherapy showed EMD 525797 to be well tolerated with potential antitumor activity [12].
Herein, the results of the first-in-human, Phase 1, randomized, double-blind, placebo-controlled study of EMD 525797 in healthy volunteers are reported, evaluating the safety, tolerability, and pharmacokinetics (PK) of single ascending intravenous doses of EMD 525797 up to 1500 mg.
Methods
Subjects
Eligible subjects were healthy male volunteers aged 18 to 45 years who had given their written informed consent. They were required to be Caucasian with a weight of 55 to 105 kg and a body mass index of 19 to 29.9 kg/m2. The subjects had to be healthy, particularly with respect to physical examination, lung function, vital signs, 12-lead electrocardiogram (ECG), and laboratory tests (such as hematologic, biochemistry, coagulation, and urine analyses). Main exclusion criteria were evidence of clinically relevant pathology, particularly severe hepatic or renal impairment, presence of any immunologic disease, history of moderate or severe hypertension, hypotension, or bleeding disorders, history of neurologic disorders and/or epilepsy; history of hypersensitivity to immune globulin preparations; regular smoking consumption of more than 5 cigarettes or equivalent per day; consumption of large quantities of methylxanthine-containing beverages and inability to abstain from caffeine consumption during study participation; history of thromboembolic events; and major surgery within 6 months of screening.
Study design
Healthy volunteers were consecutively assigned to 6 ascending single-dose cohorts of 35, 100, 250, 500, 1000, and 1500 mg EMD 525797. EMD 525797 or placebo was administered over 1 h as an intravenous 250-mL infusion without premedication. Within each dose group, 6 volunteers were randomized to receive EMD 525797 and 3 to receive placebo. The study drug was sequentially administered to subgroups of 2 then 3 then 4 subjects (including a single subject receiving placebo in each subgroup), with the decision to expose more subjects within each cohort to study drug based on assessment of safety and tolerability findings at the end of a 2-day observation period. Escalation to the next dose level was based on evaluation of safety, tolerability, and PK data. Subjects were followed for up to 42 days.
Study objectives
The primary objectives were to assess the safety, tolerability, and PK profile of EMD 525797 administered as one single dose in healthy male volunteers at various dose levels. The secondary objective was to evaluate the influence of EMD 525797 on pharmacodynamic parameters.
Study assessments
Safety and tolerability measures included the assessment of adverse events (AEs), vital signs, physical examination, and (safety) laboratory parameters. Blood samples were obtained for assessment of serum concentrations of EMD 525797 using a validated ELISA method with a quantification range of 233 to 10,000 ng/mL. PK parameters were calculated according to standard noncompartmental methods using KINETICA™ software, version 4.4.1. The presence of anti-EMD 525797 antibodies was investigated using a validated semi-quantitative two-stage format ELISA assay. In addition, we investigated the influence of EMD 525797 on pharmacodynamic parameters and safety biomarkers such as platelet activation, complement activity, endogenous thrombin potential (ETP), D-dimer, circulating endothelial cells, and circulating endothelial progenitor cells. The study drug was administered at an in-house facility, with subjects receiving supervision until 72 h after the start of infusion. Two additional in-house stays were from day 7 to 8 and day 41 to 42 to guarantee appropriate 24-hour urine collection for determination of the glomular filtration rate and α1-microglobulin, and an ophthalmologic examination on day 41.
Statistical analysis
Study results were analyzed using descriptive statistics. The safety population included all randomized subjects who had taken study drug. The PK population included all subjects randomized to receive EMD 525797 and who provided sufficient data for PK assessment of EMD 525797. AEs were listed per dose level and analyzed by intensity and relationship to the study drug. All other variables were analyzed descriptively per dose level. Dose proportionality for PK parameters was examined using the power model and by graphic means. No formal statistics comparisons were planned.
Results
Baseline characteristics and subject disposition
A total of 55 healthy male subjects ranging from 18 to 45 years of age were randomized, with 37 subjects receiving infusions of EMD 525797 and 18 subjects receiving placebo. A single subject assigned to receive EMD 525797 at the 1500-mg dose level withdrew for personal reasons. Therefore, 54 subjects completed the study according to protocol. Across all EMD 525797 dose groups and compared with placebo, demographic characteristics were similar (Table 1).
Table 1.
Baseline demographics of the healthy volunteers
| EMD 525797 | Placebo (Pooled) N = 18 | |||||||
|---|---|---|---|---|---|---|---|---|
| 35 mg | 100 mg | 250 mg | 500 mg | 1000 mg | 1500 mg | Overall | ||
| N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 7 | N = 37 | ||
| Age, years | ||||||||
| Mean (SD) | 33.7 (7.3) | 37.5 (7.2) | 29.3 (7.9) | 32.0 (9.3) | 36.7 (6.8) | 31.4 (7.3) | 33.4 (7.7) | 31.2 (7.6) |
| Range | 26–42 | 26–45 | 18–38 | 19–43 | 27–44 | 24–41 | 18–45 | 19–45 |
| Weight, kg | ||||||||
| Mean (SD) | 90.9 (6.5) | 81.9 (9.2) | 78.0 (6.0) | 67.9 (4.9) | 76.1 (7.0) | 76.6 (7.9) | 78.5 (9.5) | 79.7 (9.5) |
| Range | 81.8–97.3 | 72.7–98.4 | 69.9–85.1 | 63.2–76.4 | 68.4–88.6 | 67.4–92.2 | 63.2–98.4 | 65.3–97.5 |
| Height, cm | ||||||||
| Mean (SD) | 184 (8.1) | 183 (4.8) | 180 (6.1) | 177 (7.5) | 178 (4.5) | 182 (5.4) | 181 (6.3) | 181 (6.4) |
| Range | 171–195 | 178–191 | 168–184 | 167–188 | 171–183 | 174–189 | 167–195 | 171–196 |
| BMI, kg/m2 | ||||||||
| Mean (SD) | 27.0 (1.7) | 24.4 (2.7) | 24.0 (1.4) | 21.7 (1.2) | 24.1 (1.6) | 23.2 (2.8) | 24.0 (2.5) | 24.3 (2.4) |
| Range | 25.0–29.5 | 22.2–29.7 | 21.8–25.5 | 19.9–23.1 | 22.1–26.5 | 21.2–29.4 | 19.9–29.7 | 19.9–28.2 |
BMI body mass index; SD standard deviation
Safety
Twenty-seven of 37 (73 %) subjects randomized to EMD 525797 reported a total of 61 AEs and 14 of 18 (78 %) subjects randomized to placebo reported a total of 35 AEs (Table 2). Thirty-eight AEs reported by 17 (46 %) subjects who received EMD 525797 and 21 AEs by 12 subjects (67 %) with placebo were considered by the investigator to be likely related to study drug. All but one of the AEs reported were considered by the investigator to be of mild or moderate intensity (Table 2); only an increased C-reactive protein (CRP) value observed in a subject in the EMD 525797 500-mg dose group was assessed as severe. This subject was additionally suffering from back pain, headache, increased body temperature with sweating and shivering, stomach cramps, and vomiting within 24 h and 6 days after EMD 525797 dosing, all AEs of moderate intensity. No serious AEs occurred and no subject died. Across all dose groups, the majority of AEs occurred within 48 h of dosing and all were resolved at the end of the study. In general, AEs occurred at a similar incidence in the overall EMD 525797 group and the placebo group. The most commonly occurring treatment-emergent AEs according to system organ class were abnormal laboratory values (investigations), gastrointestinal disorders, infections and infestations, nervous system disorders, and general disorders and administration site conditions (Table 3).
Table 2.
Overview of reported AEsa, relationship to drug and intensity
| EMD 525797 | Placebo (Pooled) N = 18 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 35 mg | 100 mg | 250 mg | 500 mg | 1000 mg | 1500 mg | Overall | ||||||||||
| N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 7 | N = 37 | ||||||||||
| n (%) | E | n (%) | E | n (%) | E | n (%) | E | n (%) | E | n (%) | E | n (%) | E | n (%) | E | |
| Any AE | 4 (67) | 6 | 4 (67) | 6 | 3 (50) | 5 | 6 (100) | 18 | 5 (83) | 17 | 5 (71) | 9 | 27 (73) | 61 | 14 (78) | 35 |
| Relationship to drug | ||||||||||||||||
| Likely | 3 (50) | 5 | 3 (50) | 3 | 1 (17) | 2 | 4 (67) | 13 | 4 (67) | 12 | 2 (29) | 3 | 17 (46) | 38 | 12 (67) | 21 |
| Not likely | 1 (17) | 1 | 3 (50) | 3 | 3 (50) | 3 | 5 (83) | 5 | 3 (50) | 5 | 4 (57) | 6 | 19 (51) | 23 | 8 (44) | 14 |
| Intensity | ||||||||||||||||
| Mild | 1 (17) | 1 | 3 (50) | 3 | 2 (33) | 2 | 5 (83) | 6 | 4 (67) | 6 | 3 (43) | 4 | 18 (49) | 22 | 9 (50) | 15 |
| Moderate | 4 (67) | 4b | 3 (50) | 3 | 1 (17) | 3 | 4 (67) | 11 | 4 (67) | 11 | 4 (57) | 5 | 20 (54) | 37 | 13 (72) | 19 |
| Severe | - | - | - | - | - | - | 1 (17) | 1 | - | - | - | - | 1 (3) | 1 | - | - |
aSubjects could report more than 1 AE
bOf 2 observed AEs “creatinine renal clearance decreased,” only 1 was counted
AE, adverse event; E, number of events; N/n, number of subjects reporting AEs
Table 3.
Overview of subjects reporting TEAEs according to MedDRA System Organ Class and Preferred Term
| EMD 525797 | Placebo (Pooled) N = 18 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 35 mg | 100 mg | 250 mg | 500 mg | 1000 mg | 1500 mg | Overall | ||||||||||
| N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 7 | N = 37 | ||||||||||
| n (%) | E | n (%) | E | n (%) | E | n (%) | E | n (%) | E | n (%) | E | n (%) | E | n (%) | E | |
| Eye disorders | ||||||||||||||||
| Eye irritation | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 (6) | 1 |
| Gastrointestinal disorders | ||||||||||||||||
| Abdominal pain | - | - | - | - | - | - | 1 (17) | 1 | - | - | - | - | 1 (3) | 1 | - | - |
| Abdominal pain (upper) | - | - | - | - | - | - | 1 (17) | 1 | - | - | - | - | 1 (3) | 1 | - | - |
| Diarrhea | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 (6) | 1 |
| Flatulence | - | - | - | - | - | - | - | - | 1 (17) | 1 | - | - | 1 (3) | 1 | - | - |
| Nausea | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 (6) | 2 |
| Toothache | - | - | - | - | - | - | 1 (17) | 1 | - | - | - | - | 1 (3) | 1 | - | - |
| Vomiting | - | - | - | - | - | - | 1 (17) | 1 | - | - | - | - | 1 (3) | 1 | - | - |
| General disorders and administration site conditions | ||||||||||||||||
| Asthenia | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 (6) | 1 |
| Chest discomfort | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 (6) | 1 |
| Chills | - | - | - | - | - | - | 1 (17) | 1 | 1 (17) | 1 | - | - | 2 (5) | 2 | - | - |
| Infusion site erythema | - | - | 1 (17) | 1 | - | - | - | - | - | - | - | - | 1 (3) | 1 | - | - |
| Infusion site pain | - | - | - | - | - | - | - | - | - | - | 1 (14) | 1 | 1 (3) | 1 | 1 (6) | 1 |
| Infusion site swelling | - | - | - | - | 1 (17) | 1 | - | - | - | - | - | - | 1 (3) | 1 | - | - |
| Infections and infestations | ||||||||||||||||
| Nasopharyngitis | - | - | 1 (17) | 1 | - | - | 1 (17) | 1 | 1 (17) | 1 | 4 (57) | 4 | 7 (19) | 7 | 5 (28) | 5 |
| Rhinitis | - | - | - | - | - | - | 1 (17) | 1 | 1 (17) | 1 | - | - | 2 (5) | 2 | - | - |
| Investigations | ||||||||||||||||
| Blood creatine phosphokinase increased | - | - | - | - | - | - | - | - | 1 (17) | 1 | - | - | 1 (3) | 1 | 1 (6) | 1 |
| Blood glucose decreased | - | - | - | - | - | - | - | - | 1 (17) | 1 | - | - | 1 (3) | 1 | - | - |
| Blood magnesium increased | - | - | - | - | - | - | - | - | 1 (17) | 1 | - | - | 1 (3) | 1 | - | - |
| Blood triglycerides increased | - | - | - | - | - | - | - | - | 1 (17) | 1 | - | - | 1 (3) | 1 | - | - |
| Body temperature increased | - | - | - | - | - | - | 1 (17) | 1 | 1 (17) | 1 | - | - | 2 (5) | 2 | - | - |
| C-reactive protein increased | - | - | - | - | - | - | 1 (17) | 1 | 1 (17) | 1 | - | - | 2 (5) | 2 | 1 (6) | 1 |
| Complement factor decreased | - | - | - | - | - | - | - | - | - | - | 1 (14) | 1 | 1 (3) | 1 | - | - |
| Creatinine renal clearance decreased | 1 (17) | 2 | - | - | 1 (17) | 1 | - | - | - | - | 1 (14) | 1 | 3 (8) | 4 | 3 (17) | 3 |
| Glutamate dehydrogenase increased | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 (6) | 1 |
| High-density lipoprotein decreased | - | - | - | - | - | - | - | - | 1 (17) | 1 | - | - | 1 (3) | 1 | - | - |
| Interleukin 8 level increased | 2 (33) | 2 | 3 (50) | 3 | 1 (17) | 1 | 1 (17) | 1 | - | - | - | - | 7 (19) | 7 | 6 (33) | 6 |
| Lymphocyte count decreased | - | - | - | - | - | - | 1 (17) | 1 | - | - | - | - | 1 (3) | 1 | - | - |
| Neutrophil count increased | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 (6) | 1 |
| White blood cell count increased | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 (6) | 1 |
| TNFα increased | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 (6) | 1 |
| Metabolism and nutrition disorders | ||||||||||||||||
| Appetite decreased | 1 (17) | 1 | - | - | - | - | - | - | 1 (17) | 1 | - | - | 2 (5) | 2 | - | - |
| Musculoskeletal and connective tissue disorders | ||||||||||||||||
| Back pain | - | - | - | - | - | - | 1 (17) | 1 | 1 (17) | 1 | - | - | 2 (5) | 2 | - | - |
| Pain in extremity | - | - | - | - | - | - | - | - | 1 (17) | 1 | - | - | 1 (3) | 1 | 1 (6) | 1 |
| Nervous system disorders | ||||||||||||||||
| Headache | 1 (17) | 1 | 1 (17) | 1 | - | - | 4 (67) | 4 | 1 (17) | 1 | - | - | 7 (19) | 7 | 2 (11) | 2 |
| Hypoaesthesia | - | - | - | - | 1 (17) | 1 | - | - | - | - | - | - | 1 (3) | 1 | - | - |
| Respiratory, thoracic, and mediastinal disorders | ||||||||||||||||
| Pharyngolaryngeal pain | - | - | - | - | - | - | 1 (17) | 1 | 1 (17) | 1 | - | - | 2 (5) | 2 | 1 (6) | 1 |
| Skin and subcutaneous tissue disorders | ||||||||||||||||
| Hyperhidrosis | - | - | - | - | - | - | 1 (17) | 1 | 1 (17) | 1 | - | - | 2 (5) | 2 | - | - |
| Rash papular | - | - | - | - | - | - | - | - | - | - | 1 (14) | 1 | 1 (3) | 1 | - | - |
| Skin irritation | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 (6) | 1 |
| Vascular disorders | ||||||||||||||||
| Hematoma | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 2 (11) | 2 |
| Hot flush | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 (6) | 1 |
MedDRA medical dictionary for regulatory activities; TEAE treatment-emergent adverse event; TNFα tumor necrosis factor alpha
Overall, treatment with EMD 525797 in ascending doses from 35 to 1500 mg was safe and well tolerated. Although the majority of AEs occurred in the 500-mg (n = 18, 30 %) and 1000-mg (n = 17, 28 %) dose groups, there were no obvious trends suggesting a possible dose relationship. There was no evidence of accumulation of any specific event within the individual dose cohorts as the number of events in the 1500-mg dose group (n = 9, 15 %) was similar to the number of events in each of the 35-mg (n = 6, 10 %), 100-mg (n = 6, 10 %), and 250-mg (n = 5, 8 %) dose groups.
Clinical laboratory evaluations, including hematology, biochemistry, coagulation, and urinalysis, did not reveal any dose- or treatment-related changes over time after infusion of EMD 525797 or placebo. Similarly, there were no clinically relevant findings based on vital signs, 12-lead ECG, telemetry and spirometry data, or physical or ophthalmologic examinations to indicate a signal that could be explained by exposure to study drug. Finally, anti-EMD 525797 antibodies were detected in 7 of 37 (19 %) of subjects. However, the presence of such antibodies was not associated with clinical signs or symptoms.
The healthy volunteers rated the safety and tolerability of the received treatment exclusively as good and very good overall. Except for a moderate assessment for the subject of the 500-mg dose group with increased CRP values, the investigator’s assessment of the safety and tolerability of the administered treatment was also very good to good. The local tolerability was considered to be good to very good as well.
Pharmacokinetics
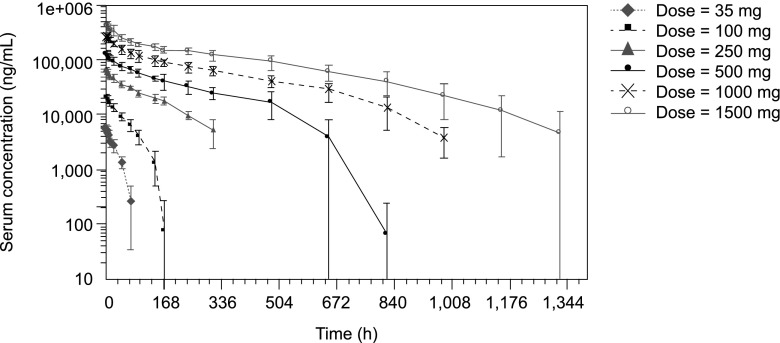
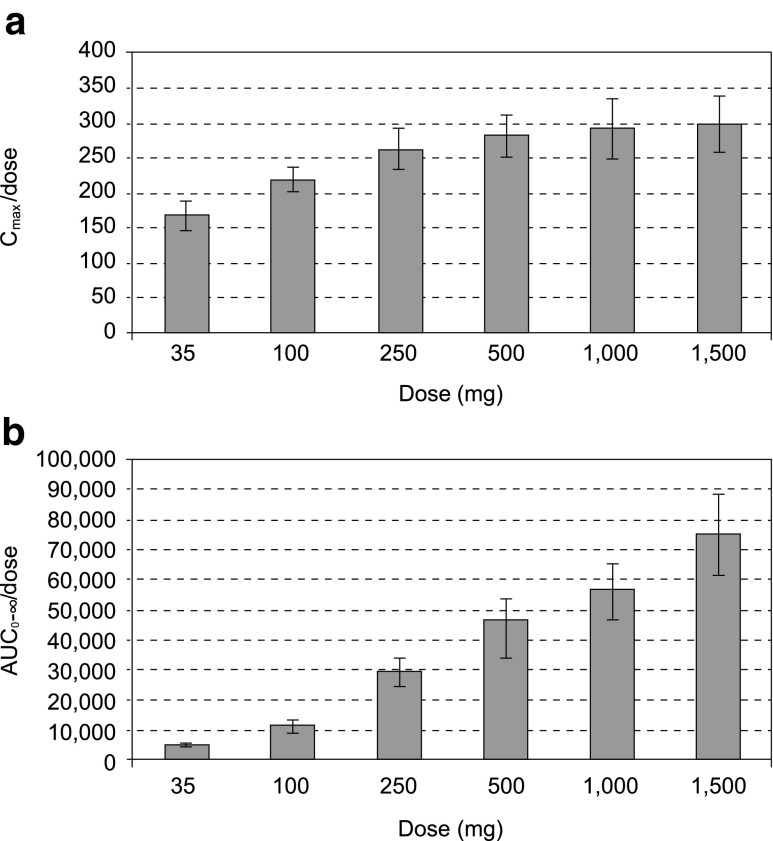
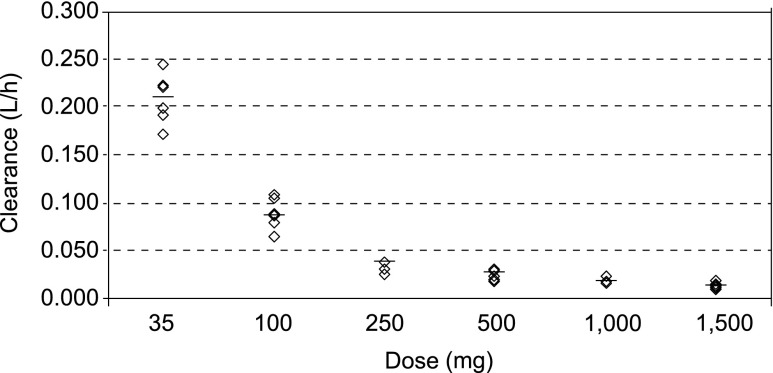
Maximum serum concentrations of EMD 525797 were reached 1 to 2 h after start of administration (range 1−8 h over the dose groups) with maximum serum concentration (Cmax) values increasing nearly in proportion to dose (Figs. 1 and 2a). Exposure expressed as area under the serum concentration-time curve (AUC) increased more than proportionally across the 35-mg to 1500-mg dose range (Fig. 2b). EMD 525797 was eliminated from serum with a half-life of 19.3 h in the 35-mg dose group and 246.1 h in the 1500-mg dose group (Table 4). Accordingly, total clearance (CL) decreased from 0.209 L/h at the 35-mg dose to 0.014 L/h at 1500 mg. This dose-dependency was more pronounced in the lower-dose groups, but at doses above 250 mg CL started to level off, indicating a first-order and saturable elimination pathway (Fig. 3). The volume of distribution (Vz) was small and did not markedly change with dose. Mean Vz values ranged from 4.1 to 5.9 L, indicating a low distribution of EMD 525797 into the extravascular space (Table 4). The presence of anti-EMD 525797 antibodies, as detected in 19 % of subjects, did not influence the PK of EMD 525797.
Fig. 1.
Serum concentration-time courses of EMD 525797 for all investigated dose groups. Values are presented as mean ± standard deviation on a semi-logarithmic scale
Fig. 2.
Dose-adjusted Cmax (a) and AUC (b) of EMD 525797 for all dose groups. Values are presented as mean ± standard deviation. AUC, area under the concentration-time curve; Cmax, maximum serum concentration
Table 4.
EMD 525797 PK data
| EMD 525797 | ||||||
|---|---|---|---|---|---|---|
| 35 mg | 100 mg | 250 mg | 500 mg | 1000 mg | 1500 mg | |
| N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | |
| Cmax, μg/mL | ||||||
| Mean | 5.9 | 21.9 | 65.7 | 141 | 292 | 449 |
| (SD) | (0.76) | (1.75) | (7.48) | (15.2) | (42.0) | (58.7) |
| Range | 4.9–6.5 | 19.3–24.5 | 54.8–75.1 | 122–158 | 229–358 | 368–521 |
| Tmax, h | ||||||
| Median | 1.3 | 2.0 | 1.5 | 1.8 | 3.5 | 2.0 |
| Range | 1.0–4.0 | 1.5–3.0 | 1.0–2.0 | 1.0–4.0 | 1.5–8.0 | 1.0–6.0 |
| AUC0-∞, μg/mL•h | ||||||
| Mean | 169 | 1155 | 7350 | 21834 | 55811 | 112023 |
| (SD) | (20.2) | (212.6) | (1158) | (4936) | (9042) | (20183) |
| Range | 144–201 | 932–1513 | 6538–9421 | 17438–28809 | 43219–64439 | 81964–141859 |
| t1/2, h | ||||||
| Mean | 19.3 | 32.5 | 92.6 | 128.7 | 206.3 | 246.1 |
| (SD) | (3.5) | (6.8) | (24.4) | (35.1) | (24.8) | (79.1) |
| Range | 13.3–23.2 | 20.9–40.3 | 61.2–129.3 | 95.7–174.4 | 178.0–248.0 | 156.7–356.2 |
| CL, L/h | ||||||
| Mean | 0.209 | 0.089 | 0.035 | 0.024 | 0.018 | 0.014 |
| (SD) | (0.024) | (0.015) | (0.005) | (0.005) | (0.003) | (0.003) |
| Range | 0.18–0.24 | 0.07–0.11 | 0.03–0.04 | 0.02–0.03 | 0.02–0.02 | 0.01–0.02 |
| Vz, L | ||||||
| Mean | 5.9 | 4.1 | 4.5 | 4.3 | 5.4 | 4.7 |
| (SD) | (1.5) | (0.6) | (0.9) | (0.9) | (0.9) | (0.9) |
| Range | 4.2–8.1 | 3.1–4.8 | 3.4–5.8 | 3.2–5.8 | 4.4–6.5 | 3.5–5.7 |
AUC area under the concentration-time curve; CL clearance; C max maximum serum concentration; PK pharmacokinetic; SD standard deviation; t1/2, half-life; T max time to maximum serum concentration; V z volume of distribution
Fig. 3.
Clearance of EMD 525797 by dose group
Pharmacodynamics and safety biomarkers
Assessments indicated that EMD 525797 did not induce clinically relevant changes in endogenous thrombin potential, D-dimer, platelet activation, tumor necrosis factor alpha (TNFα), interleukin 8 (IL-8), or 50 % hemolytic complement (CH50) in this population of healthy subjects. No apparent relationship between EMD 525797 serum concentrations and these various pharmacodynamic parameters and safety biomarkers could be detected. It was not possible to draw statistically affirmed conclusions concerning the counts of circulating endothelial cells and circulating endothelial progenitor cells per volume of blood due to the high standard deviations and low sample size.
Discussion
The results of this first-in-human, Phase 1, single-center, randomized, double-blind, placebo-controlled study showed that EMD 525797 at single ascending intravenous doses ranging from 35 to 1500 mg did not raise any major safety issues and was well tolerated in healthy male subjects.
No dose dependency was confirmed in the distribution of AEs and there was no evidence of accumulation of any specific event within the individual dose cohorts. Furthermore, there were no clinically relevant dose-related changes in any of the safety parameters assessed. Seventeen subjects experienced AEs that were considered related to EMD 525797 treatment. There were no serious AEs and no deaths. The majority of AEs occurred within 48 h of dosing and was of a mild or moderate intensity in all subjects, except for an increase in CRP level assessed as severe in one subject treated with 500 mg EMD 525797. All AEs were resolved without exception at the end of the study. All classes of AE were reported at a similar level to placebo, except for headaches (nervous system disorder class), an AE that occurred at a slightly higher frequency in the EMD 525797 group compared to the placebo group (7 of 37 vs 2 of 18 subjects). However, no dose relationship observed. Headache is frequently seen in early drug development studies and a pattern as observed in this study is common.
Single-dose administration of EMD 525797 showed that the PKs of this compound were dose-dependent, particularly at lower doses. This nonlinear PK behavior was driven by first-order and saturable elimination, which was mostly evident in the range of 35 to 250 mg. In this dose range, antibody half-life was not constant but increased markedly with dose. Thus, at low concentrations the level of receptor binding predominantly determines antibody clearance, whereas at doses above 250 mg where receptors can be assumed to be saturated, catabolism is the primary mechanism associated with antibody clearance. Total clearance started to level off at doses above 250 mg, with a further slight trend to decrease with dose. This was also reflected in the dose-dependency of the area under the curve extrapolated to infinity, for which levels increased more than proportionally between 35 and 1500 mg.
Although anti-EMD 525797 antibodies were detected in 7 of 37 (19 %) subjects, no impact on either PK or safety was observed. Moreover, EMD 525797 did not produce any clinically relevant changes in various pharmacodynamic and safety measures, including ETP, D-dimer, platelet activation, and several immunologic parameters. These findings suggest there was no apparent relationship between EMD 525797 serum concentrations and the assessed pharmacodynamics and safety parameters.
In conclusion, ascending single doses of EMD 525797 (ranging from 35 to 1500 mg) were shown to be safe and well tolerated in this first-in-human study of healthy subjects, with most reported AEs of a similar incidence for EMD 525797 and placebo. No safety concerns were identified and the PK findings suggest that EMD 525797 is eliminated in a manner consistent with other antibodies targeting membrane-associated receptors. This study supported further investigation of EMD 525797 in randomized trials and provided a basis for dose selection in currently ongoing trials of EMD 525797 in metastatic castrate-resistant prostate cancer and colorectal cancer patients.
Acknowledgments and disclosures
This trial was sponsored by Merck KGaA, Darmstadt, Germany. Editorial and medical writing assistance in the preparation of this manuscript was provided by Anna Hooijkaas, PhD, and Marianne Jenal-Eyholzer, PhD, CMPP, TRM Oncology, The Hague, The Netherlands, funded by Merck KGaA, Darmstadt, Germany.
Conflicts of interest
WU, MZ, AK: Employees of Merck KGaA, Darmstadt, Germany; UF: Former employee of Merck KGaA, Darmstadt, Germany; TK: no conflicts of interest.
Contributor Information
Wolfgang Uhl, Phone: +49-6151-727042, FAX: +49-6151-723163, Email: Wolfgang.Uhl@merckgroup.com.
Michael Zühlsdorf, Phone: +49-6151-727493, FAX: +49-6151-72917493, Email: michael.zuehlsdorf@merckgroup.com.
Thomas Koernicke, Phone: +49-30-306859676, FAX: +49-30-306857017, Email: Thomas.Koernicke@parexel.com.
Ulf Forssmann, Phone: +49-30-468192055, Email: ulf.forssmann@bayer.com.
Andreas Kovar, Phone: +49-6151-726690, Email: andreas.kovar@merckgroup.com.
References
- 1.Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci. 2012;33:405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11:48–53. doi: 10.1016/S0959-437X(00)00155-6. [DOI] [PubMed] [Google Scholar]
- 3.Holly SP, Larson MK, Parise LV. Multiple roles of integrins in cell motility. Exp Cell Res. 2000;261:69–74. doi: 10.1006/excr.2000.5040. [DOI] [PubMed] [Google Scholar]
- 4.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 5.Legate KR, Montañez E, Kudlacek O, Fässler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth JA, Nakada MT, Trikha M, Lang Z, Gordon MS, Jayson GC, Corringham R, Prabhakar U, Davis HM, Beckman RA. Alpha-v integrins as therapeutic targets in oncology. Cancer Invest. 2007;25:632–646. doi: 10.1080/07357900701522638. [DOI] [PubMed] [Google Scholar]
- 7.Chen K, Chen X. Integrin targeted delivery of chemotherapeutics. Theranostics. 2011;1:189–200. doi: 10.7150/thno/v01p0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20:321–331. doi: 10.1023/A:1015547830323. [DOI] [PubMed] [Google Scholar]
- 9.Slack-Davis JK, Parsons JT. Emerging views of integrin signaling: implications for prostate cancer. J Cell Biochem. 2004;91:41–46. doi: 10.1002/jcb.10665. [DOI] [PubMed] [Google Scholar]
- 10.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami RK, Bajjuri KM, Forsyth JS, Das S, Hassenpflug W, Huang ZZ, Lerner RA, Felding-Habermann B, Sinha SC. Chemically programmed antibodies targeting multiple alpha(v) integrins and their effects on tumor-related functions in vitro. Bioconjug Chem. 2011;22:1535–1544. doi: 10.1021/bc2000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wirth M, Heidenreich A, Gschwend JE, Gil T, Zastrow S, Laniado M, Gerloff J, Zühlsdorf M, Mordenti G, Uhl W, Lannert H. A multicenter phase 1 study of EMD 525797 (DI17E6), a novel humanized monoclonal antibody targeting αv integrins, in progressive castration-resistant prostate cancer with bone metastases after chemotherapy. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.05.051. [DOI] [PubMed] [Google Scholar]