Abstract
In Ph− myeloproliferative neoplasms, the quantification of the JAK2V617F transcripts may provide some advantages over the DNA allele burden determination. We developed a q-RT-PCR to assess the JAK2WT and JAK2V617F mRNA expression in 105 cases (23 donors, 13 secondary polycythemia, 22 polycythemia vera (PV), 38 essential thrombocythemia (ET), and 9 primary myelofibrosis (PMF)). Compared with the standard allele-specific oligonucleotide (ASO)-PCR technique, our assay showed a 100 % concordance rate detecting the JAK2V617F mutation in 22/22 PV (100 %), 29/38 (76.3 %) ET, and 5/9 (55.5 %) PMF cases, respectively. The sensitivity of the assay was 0.01 %. Comparing DNA and RNA samples, we found that the JAK2V617F mutational ratios were significantly higher at the RNA level both in PV (p = 0.005) and ET (p = 0.001) samples. In PV patients, JAK2WT expression levels positively correlated with the platelets (PLTs) (p = 0.003) whereas a trend to negative correlation was observed with the Hb levels (p = 0.051). JAK2V617F-positive cases showed the lowest JAK2WT and ABL1 mRNA expression levels. In all the samples, the expression pattern of beta-glucoronidase (GUSB) was more homogeneous than that of ABL1 or β2 microglobulin (B2M). Using GUSB as normalizator gene, a significant increase of the JAK2V617F mRNA levels was seen in two ET patients at time of progression to PV. In conclusion, the proposed q-RT-PCR is a sensitive and accurate method to quantify the JAK2 mutational status that can also show clinical correlations suggesting the impact of the residual amount of the JAK2WT allele on the Ph− MPN disease phenotype. Our observations also preclude the use of ABL1 as a housekeeping gene for these neoplasms.
Electronic supplementary material
The online version of this article (doi:10.1007/s00277-013-1920-0) contains supplementary material, which is available to authorized users.
Keywords: Ph− myeloproliferative neoplasms, JAK2WT level, JAK2V617F level, Housekeeping gene, q-RT-PCR
Introduction
Philadelphia chromosome-negative myeloproliferative neoplasms (Ph− MPNs) are clonal myeloid disorders characterized by an increased production of terminally differentiated cells. The mechanisms of MPN initiation and progression have been extensively studied. A number of genetic and epigenetic abnormalities associated with Ph− MPNs have been reported [1–5]. The somatic mutation of JAK2V617F is the most frequent genetic alteration in these diseases and is presently considered as a major diagnostic criterion for Ph− MPNs. The frequency of this mutation is greater than 95 % in patients with polycythemia vera (PV) and around 50 % in patients with essential thrombocythemia (ET) or primary myelofibrosis (PMF).
JAK2V617F-positive Ph− MPNs show a biological continuum with clinical presentation, which is in part influenced by the JAK2V617F mutational load [6]. A relationship between JAK2V617F mutational burden and disease phenotype has been reported [7].
Although the clinical relevance of the correct quantification of the JAK2 allele burden is still not clearly stated, the JAK2 tyrosine kinase activity is now a therapeutic target for innovative and more specific treatment of these diseases [8]. The still debated pathogenesis of MPNs forces to seek methods which are increasingly specific and sensitive for the quantification of the JAK2 allele burden at diagnosis and during treatment. To date, the detection and quantification of the JAK2WT and JAK2V617F alleles are usually assessed using genomic DNA. However, the quantification of JAK2V617F mRNA transcripts by a real-time quantitative RT-PCR (q-RT-PCR) may provide some advantages over the DNA allele burden [9]. In PV mononuclear cell samples, Zhao et al. [10] demonstrated that the ratio JAK2V617F/JAK2WT is higher in cDNA than in genomic DNA. Using an ARMS assay on cDNA originated from granulocyte mRNAs, Vannucchi et al. [11] identified more JAK2-mutated transcripts in MPN patients (9 %), as compared to conventional allele-specific PCR. Moreover, the mRNA (but not DNA) can be extracted from platelets present in buffy coats, and this may increase the JAK2V617F assay sensitivity in ET patient samples. Finally, in transgenic mice models, the JAK2V617F transcript levels were found to be strictly correlated with the Ph− MPN phenotypes [12]. By contrast, q-RT-PCR requires a parallel amplification of a housekeeping gene (HKG) as control gene (CG) to correct variations in RNA quality and quantity and to calculate the sensitivity of each measurement. Therefore, to better investigate the pathogenesis complexity of the Ph− MPNs and to provide a useful assay to monitor minimal residual disease (MRD), we set up an absolute q-RT-PCR method for the quantification of JAK2WT and JAK2V617F mRNA. The data herein reported show that this method is highly specific and sensitive. Moreover, the observation of a significant variability within the sample groups of the expression levels of ABL1, one of the most commonly used HKGs, leads us to test other genes as more appropriate CGs in this clinical setting.
Materials and methods
Patients and samples
Peripheral blood buffy coat specimens were collected from 105 individuals. Twenty-two of them were diagnosed as having PV, 38 ET, and 9 PMF according to the 2008 WHO diagnostic criteria [13]. Thirteen patients with secondary polycythemia (SP) and 23 healthy blood donors were included as controls. For three patients with initial diagnosis of ET who progressed to PV, paired samples of both disease phases were available. All specimens were collected after patients had signed an informed consent. The study was approved by our IRB at AUSL Latina (no. 6315/A001/2012).
DNA, RNA extraction, and cDNA synthesis
Cell pellets were either processed for DNA purification using the Wizard Genomic DNA Purification Kit (Promega Corporation, Madison, WI), according to the manufacturer’s instructions, or resuspended in guanidine isothiocyanate for RNA extraction [14, 15]. Equal amounts of RNA (1 μg) were reverse transcribed into cDNAs with random hexamers and MuLV reverse transcriptase (Applied Biosystems, Monza, Italy).
JAK2V617F mutation analysis and allele burden assay
JAK2V617F mutation was detected in DNA samples using the allele-specific PCR method previously described by Baxter et al. [16] The quantification of JAK2V617F allele burden on genomic DNA was performed using the JAK2V617F MutaQuant® Allele kit (Qiagen, Milano, Italy), according to the manufacturer’s instructions.
Allele-specific q-RT-PCR
To obtain reference curves, standard plasmids were manufactured to contain JAK2WT or JAK2V617F cDNA sequences. cDNAs were obtained from RNA extracted from human K562 cells (homozygous for JAK2WT allele) and HEL cells (homozygous for JAK2V617F allele). We designed specific primers (forward: TTCTGGATAAAGCACACAGAAA; reverse: CCAAATTTTACAAACTCCTGAACC), in order to amplify 150 bp including the V617F codon (NM_004972.3:c.1745-1895), using the Primer 3 software (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi). One-step cloning strategy was performed by using the TOPO TA Cloning® kit (Invitrogen, Monza, Italy). Each plasmid vector was checked by sequencing and includes five 10-fold serial dilutions, ranging from 105 to 10 targets per well.
JAK2WT and JAK2V617F mRNAs were measured as described by Merker et al. [17]. Primers and probe concentrations were optimized at concentrations of 300 and 200 nM, respectively, in a final volume of 25 μl. q-RT-PCR was performed in the ABI 7900 (Applied Biosystems) with an initial 10-min incubation at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. All samples and no-template controls were run in triplicates.
For each sample, the ABL1, B2M, and GUSB copy numbers were determined by using standard endogenous plasmid controls (FusionQuant® Standards Ipsogen, Qiagen), according to the manufacturer’s instructions and in accordance with the Europe Against Cancer (EAC) network [18].
JAK2 mutational burden evaluation
The mutational burden ratio of JAK2 was calculated using the DNA and RNA copy numbers according to the following formula: JAK2V617F/(JAK2V617F + JAK2WT).
Statistical analysis
Statistical analyses and graphs were generated using SPSS-10 software. Further, targeted pairwise comparisons to evaluate the significance between individual diseases were performed by the two-sided Student’s t test. Results were expressed as means ± SEM. The level of significance from t test was p < 0.05.
Results
Using the standard qualitative allele-specific oligonucleotide (ASO)-PCR method [17], the JAK2V617F was detected in 22/22 PV (100 %), in 29/38 (76.3 %) ET, and in 5/9 (55.5 %) PMF cases, respectively (Table 1). Our q-RT-PCR method confirmed the presence/absence of the JAK2V617F mutation in all the cases, resulting in a 100 % concordance rate with the standard assay. In addition, we did not observe nonspecific amplification in either JAK2WT or JAK2V617F plasmids or in positive or negative controls and JAK2V617F-positive and negative samples (Fig. S1 A, B). cDNA carrying a predetermined JAK2V617F copy number was diluted with cDNA from a healthy control to establish the sensitivity of our q-RT-PCR method. Serial dilution experiments demonstrated a detection limit of 0.01 % (Fig. S2 A, B).
Table 1.
The diagnostic main clinical–hematologic characteristics of the 105 individuals included in the present study grouped according to diagnosis
| Characteristics | Donors (n = 23) | SP (n = 13) | PV (n = 22) | ET (n = 38) | PMF (n = 9) | ||
|---|---|---|---|---|---|---|---|
| JAK2WT/V617F (n = 0) (n = 22; 100 %) | JAK2WT/V617F (n = 9; 19.5 %) (n = 29; 80.5 %) | JAK2WT/V617F (n = 4) (n = 5) | |||||
|
Age, median (years) (range) |
45 (26–61) |
59 (27–90) |
65 (52–88) |
63 (35–80) |
62 (38–81) |
75 (70–80) |
68 (47–83) |
| Gender, male/female | 16/7 | 13/0 | 17/5 | 4/5 | 20/29 | 2/4 | 3/5 |
|
Hb (g/dl), median (range) |
15 (11.5–16.9) |
17 (16–18.2) |
18 (17.6–20.2) |
12.5 (11.6–14) |
15 (12–19) |
9.6 (8.9–10) |
15.4 (13.8–17.3) |
|
WBC (1 × 109/l), median (range) |
6.6 (4.7–9.3) |
7.1 (4.8–11.4) |
10.6 (4.4–15) |
8.5 (6–10) |
9.9 (7.1–18) |
8 (1.3–16.8) |
20.6 (20–10.8) |
|
PLTs (1 × 109/l), median (range) |
232 (192–349) |
200.5 (91–274) |
370 (75–712) |
792 (631–1,420) |
718 (256–1,318) |
364 (129–489) |
555 (197–946) |
|
Neutrophils (1 × 109/l), median (range) |
4.4 (1.4–7) |
4.6 (1.4–7.5) |
7.9 (2.7–13) |
4.6 (2.8–7.6) |
6 (1.4–13.5) |
5.1 (1–9.4) |
15.9 (8.4–19.5) |
|
JAK2WT copy numbers (1 × 103), median (range) |
7.7 (1.2–38.5) |
8.7 (2.5–30.9) |
6.9 (0.02–22.6) |
15.6 (6.2–34.5) |
6.0 (0.9–16.7) |
11.2 (3.8–18.5) |
3.0 (0.3–4.5) |
|
JAK2V617F copy numbers (1 × 103), median (range) |
– | – |
26.9 (0.2–203.6) |
– |
6.9 (0.7–52.8) |
– |
29.4 (1.4–78.7) |
|
ABL1 copy number (1 × 103), median (range) |
4.6 (1.6–11.9) |
5.0 (2.4–20.6) |
2.9 (0.9–12.7) |
7.4 (1.8–17.7) |
3.8 (0.3–8.7) |
8.6 (4.6–14.9) |
3.3 (1.7–4.6) |
|
B2M copy number (1 × 106), median (range)a |
8.0 (2.8–10.1) |
4.8 (2.5–13.0) |
8.8 (2.4–17.7) |
6.1 (2.4–7.9) |
8.1 (2.4–17.3) |
6.0 |
7.0 (2.3–13.4) |
|
GUSB copy number (1 × 103), median (range)a |
37.6 (7.4–53.2) |
34.5 (3.2–58.7) |
37.8 (0.2–56.7) |
39.8 (8.6–71.6) |
33.2 (5.8–59.1) |
23.4 |
41.0 (19.3–93.5) |
a B2M and GUSB copy numbers were detected in donor (n = 11), SP (n = 5), PV (n = 16), ET (WT n = 7; V617F n = 24), and PMF (WT n = 1; V617F n = 4) samples
The main demographic, clinical, and laboratory characteristics of donors and patients, grouped according to initial diagnosis, together with their JAK2WT, JAK2V617F, ABL1, B2M, and GUSB median absolute copy numbers are reported in Table 1. Several lines of evidence demonstrate that the transition between PV, ET, and PMF is frequent in JAK2V617F-positive Ph− MPN patients, suggesting for these disease entities a biologic continuum [6]. Therefore, the analysis of the expression levels of the JAK2WT, ABL1, B2M, and GUSB genes was done, grouping Ph− MPN cases according to the presence/absence of the JAK2V617F mutation.
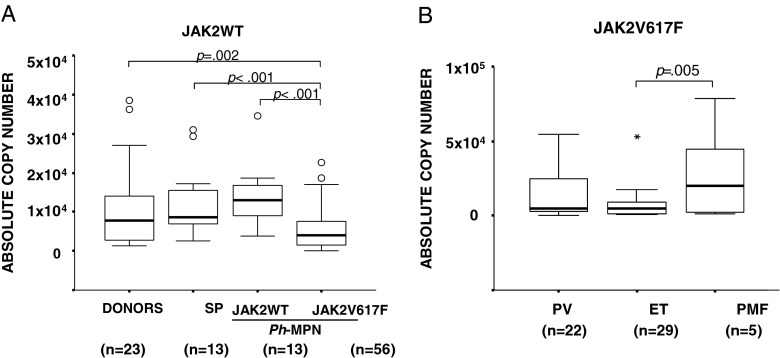
The median of absolute JAK2WT mRNA copy numbers detected in the donor, SP, and JAK2V617F-positive and JAK2V617F-negative Ph− MPN groups is depicted in Fig. 1a. JAK2V617F-positive patients presented a significantly lower JAK2WT mRNA expression compared with donors and SP and JAK2V617F-negative patients (p = 0.002; p < 0.001; p < 0.001, respectively). Significantly higher JAK2V617F mRNA expression levels were detectable in PMF patients when compared with ET cases; differences between PV and ET samples were not statistically significant (Fig. 1b).
Fig. 1.
JAK2WT and JAK2V617F mRNA expression levels detected by q-RT-PCR method. a JAK2WT absolute copy numbers, in donors, SP cases, and Ph− MPN patients. b JAK2V617F absolute copy numbers in JAK2V617F-positive Ph− MPN patients. Circles indicate the outlier values. Asterisks indicate the extreme values
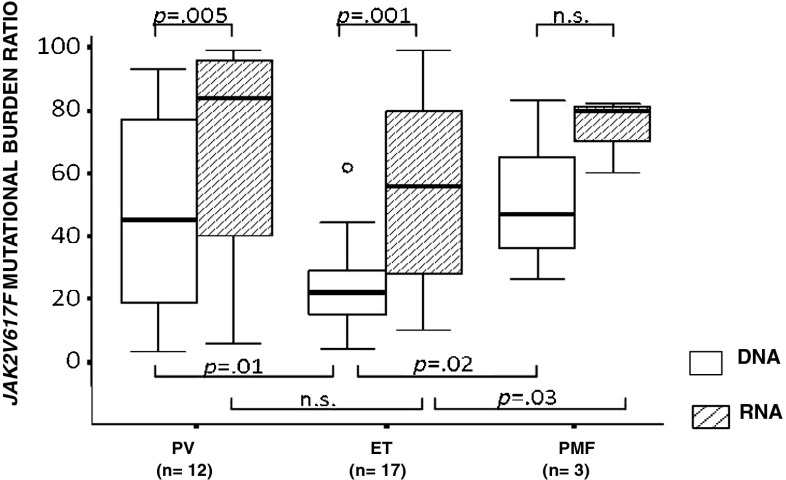
In a group of 12 PV, 17 ET, and 3 PMF, we matched the JAK2V617F mutational ratios, in patient-derived DNA and RNA samples. As shown in Fig. 2, with respect to DNA, ratios were significantly higher when assessed at the RNA level both in PV (p = 0.005) and ET (p = 0.001) samples and not statistically significant in PMFs (three cases). The comparisons of the DNA or RNA JAK2V617F mutational burden ratios showed significant differences whether they were calculated at the DNA or RNA level. At the DNA level, ET patients showed significantly lower JAK2V617F allele burden ratios as compared to both PV and PMF (22 % vs. 45 % and vs. 47 %, respectively [ET vs. PV p = 0.01; ET vs. PMF p = 0.02]) whereas, at the RNA level, significant differences were recorded only between ET and PMF patient groups (56 vs. 80 %; p = 0.03) (Fig. 2).
Fig. 2.
JAK2V617F mutational burden ratio, calculated at the DNA and RNA levels. The circle indicates the outlier values
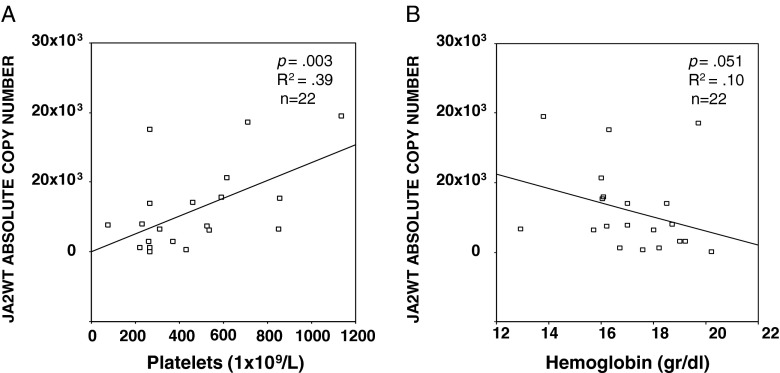
We did not observe significant associations correlating the JAK2WT or JAK2V617F expression levels and Hb, PLTs, WBC, splenomegaly, or occurrence of thrombosis in the entire patient group. Instead, considering the sole PV group, we observed a positive statistically significant correlation between the JAK2WT expression levels and PLT count (R 2 = 0.39; p = 0.003) and a trend to negative correlation between the JAK2WT expression levels and the Hb levels (R 2 = 0.10; p = 0.051) (Fig. 3a, b).
Fig. 3.
Correlations between the JAK2WT expression levels and PLT count (a) and Hb levels (b) in the PV patient group
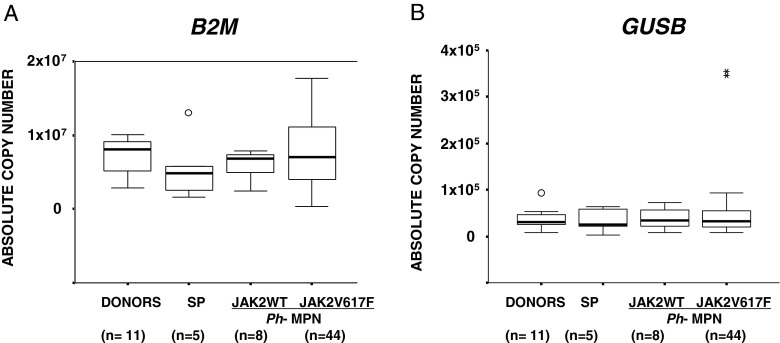
We also found that the ABL1 gene was not uniformly expressed among healthy donors and patient groups, being expressed at higher levels in donors and SP and JAK2V617F-negative patients compared to JAK2V617F-positive cases (Fig. 4a). A significant positive correlation between the copy numbers of ABL1 and JAK2WT was measured in JAK2V617F-positive patients (Fig. 4b). These observations raise several doubts about the appropriateness of using ABL1 as CG in Ph− MPN patients. Therefore, to identify CGs more suitable than ABL1, using the q-RT-PCR protocols optimized by the EAC program, we evaluated the B2M and GUSB expression levels in a group of 68 samples (donors = 11, SP = 5, PV = 16, ET = 31, and PMF = 5) with remaining available RNA. Both B2M and GUSB showed similar expression levels within all the groups examined. However, GUSB showed the most homogeneous medians and the narrowest distribution of values also in the JAJ2V617F-positive cases (Fig. 5a, b) where the expression levels of ABL1 were significantly affected. In 11 control samples, we assessed the Ct values of ABL1, B2M, GUSB, and JAK2 genes. In all cases, the variations in Ct values turned out to be comparable, and all fell within three Ct values. However, the Ct values of B2M were lower than those of ABL1, GUSB, and JAK2, thus indicating the higher level of expression of B2M in these samples (Fig. S3).
Fig. 4.
a ABL-1 absolute copy numbers in donors, SP cases, and Ph− MPN patients grouped according to the presence/absence of the JAK2V617F mutation. Circles indicate the outlier values. The asterisks indicate the extreme values. b Correlation between the ABL-1 and JAK2WT expression levels in the JAK2V617F-positive Ph− MPN patient group
Fig. 5.
B2M (a) and GUSB (b) absolute copy numbers in donors, SP cases, and Ph− MPN patients grouped according to the presence/absence of the JAK2V617F mutation. The circle indicates the outlier values. The asterisks indicate the extreme values
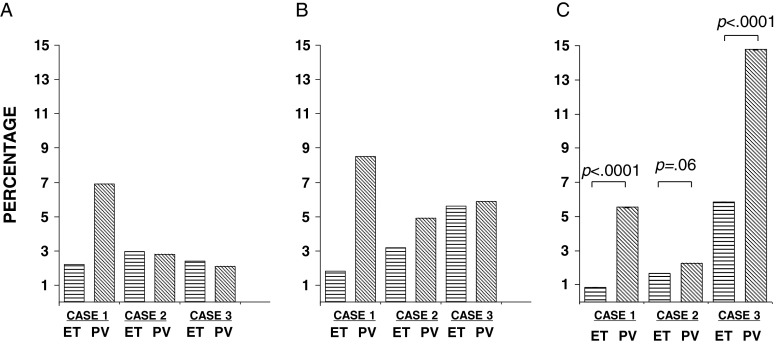
In three ET patients, who progressed to PV during this study, we could serially assess at ET diagnosis and PV progression the DNA and RNA JAK2V617F mutational ratios and the JAK2V617F expression levels normalized with GUS. The clinical–biologic parameters of these three patients are reported in Table 2. As illustrated in Fig. 6, we observed a significant increase of the JAK2V617F expression levels at PV progression in two of the three cases (case 1 and 3, Fig. 6c) and a trend toward statistical significance in the remaining case. By contrast, the JAK2 mutational ratios at DNA and RNA were increased only in case 1 (Fig. 6a, b and Table 2).
Table 2.
The hematologic characteristics of the three ET patients who progressed to PV referred at the time of ET diagnosis and at PV progression (pPV)
| Characteristics | Case 1 | Case 2 | Case 3 | |||
|---|---|---|---|---|---|---|
| ET | pPV | ET | pPV | ET | pPV | |
| WBC (1 × 109/l), median (range) | 9.9 | 26.2 | 7.1 | 12 | 9.2 | 10.69 |
| PLTs (1 × 109/l), median (range) | 696 | 1,003 | 1,184 | 1,500 | 519 | 516 |
| Neutrophils (1 × 109/l), median (range) | 7.5 | 21 | 5.6 | 7.6 | 5.4 | 6.73 |
| JAK2WT copy number (1 × 103), mean ± SEM | 5.8 ± 0.7 | 1 ± 0.01 | 5.4 ± 0.25 | 3.7 ± 0.2 | 3.2 ± 0.08 | 7.1 ± 1 |
| JAK2V617F copy number (1 × 103), mean ± SEM | 1.3 ± 0.3 | 5.7 ± 0.5 | 2.6 ± 0.1 | 3.6 ± 0.6 | 4.1 ± 0.5 | 10.2 ± 0.3 |
| DNA mutational burden ratio | 22 | 69 | 29.4 | 28 | 24 | 21 |
| mRNA mutational burden ratio | 18 | 85 | 32 | 49 | 56 | 59 |
| B2M copy number (1 × 103), mean ± SEM | 1,751 ± 27 | 2,256 ± 500 | 1,974 ± 75 | 1,953 ± 71 | 1,376 ± 142 | 1,749 ± 163 |
| GUSB copy number (1 × 103), mean ± SEM | 15.5 ± 0.6 | 10.4 ± 1.1 | 15.6 ± 0.8 | 16.1 ± 0.3 | 7.0 ± 0.06 | 6.9 ± 0.2 |
Fig. 6.
The JAK2V617F mutational burden ratio in the three ET patients who progressed to PV evaluated by the following three different methods: a the absolute allele-specific PCR, b the present absolute allele-specific q-RT-PCR, and c using GUSB to normalize the values achieved by the present q-RT-PCR assay
Discussion
Our study shows that, in the diagnostic approach to Ph− MPN patients, a q-RT-PCR assay to detect JAK2V617F expression levels offers comparable specificity than ASO-PCR and allele burden quantitative assays. Moreover, this method is probably more sensitive than these “standard” methods, as supported by the fact that the JAK2V617F mutational ratios were steadily superior at the RNA than at the DNA level in all the groups studied. These latter findings, showing an impact of the type of nucleic acid on the determination of the JAK2V617F burden, are in apparent contrast to those reported by Vannucchi et al. [11] who did not see differences with respect to the use of RNA or DNA. A likely explanation of these discrepancies may rely on the fact that we used buffy-coat preparations, which include platelets instead of the isolated leukocytes. These findings might also suggest that, using RNA, it is possible to avoid the cost-effective and time-consuming methods for leukocyte isolation.
The higher sensitivity to detect the JAK2V617F mutation at the RNA level was firstly demonstrated by the above mentioned study of Vannucchi et al. [11], who reported an increased percentage (9 %) of JAK2-mutated ET patients by using RNA instead of DNA. Thus, the mRNA template should be recommended for the diagnosis of ET patients, usually presenting a lower JAK2V617F allele burden, or in the monitoring of Ph− MPN treatment response.
The present q-RT-PCR method was derived from that recently proposed by Merker et al. [17] for the quantification of the JAK2WT and JAK2V617F transcript levels in Ph− MPNs. However, after testing more than 100 samples of patients with and without Ph− MPNs, these authors reported a low level of nonspecific amplifications in samples containing a high copy number of standard plasmids and in blood specimens from patients without Ph− MPNs. To eliminate these undesired amplifications, Merker et al. [17] established a mutant to wild type cutoff of <0.0005. We optimized Merker’s amplification reaction by reducing the amount of final concentrations of primers from 800 to 300 nM and of probes to 400 to 200 nM, respectively. We did not observe nonspecific amplifications in JAK2WT or JAK2V617F reaction by using plasmid standards, K562 and HEL cell lines, or JAK2V617F-positive and negative samples, tested as positive or negative controls (Fig. S1 A, B). The lack of nonspecific amplifications justifies the use of this test for diagnostic purposes.
Although the analysis of DNA is in principle technically simpler than that of RNA, discrepant results are often reported even after DNA-based techniques, so that Lippert et al. [19] have emphasized the need of using positive and negative quality controls, and calibration to a reference standard to improve reproducibility. More recently, the European Leukemia Net/MPN&MPNr-EuroNet group, to avoid that the variations in the performance of the plethora of qPCR assays routinely used to detect JAK2V617F could potentially impact on their clinical utility, selected the most sensitive and performing of nine DNA-based quantitative PCR assays as the optimal quantitative-polymerase chain reaction method for routine diagnosis and tracking of minimal residual disease in JAK2V617F-associated myeloproliferative neoplasms [20].
Unlike reports measuring DNA levels, we did not detect higher JAK2V617F mRNA expression levels in samples from ET patients with respect to PV. One possible explanation of this discrepancy may be that the higher sensitivity of the present q-RT-PCR method, by increasing the JAK2V617F copy numbers detected in ET samples, has canceled this difference.
To evaluate the putative prognostic value of the JAK2WT and JAK2V617F expression levels detected by the present assay, we search for significant correlations with the patient’s clinical–biologic parameters. The relatively small number of cases did not allow us to draw conclusive remarks on this issue. However, it is interesting to note that in PV patients, we observed a significant correlation between the JAK2WT expression levels and PLT count and a trend to a negative correlation with the Hb values. These latter findings may confirm the assumptions that even the residual amount of the normal JAK2 allele may impact on the different Ph-negative MPN entities [21].
Aiming to set up a sensitive q-RT-PCR method allowing the quantification of gene expression, and having to compensate for potential variability of this procedure, we firstly used ABL1 as HKG. However, we found that the ABL1 gene was not uniformly expressed among donor and JAK2V617F-negative and positive patient groups, being significantly lower in the group characterized by the JAK2V617F mutation. This finding clearly contradicts the most important requisite of a HKG that should be expressed at a similar level in all the tested samples. Therefore, although ABL1 is one of the most commonly used HKG in hematologic neoplasms [22], at least in Ph− MPNs, it should not be chosen for gene expression analysis. To identify CGs more suitable than ABL1, we evaluated two other widely used HKGs as B2M and GUSB. Both these genes showed similar expression levels within all the groups examined, but GUSB showed the most homogeneous medians and the narrowest distribution of values also in the JAK2V617F-positive cases (Fig. 4). Moreover, we confirmed that in control samples, JAK2, ABL1, and B2M and GUSB have comparable Ct variations, falling in all cases within three values and that B2M, compared to the other three genes, is clearly overexpressed (Fig.S3). The fact that these latter data exactly reproduced those achieved by the EAC program in a much larger number of cases than ours confirmed their reliability. Based on these observations, we would suggest GUSB, but not ABL1, as the most appropriate GC for this clinical setting.
The observed positive correlation between the expression levels of JAK2WT and ABL1 in the JAK2V617F-positive patient group further suggests the issue that JAK2V617F-positive MPN cases may represent a distinct biologic entity. In addition, this finding may support the model recently proposed by Irino et al. [23]. These authors, focusing on genes involved in the JAK-STAT signaling pathway, identified two upregulated genes in MPN patients: SOCS3, a known target of the JAK-STAT axis and a potentially novel target, and SPI1, encoding PU.1. The latter gene is a regulator of proliferation and differentiation of hematopoietic cells [24]. The pathogenic effect of JAK2 mutation appears mediated, at least in part, through the upregulation of PU.1. In addition, they showed that PU.1 is regulated by both JAK2 and ABL1, and suggested that SOCS3 and PU.1 are common downstream targets of both JAK2 and ABL1. Together, these observations and our results link the constitutive activation of JAK2 to the downregulation of the expression of ABL1.
The potential usefulness of GUSB as CG might also be supported by the additional findings observed in the three ET patients who progressed to PV. The serial monitoring of the DNA and RNA JAK2V617F mutational ratios and the JAK2V617F expression levels normalized with GUSB allowed to observe a significant increase of the JAK2V617F expression levels at PV progression in two of the three cases (case 1 and 3, Fig. 6c) and a trend toward statistical significance in the remaining case. By contrast, the JAK2 mutational ratios at DNA and RNA were increased only in case 1 (Fig. 6a, b). These data support in humans the findings recently demonstrated in transgenic mice showing that the levels of JAK2V617F expression influence the Ph− MPN phenotype: higher levels favor erythrocytosis whereas lower levels favor thrombocytosis [12]. Similarly, Barosi et al. observed a progression toward JAK2V617F homozygosity in serial DNA samples collected from 64 patients with PMF [25].
In conclusion, the q-RT-PCR assay hereby reported is a sensitive and accurate method to quantify the JAK2 mutational status that can also show clinical correlations suggesting the impact of the residual amount of the JAK2WT allele on the Ph− MPN disease phenotype. In addition, for the first time, we provided evidences that ABL1 is not a useful CG to obtain reliable JAK2V617F quantifications in Ph− MPN patients whereas GUSB turned out be more appropriate for this purpose. These findings might become clinically relevant in light of the availability of several new and effective targeted therapies which require sensitive and precise assessment of the patient’s response to treatments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 266 kb)
Acknowledgments
This work was partially supported by research funding from the University of Roma “La Sapienza” (to C.N. e G.C.), Fondazione Roma (to C.N. e G.C.), Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN), and the Italian Association for Cancer Research (IG-11949 to C.N.).
Conflict of interest
The authors report no potential conflicts of interest.
References
- 1.Lacout C, Pisani DF, Tulliez M, Gachelin FM, Vainchenker W, Villeval JL. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Wahab O. Genetics of the myeloproliferative neoplasms. Curr Opin Hematol. 2011;18:117–123. doi: 10.1097/MOH.0b013e328343998e. [DOI] [PubMed] [Google Scholar]
- 3.Klampfl T, Harutyunyan A, Berg T, Gisslinger B, Schalling M, Bagienski K, Olcaydu D, Passamonti F, Rumi E, Pietra D, Jäger R, Pieri L, Guglielmelli P, Iacobucci I, Martinelli G, Cazzola M, Vannucchi AM, Gisslinger H, Kralovics R. Genome integrity of myeloproliferative neoplasms in chronic phase and during disease progression. Blood. 2011;118:167–176. doi: 10.1182/blood-2011-01-331678. [DOI] [PubMed] [Google Scholar]
- 4.Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OA. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;118:1723–1735. doi: 10.1182/blood-2011-02-292102. [DOI] [PubMed] [Google Scholar]
- 5.Zhan H, Cardozo C, Yu W, Wang A, Moliterno AR, Dang CV, Spivak JL. MicroRNA deregulation in polycythemia vera and essential thrombocythemia patients. Blood Cells Mol Dis. 2013;50:190–195. doi: 10.1016/j.bcmd.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafer AI. Molecular basis of the diagnosis and treatment of polycythemia vera and essential thrombocythemia. Blood. 2006;107:4214–4222. doi: 10.1182/blood-2005-08-3526. [DOI] [PubMed] [Google Scholar]
- 7.Passamonti F, Rumi E. Clinical relevance of JAK2 (V617F) mutant allele burden. Haematologica. 2009;94:7–10. doi: 10.3324/haematol.2008.001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vannucchi AM, Pieri L, Susini MC, Guglielmelli P. BCR-ABL1-negative chronic myeloid neoplasms: an update on management techniques. Future Oncol. 2012;8:575–593. doi: 10.2217/fon.12.50. [DOI] [PubMed] [Google Scholar]
- 9.Kim HR, Choi HJ, Kim YK, Kim HJ, Shin JH, Suh SP, Ryang DW, Shin MG. Allelic expression imbalance of JAK2 V617F mutation in BCR-ABL negative myeloproliferative neoplasms. PLoS ONE. 2013;8:512–518. doi: 10.1371/journal.pone.0052518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, Zhao ZJ. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vannucchi AM, Pancrazzi A, Bogani C, Antonioli E, Guglielmelli P. A quantitative assay for JAK2(V617F) mutation in myeloproliferative disorders by ARMS-PCR and capillary electrophoresis. Leukemia. 2006;20:1055–1060. doi: 10.1038/sj.leu.2404209. [DOI] [PubMed] [Google Scholar]
- 12.Skoda RC, Tiedt R, Hao-Shen H, Sobas M, Looser R, Dirnhofer S, Schwaller J. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD and phenotypes in transgenic mice. Blood. 2008;111:3931–3940. doi: 10.1182/blood-2007-11-123810. [DOI] [PubMed] [Google Scholar]
- 13.Thiele J, Kvasnicka HM. The 2008 WHO diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Curr Hematol Malignancy Rep. 2009;4:33–40. doi: 10.1007/s11899-009-0005-6. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- 15.Elia L, Gottardi E, Floriddia G, Grillo R, Ciambelli F, Luciani M, Chiusolo P, Invernizzi R, Meloni G, Foà R, Saglio G, Cimino G. Retrospective comparison of qualitative and quantitative reverse transcriptase polymerase chain reaction in diagnosing and monitoring the ALL1-AF4 fusion transcript in patients with acute lymphoblastic leukaemia. Leukemia. 2004;18:1824–1830. doi: 10.1038/sj.leu.2403448. [DOI] [PubMed] [Google Scholar]
- 16.Baxter EJ, Scott LM, Campbell P, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 17.Merker JD, Jones CD, Oh ST, Schrijver I, Gotlib J, Zehnder L. Design and evaluation of a real-time PCR assay for quantification of JAK2 V617F and wild-type JAK2 transcript levels in the clinical laboratory. J Mol Diagn. 2010;12:58–64. doi: 10.2353/jmoldx.2010.090068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beillard E, Pallisgaard N, van der Velden VHJ, Bi W, Dee R, van der Schoot E, Delabesse E, Macintyre E, Gottardi E, Saglio G, Watzinger F, Lion T, van Dongen JJ, Hokland P, Gabert J. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)—a Europe Against Cancer program. Leukemia. 2003;17:2474–2486. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 19.Lippert E, Girodon F, Hammond E, Jelinek J, Reading NS, Fehse B, Hanlon K, Hermans M, Richard C, Swierczek S, Ugo V, Carillo S, Harrivel V, Marzac C, Pietra D, Sobas M, Mounier M, Migeon M, Ellard S, Kröger N, Herrmann R, Prchal JT, Skoda RC, Hermouet S. Concordance of assays designed for the quantification of JAK2V617F: a multicenter study. Haematologica. 2009;94:38–45. doi: 10.3324/haematol.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jovanovic JV, Ivey A, Vannucchi AM, Lippert E, OppligerLeibundgut E, Cassinat B, Pallisgaard N, Maroc N, Hermouet S, Nickless G, Guglielmelli P, van der Reijden BA, Jansen JH, Alpermann T, Schnittger S, Bench A, Tobal K, Wilkins B, Cuthill K, McLornan D, Yeoman K, Akiki S, Bryon J, Jeffries S, Jones A, Percy MJ, Schwemmers S, Gruender A, Kelley TW, Reading S, Pancrazzi A, McMullin MF, Pahl HL, Cross NC, Harrison CN, Prchal JT, Chomienne C, Kiladjian JJ, Barbui T, Grimwade D. Establishing optimal quantitative-polymerase chain reaction assays for routine diagnosis and tracking of minimal residual disease in JAK2-V617F-associated myeloproliferative neoplasms: a joint European Leukemia Net/MPN&MPNr-EuroNet (COST action BM0902) study. Leukemia. 2013;27:2032–2039. doi: 10.1038/leu.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT, Duffy A, Boyd EM, Bench AJ, Scott MA, Vassiliou GS, Milligan DW, Smith SR, Erber WN, Bareford D, Wilkins BS, Reilly JT, Harrison CN, Green AR. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366:1945–1953. doi: 10.1016/S0140-6736(05)67785-9. [DOI] [PubMed] [Google Scholar]
- 22.Gabert J, Beillard E, van der Velden VHJ, Bi W, Grimwade D, Pallisgaard N, Barbany G, Cazzaniga G, Cayuela JM, Cavé H, Pane F, Aerts JL, De Micheli D, Thirion X, Pradel V, González M, Viehmann S, Malec M, Saglio G, van Dongen JJ. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe Against Cancer Program. Leukemia. 2003;17:2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 23.Irino T, Uemura M, Yamane H, Umemura S, Utsumi T, Kakazu N, Shirakawa T, Ito M, Suzuki T, Kinoshita K. JAK2 V617F-dependent upregulation of PU.1 expression in the peripheral blood of myeloproliferative neoplasm patients. PloS ONE. 2011;6:2214822. doi: 10.1371/journal.pone.0022148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koschmieder S, Rosenbauer F, Steidl U, Owens BM, Tenen DG. Role of transcription factors C/EBPalpha and PU.1 in normal hematopoiesis and leukemia. Int J Hematol. 2005;81:368–377. doi: 10.1532/IJH97.05051. [DOI] [PubMed] [Google Scholar]
- 25.Barosi G, Bergamaschi G, Marchetti M, Vannucchi AM, Guglielmelli P, Antonioli E, Massa M, Rosti V, Campanelli R, Villani L, Viarengo G, Gattoni E, Gerli G, Specchia G, Tinelli C, Rambaldi A, Barbui T. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood. 2007;110:4030–4036. doi: 10.1182/blood-2007-07-099184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 266 kb)